Abstract
Every year, close to 8 million tons of waste crab, shrimp and lobster shells are produced globally, as well as 10 million tons of waste oyster, clam, scallop and mussel shells. The disposed shells are frequently dumped at sea or sent to landfill, where they modify soils, waters and marine ecosystems. Waste shells are a major by-product, which should become a new raw material to be used to the best of their potential. There are a number of applications for waste shells in many fields, such as agriculture, medicine, chemical production, construction, environmental protection, cosmetic industry, food and feed industry, and a plethora of other (often niche) applications, which are being developed by the day. This review provides a broad picture of crustacean and mollusc shell waste management and reutilization possibilities, reviewing well established, current, and potential strategies, particularly from the standpoint of sustainability challenges and energy demand.
1. Introduction
The total world fisheries and aquaculture production showed a 41% growth in the 2000–2019 period, reaching 178 million tons in 2019, and representing an expansion of 52 million tons compared to the year 2000. Of the total production, molluscs comprised 13%, while crustaceans comprised 9% [1]. Every year, close to 8 million tons of waste crab, shrimp and lobster shells are produced globally, comprising up to 60% of overall crab mass [2]. On top of that, over 10 million tons of mollusc shells are produced each year, of which over 70% is represented by oyster, clam, scallop and mussel shells [3]. Mollusc shells account for 65–90% of live weight, depending on the species [4,5]. Mussels in particular have the greatest increase in production and shell contribution compared to other shellfish species [6]. A significant portion relates also to abalone and other gastropod shells [7]. Shells are thus a major by-product, which should not become waste, but rather a new raw material to be used to the best of their potential.
However, sadly, disposed shells are primarily dumped at sea or sent to landfill [8]. Thereafter they modify soils, waters and marine ecosystems, particularly if their disposal is uncontrolled [9]. Consequently, piles of shells can be found all over the world, causing environmental damage by the decomposition of residual tissues attached to shells, emanating foul odors, as well as adding to visual pollution [7]. Shells are not only disposed as pre- or post-consumer residues, but also as losses or wastes of harvest and sorting in both fisheries and aquaculture, often due to inadequate preservation infrastructure in low-income countries [10]. Furthermore, shells can be detached from flesh at various processing steps, by the aquaculture producers, processing companies, restaurants or by individual consumers. Consummation habits regarding molluscs and crustaceans vary in different countries, as they can be sold and served in full shell or processed. Shell waste is thus produced in a number of various locations, making large-scale valorization difficult [5]. Shells should therefore be disposed of in relation to geographical distances between their source and processing facilities, considering the cost-effectiveness of transport, fossil fuels, and potential applications [11]. Shells are composed mainly of chemically stable calcium carbonate (CaCO3) (Table 1). The decomposition of shells is impossible without any treatment. Their thermal decomposition requires high temperatures of over 1000 °C, resulting in high-energy consumption and the frequent emission of greenhouse gases [12]. The disposal and handling of waste shells can thus become a major operational and financial burden.

Table 1.
Chemical composition (%) of various raw bivalve shells. Adapted from Zhan et al. [7]. Their habitat location and shell type determine their exact chemical composition. When calcined (pyrolysed), shell chemical composition modifies, as calcium carbonate (CaCO3) converses into lime (CaO).
People have traditionally always given value to shells, which is a practice to retain from environmental, economic, and social standpoints. As far as we know from archeological findings of durable remains, shells have a long history of recognition as a valuable resource. They were used already in Paleolithic times [13,14,15], as tools or ornaments. In many cultures, shells and shell beads have been used for money [16,17], even to date [18] demonstrating how standards determine value and illustrating the forms of social authority that constitute worth. In modern times, some mollusc species are in high demand due to the aesthetic value of their shells. It is estimated that at least 5000 mollusc species are marketed for ornamental purposes, mostly gastropods, the trade which has not been thoroughly documented nor quantified [19,20]. Additionally, several million tropical gastropods are exported each year in the scope of the marine ornamental trade to become clean-up animals in tropical aquaria in their life-span, after which the issue of their shells is vague [21]. Communities try to contribute to resolving the issue of sustainable use of existing mollusc shell waste and numerous projects are launched worldwide to sensitize the local population on their reuse as decorative and useful items. However, those practices are just a drop in the sea until the valorization of shells and the unlocking of markets for new products thereof becomes the prime consideration of the industry (Figure 1).
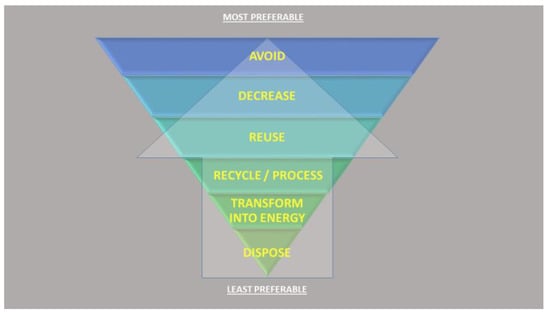
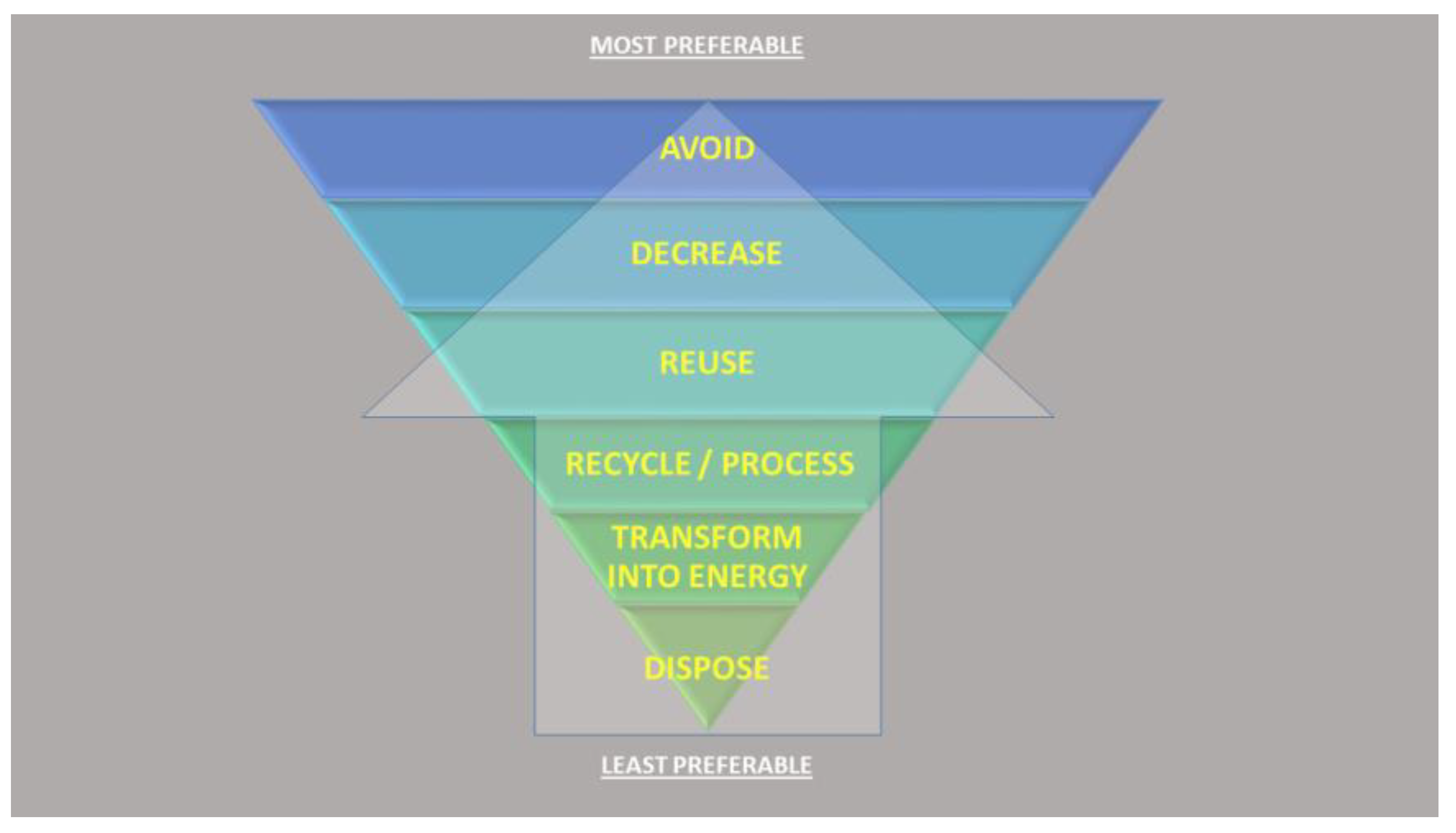
Figure 1.
The hierarchy of waste disposal. With reference to the increase in food demand, shell waste could only be considered from the point of reuse down to dispose, as adapted from the European Commission’s Green Growth and Circular Economy package of the Europe 2020 Strategy (https://ec.europa.eu/environment/green-growth/index_en.htm).
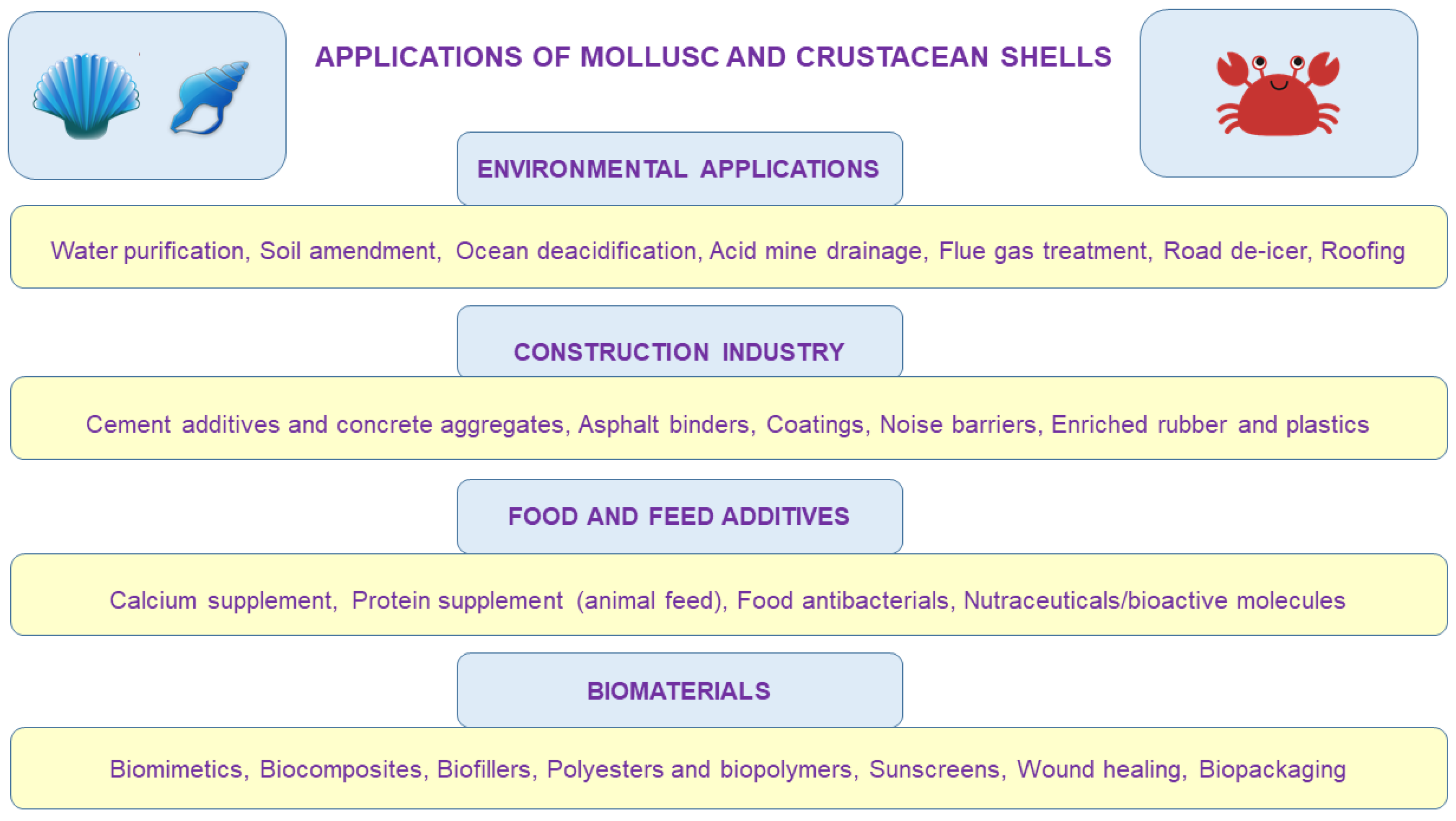
The purpose of this paper is thus to provide a broad picture of shell waste reutilization possibilities, reviewing well established, current, and potential strategies (Figure 2), particularly from the standpoint of sustainability and energy demand. Our goals were to investigate the importance and status of mollusc and crustacean shell waste by reviewing previous studies, which will help in understanding the complexity of shell reutilization; and to investigate their valorization options in terms of bioeconomy prerequisites. There is a number of other waste shell application possibilities than the ones described in this work, such as small-scale niche valorizations, or unmentioned potential biomedical applications that are mainly based on laboratory experiments, which may be considered as limitations of this review. However, unlike other papers on the topic of shell valorization, this review lays out shell applications with global perspective, as well as critical description of the sustainability challenges for each waste shell reutilization strategy described.
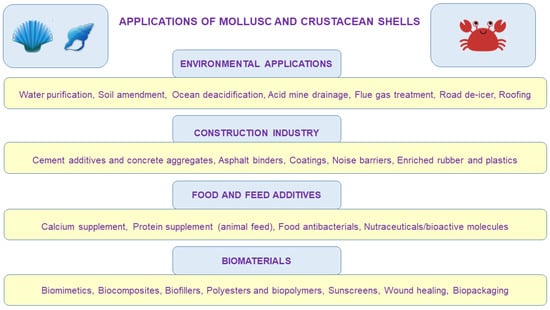
Figure 2.
Conceptual diagram describing some of the numerous potential applications of waste mollusc and crustacean shells. To a larger or lesser extent, they are reutilized for applications in agriculture, medicine, chemical production, construction, environmental protection, cosmetic industry, food and feed industry, and a plethora of other (often niche) applications, which are being developed by the day.
Methodology
The bibliometric data, including research articles, books, book chapters, reviews, yearbooks, handbooks, and proceedings were accessed from Scopus database (https://www.scopus.com/search/form.uri?display=basic#basic) in English. In this review, publications from 1990 up to 2022 were included. Publications dating from year 1990 till 1999 comprise 4.3%, from 2000 till 2009 13.7%, from 2010 till 2019 40.2%, while publications dating from year 2020 till November 2022 comprise 44.44%. Thus, approximately 84% of presented results were published in the relatively short period of 12 years. Five publications were included after cross-citation analysis of the selected documents. The key words for searching the database of available literature were: shell waste application (2776 document results); shell waste valorization (304 results); mollusc waste (1525 results); mollusc shell waste (483 results); crustacean waste (1442 results); crustacean shell waste (265 results) without inserting values between Boolean operators. The document results were further narrowed based on their title and abstract, in order to be selected and appropriately included in this review. It is notable that the area primarily represented in search hits was the construction sector, referring mostly to the use of shell waste as concrete components, followed by environmental applications, primarily agriculture. The novel literature available, dating from the previous three years, reports largely on biomedical and cosmetic applications, fields that are growing exponentially.
2. Cleaning Shells
Within the meaning of Regulation (EU) No 1069/2009, shells with soft tissue and flesh completely removed are not considered as by-products, but as waste. For some reutilization purposes, such waste shells need to be cleaned prior to their industrial use. Particular applications call for specific ways of pretreating and cleaning. Namely, for using bivalve shells as a source of CaCO3, any residual organic material would decrease the purity of the CaCO3 [8,22,23]. Shells with organic residue attached can be washed with large amounts of freshwater to remove salt, and after that heated at 500 °C to burn off the residual organic material [24]. The more energy efficient and environmentally friendly method for shell cleaning is using the enzymatic cleaning, as enzymes can digest proteins at temperatures close to ambient and may also produce an additional product stream, such as protein hydrolysate [8]. Enzymatic hydrolysis can also be applied for cleaning of shrimp shells and bio-conversing them into valuable nitrogen-containing compounds, although intact shells are poorly hydrolyzed by chitinases unless pre-demineralization and deproteinization with chemicals or proteases is conducted [25]. To facilitate the hydrolysis of crayfish shell into oligosaccharides, ball milling and ultrasound-assisted hydrogen peroxide decolorization is applied for pretreating [26].
For ammonia nitrogen removal in a biological nitrifying system using oyster shells as alkalinity-releasing filling materials, shells can be cleaned by ultrasound in water for 20 min, and then dried at room temperature [27]. To remove the attached impurities from mollusc shells, in order to synthesize aragonite needles by carbonation, shells are cleaned with water and ethyl alcohol [28]. For the purpose of catalyst preparation, crab shells can be manually cleaned to remove protein and other organic material, and washed with warm water before drying at 105 °C [29].
On a smaller scale, shells of gastropods are sometimes buried for a certain period, so that an internal cleaning process can take place through decomposition by detritivore organisms in the soil [19]. Cleaning of residual organic material from a smaller quantity of shells can also be accomplished by brushing, boiling or sun-drying [30,31].
Sustainability Challenges
Cleaning the shells with fresh water in order to reduce the salt content of the final product, to avoid the corrosion of the equipment, and to obtain a more concentrated CaCO3, is very demanding on water, as the water consumption ranges from 0.2 to 0.8 m3 per ton of shells [24].
Although biodegradable, natural, and performing targeted reactions, enzymes are very complex molecules, and often extremely costly, which questions their use in some applications [32].
3. Environmental Applications
Even nowadays, the majority of produced mollusc and crustacean shells remain a waste. Therefore, the understanding of shell valorization as a number of potential applications, which could help with decreasing the burden of waste shells, is of utmost importance. A domain where they could be vastly employed is the environmental amendment and remediation. The list below is by no means exhaustive.
3.1. Water Purification/Bio-Filters
It is known that waste shells can be successfully used in wastewater (WW) treatment, particularly in phosphate removal from WW [33]. Phosphorus, along with nitrogen and other nutrients present in WW, leads to eutrophication resulting in algal blooms and oxygen depletion, fatal for aquatic organisms [34]. Adsorption is the simplest and most cost-effective method for the removal of phosphorus, depending on the source of the adsorbent. Waste mollusc shells can act as adsorbents for both industrial and agriculture WW treatment. However, raw shells are less effective at phosphate removal than calcined shells, as they have a lack of stability in acidic environment where they decompose and form sludge, which only adds to the problem of WW [8]. The calcination or pyrolization of shells leads to lime (CaO) formation, which is a common adsorbent, typically obtained from limestone [35]. Limestone needs to be heated to 600 °C to yield lime, and lime is produced by calcination of shells just as from quarried limestone [36]. Lime dissolves in water to produce calcium hydroxide, which dissociates into calcium and hydroxide ions. These ions react with ortophosphates in water and yield calcium phosphate precipitates, one of them being a liming agent hydroxyapatite, the insoluble form of calcium phosphate [8]. Phosphate removal from recirculating marine aquaria is often neglected. However, in seawater, (oyster) shell waste is also a very efficient bioadsorbent for phosphate removal. The main mechanism for raw shells is adsorption, and for calcined shells there is also a co-mechanism of precipitation [37].
Nitrate removal from WW of fish farms can be attained with woodchip bioreactors enriched with sulfur granules and crushed seashells [38]. Shells contribute by the alkalizing effect on the low-alkaline aquaculture effluents, and compensate for the alkalinity loss from autotrophic denitrification process [39]. Additionally, the presence of crushed oyster shells was shown to accelerate the process of biological nitrification and ammonia removal in synthetic WW, by pH buffering [27].
Soft calcite, prepared from waste blue mussel shells, can act as an inorganic sponge, which adsorbs dyes from aqueous solution and absorbs crude oil from seawater with good recyclability. Soft calcite has an impressive absorption capacity of 977% ± 84% of crude oil and can be used as a high-value material for oil remediation [40].
The crustacean shell waste proved extremely successful in a number of WW applications. The demineralization and deproteination of crustacean shells yield water-insoluble chitin, with final characteristics of both cellulosic fibers and of proteins, which when deacetylated yield water-soluble chitosan [41]. Both chitin and chitosan are excellent flocculants for potable water and industrial effluents. They have been applied to remove a variety of water pollutants, such as proteins, lipids, greases, pesticides, PCBs, PAHs, textile dye residues and heavy metals [42].
The formation of insoluble metal hydroxides by metal adsorption on the surface of calcined shells contributes well to heavy metal removal from water, WW and landfill leachate [43,44,45]. It was established that the higher the temperature of calcination, the better the efficiency of calcined shells in heavy metal filtering, related to the increase in the specific surface area upon calcination [9]. The initial water pH, dosage of sorbent, and grain size may affect the heavy metal removal efficiency of shells. The shell powder in aragonite and calcite phase (razor clam and oyster shell, respectively) may remove lead (Pb2+), cadmium (Cd2+) and zinc (Zn2+) from contaminated water [46]. However, the oyster calcite has a higher biosorption capacity to Pb2+, while the razor clam aragonite has a higher capacity for Cd2+ [46].
3.2. Heavy Metal Adsorbents/Soil Amendment
Soil pollution by heavy metals might result from industrial activities, fertilizers, pesticides, livestock residues, and other wastes with high metal content. It can represent risks for ecosystems and for population, by introduction into the food chain, or by decreasing the quality of land, as metals do not undergo microbial or chemical degradation in the soil, and persist for a long time [47].
The property of calcined shells to adsorb heavy metals in water can be translated to remediation of soils contaminated with heavy metals. Contaminated soil pH is increased by CaCO3, which leads to the formation of calcium silicate hydrate and calcium aluminate hydrate, subsequently forming metal hydroxides and a relatively impermeable soil layer, thus effectively ameliorating soils [48]. For agricultural purposes, this holds many advantages. For example, calcined oyster shells applied to agricultural soil lowered Cd2+ and As2+ contents in the edible part of cabbage by 98% (Cd2+) and 73% (As2+), making it safe for consumption. However, the best results were attained by the calcination of oyster shells at up to 800 °C, not negligible in energy demand [49]. Lower temperatures for drying seashells (105 °C) seemed to have yielded a promising material for immobilizing PbS in the industrial arid soil. The decrease in soil toxicity varied relative to the dosage and time of application, however the soil remained a high environmental risk category even after the treatment [50]. For copper-rich vineyard and mine soils, the application of crushed mussel shell diminished Cu2+ desorption rates up to 86% at pH 3. Such additions could thus contribute to reduce potential hazard of copper-enriched soils under acidification events [51]. Typically, the neutralization of acidity and metal contamination in soils by liming improves fertility and oxygen level of soils, and crushed mollusc shells may be a valuable replacement for mined limestone.
3.3. Acid Mine Drainage
Acid mine drainage (AMD) is a global problem associated with sulfates and heavy metals entering the environment, mainly from coal mining when pyrite is exposed to water and oxygen. Sulfide is than oxidized to sulfuric acid, which lowers water pH and catalyzes leaching of metals [8]. Such drainage has harmful effects particularly on aquatic environment [52].
Biomaterials retrieved from dried and pulverized shrimp shell and mussel byssus had significant effects on both sorption process and pH of the AMD, and the enhanced precipitation of metal hydroxides. The kinetic studies in microcosms [53] demonstrated that only a 200 min contact time with this mixture is sufficient to transform AMD into water suitable for non-potable reuse. Clam shells also have a high capacity for removing heavy metals from simulated, synthetic AMD in lab-scale constructed wetlands [54]. Constructed wetlands have been recognized as a potential green technology for mine drainage treatment due to their effectiveness, low cost, simple maintenance, and high biodiversity value [55,56]. In a constructed wetlands study, both oyster shells and limestone had high AMD neutralization potential during a 7-month operation. Accumulation in the substrates was a principal pathway for removing heavy metals [57]. Therefore, the recycling of waste bivalve shells as substrates in constructed wetlands for AMD treatment might encourage the reduced usage of natural minerals such as limestone.
3.4. Ocean Deacidification
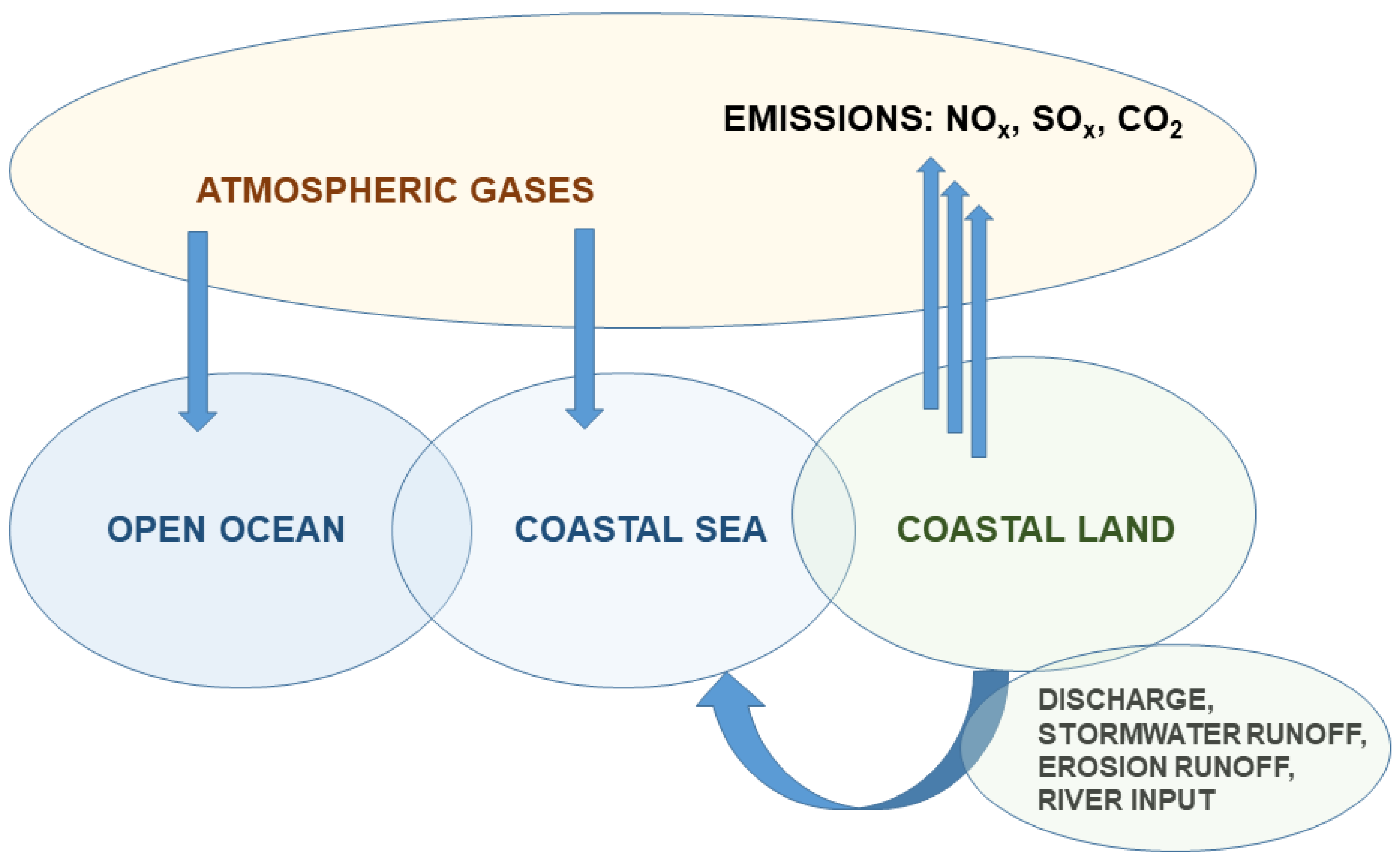
Acidification is not only related to WW and contaminated soils, it is a major problem in our seas and oceans, endangering health and the development of aquatic biota. Compared to open oceans, seas in populated coastal regions are more prone to ocean acidification (OA) due to hydrodynamic processes and human activities, often leading to rapid and extreme pH decreases [58] (Figure 3).
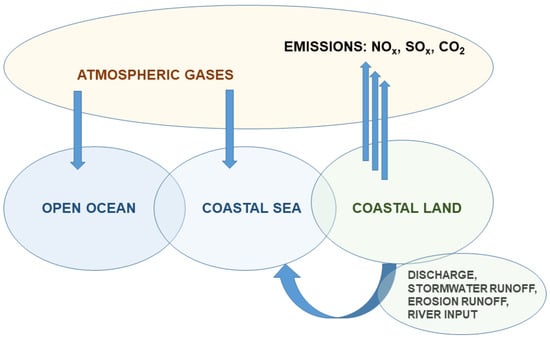
Figure 3.
Schematic diagram of the sources of ocean acidification. Coastal land is exposed to a variety of acidic sources, adding to the coastal sea, and varying across time and space, as adapted from Clements and Chopin [59].
Ocean acidification is considered detrimental to marine calcifier organisms, particularly coccolithophores, calcifying algae, and corals [60]. They appear to be more sensitive at the larval stage than at the adult stage, OA affecting both natural and farmed organisms. Shellfish production may be negatively impacted by increasing the seawater PCO2 [61]. Many actions have been proposed to increase seawater alkalinity, such as the restoration of macroalgae and seagrass beds, which remove CO2 form seawater during photosynthesis [62]. One of such actions is aimed at the utilization of waste shells as a useful material, which can provide a variety of ecosystem benefits. The placement of shell reefs adjacent to shellfish farms is a way for buffering the decreases of seawater carbonate due to OA [61]. On the North America’s Atlantic coast, the returning of crushed waste shells to mudflat sediment elevated the pH and carbonate geochemical conditions within sediment pore water, and increased clam settlement and survival in comparison to unbuffered sediments [59]. Waste shells deposited in the mussel banks not only control the benthic acidity, but also aid to avoid the speciation of heavy metals, such as Cd associated with sulfur at low pH, which prevents mineralization leading to the dissolution and migration of Cd into the water column [63]. Shells can be used to mitigate the effects of organic pollution on marine sediments, particularly in defaunated sites, as they reduce the accumulation of by-products from anaerobic metabolic pathways, decrease the release rate of ammonium to the water column and prevent the negative ecological consequences of eutrophication [64]. Waste shells in the ocean also act as settlement substrates for free-living shellfish larvae and as shelter for other aquatic organisms [65]. They can thus serve as reef restoration substrates (Figure 4) and promote biodiversity through complex habitat formation [5]. However, in spite of the agreement for improved and coordinated governance regarding OA, consistent OA policies are lacking [66].

Figure 4.
Discarded oyster shells from restaurants, usually destined for landfill, are being recycled to create shellfish reefs, in scope of the biggest community-driven reef restoration in Australia. ABC News (https://www.abc.net.au/news/2021-10-16/oyster-shell-waste-artificial-reef/100538272).
3.5. Flue Gas Treatment
The combustion of fossil fuels generates air pollutants such as carbon dioxide (CO2), nitrogen dioxide (NO2), and sulfur dioxide (SO2). In particular, SO2 is a major air pollutant causing acid rain [67]. The ability to remove pollutants such as SO2, sulfur trioxide (SO3), hydrogen sulfide (H2S), NOx, and CO2 sequestration, makes the calcined shell waste an excellent biosorbent material [9]. It can be applied to industries as acid gas cleaning agent to reduce air pollution, particularly since the SO2 removal activity and reaction rate of calcined/hydrated oyster shells are higher than those of the calcined/hydrated limestone [68]. Calcined oyster shells used to prepare CaO adsorbent had the primary conversion rate for CO2 up to 33.47% [69]. As the air pollution control is mainly focused on restriction of the SO2 emissions, the sulfur-fixing agents are mostly calcium-based, containing a mix of metal oxides. Thus, the use of the waste shell powder is applicable for flue gas desulphurization, although the accumulation of Na, Mg and other compounds from shells in the slurry can also enhance desulphurization process [7,67].
3.6. Road De-icing
Crushed freshwater bivalve shells can be used in road de-icing processes as an alternative to sodium chloride (NaCl), which leads to water pollution in times of snow melt [3,70]. A low-resolution analysis of Na/Ca in freshwater bivalve shells recorded persistent sodium pollution in streams due to the road-salt usage [70]. Road-salt is applied as NaCl as the most widely used chemical owing to its abundance and low cost, as magnesium chloride (MgCl2) brines since they have better ice melting performance at cold temperatures, and as calcium chloride (CaCl2). Chloride-based road de-icers and anti-icers can be detrimental to both urban and natural environments [71]. Interestingly, calcium-magnesium acetate, or any calcium acetate derivative showed environmentally friendly characteristics when used for road de-icing. To that end, waste CaCO3 derived from waste shells could be used to form calcium acetate for use on roads, when mixed with some vegetable waste products as acetate donors [5].
3.7. Green Roofing
In urban areas, green roofs are gaining popularity due to a growing understanding of the impact of green vegetation on environmental health. They are complex systems, with a vegetation layer covering the outermost surface of a building. Green roofs provide building insulation and energy benefit, depending on the geographical location of such roofing [72]. They have a positive impact on the reduction of runoff water as a function of the depth of substrate and its moisture prior to the rain event [73]. Mollusc shells are excellent material for green roofing structures, such as drainage layers, or to help with the neutralization of acid rain, due to their bioremediation potential [10].
3.8. Sustainability Challenges
The calcination of waste shells to yield lime requires high temperatures and special machinery. High-energy conversion of shells is not a sustainable solution for large amounts of shells. Solutions using raw shells as bio-filters should be given consideration to override their instability in acidic waters and sludge formation.
A variety of heavy metals can be found in some (waste) shells, and their levels are influenced by seawater PCO2. As shell dissolution occurs at high PCO2, some of these metals might be released into the marine water column [61], presenting a further environmental burden.
Furthermore, for any potential green roof layer, various shell types must undergo water-saturated weight tests to establish their actual feasibility on buildings [5]. In addition, a number of the mentioned waste shell applications of sustainable nature are extremely promising when conducted in laboratory- and small-scale, yet need to be up-scaled for testing of their true applicability and sustainability.
4. Food and Feed Additives; Nutraceuticals
For any potential application of the waste shells, raw material is used in the form of natural shells, calcined shells, biomolecules of the organic matrix, natural polysaccharides and derivatives (chitin and water-soluble chitosan). Natural shell powder in particular is a relevant source of calcium, and is valuable in food and nutraceutical industry, while chitin is widely used to immobilize enzymes and whole cells with applications in the food industry, such as the clarification of fruit juices and processing of milk. Furthermore, chitosan is used as a preservative, thickener and stabilizer for sauces, and has protective, fungistatic, and antibacterial properties in fruit coatings [9,74].
4.1. Calcium Supplement
Calcium supplementation from shell powders is widely used to improve bone health and blood circulation of livestock, the quality of egg-shells in laying birds, and the quality of milk products [5,7]. It is a cheaper source of CaCO3 and performing equally to limestone in Ca supplementation [5]. The CaCO3 extracted from oyster shells is also used as a food supplement for Ca replacement in humans. The extracted carbonate has efficient intestinal absorption and increases bone mineral density, especially in the lumbar region, in people with deficiency in Ca, such as elderly population [75]. The effect of Ca supplementation in osteoporotic patients in the form of oyster shell electrolysate was reported to raise serum Ca and increase urinary Ca excretion in vitamin D-deficient states more readily than CaCO3 [76].
4.2. Protein Supplement
Meals deriving from crustacean shell waste are usable feed ingredients. Shrimp waste meal (consisting of the shells, heads and appendages) is found to be rich in essential amino acids, particularly lysine [77]. Their shell fermentation product (mostly composed of protein) can be applied as a feed ingredient in the aquaculture industry, both in hatchery and grow-out systems [78]. The deproteinization process of crustacean shell waste helps to extract protein content and the transformation of chitinous waste into chitin-derived products. For deproteinization, mostly concentrated acids and bases are used, which results in structural changes of the chitin (removal of acetyl group and depolymerisation), and undesired end products, creating also waste disposal problems [79]. To overcome those impediments, a protocol of covalent immobilization of chitinase on glutaraldehyde-activated chitosan was developed [80].
4.3. Antibacterials for Food
Macromolecular chitosan, synthesized from chitin of crustacean shells, has powerful antibacterial properties. It inhibits bacterial growth on surfaces of aquatic and meat products, and has a bacteriostatic rate of 99.9%. Thus, it can increase the shelf life of such products to up to 10 days in summer, with no adverse effects on consumers [74,81]. Chitosan has inhibitory effect even on Bacillus cereus spore germination and (out)growth. In a spore test, with concentrations corresponding to 102–103 CFU/mL, which is relevant to spore concentrations in food, respectively less chitosan was needed to suppress (out)growth compared to higher spore numbers (equivalent to 108 CFU/mL) [82].
4.4. Bioactive Molecules/Antioxidants
Chitosan and chitosan oligosaccharides act as potent antioxidants by scavenging free radicals (oxygen, hydroxyl, superoxide and alkyl groups) responsible for a number of diseases. They act as hydrogen donors to prevent the damaging oxidative sequences [83]. Furthermore, crustacean waste shells are abundant sources of beta-carotene and astaxanthin, the latter being responsible for the pink pigmentation of crustaceans and wild salmonids. Astaxanthin in the form of carotenoprotein can be extracted with a yield of 49% by treating shrimp waste with proteolytic enzymes [84]. If introduced in the fish feed, astaxanthin extracted from shrimp shells can induce growth performance and skin pigmentation of orange clownfish [85]. Lobsters fed diets containing crab processing waste had higher shell mineral concentrations, although not as thick or strong as shells from animals fed the control diet [86]. Chitin and chitosan can also be used to produce glucosamine products, marketed as anti-arthritic agents [87].
4.5. Bioactive Molecules/Cholesterol Reduction
Chitosan acts as a hypocholesterolemic agent, being effective in the reduction of low-density lipoprotein (LDL)-cholesterol levels and its fat-binding properties [88]. Ingesting chitosan oligosaccharide for two weeks resulted in a decrease in LDL-cholesterol by 6% and a boost in high-density lipoprotein (HDL)-cholesterol by 10% [89]. Chitosan and chitosan oligosaccharide act as fat scavengers in the digestive tract by removing cholesterol via ionic binding with bile salts and acids [89]. However, although used for weight loss, chitosan is non-absorbable and active only in the gastrointestinal tract, thus the weight loss associated with chitosan is most likely correlated to increased fecal excretion of neutral fats [90].
4.6. Bioactive Molecules/Blood Pressure Reduction
Chitosan oligosaccharides were found to have angiotensin-converting-enzyme (ACE) inhibitory activity, reducing peripheral blood pressure and having an anti-hypertensive effect [83]. Chitosan is a potential carrier of antihypertensive drugs due to its muco-adhesive attribute, permeation enhancement, biocompatibility and biodegradability [91]. When carboxylating chitosan oligosaccharides with -COCH2CH2COO− groups to obtain chemical structures similar to the commercially available ACE inhibitor, ACE inhibition increased with increased degrees of substitution [92]. Specifically, chitin derivative designated as aminoethyl-chitin (AEC) with 50% degree of deacetylation (AEC50), was found a competitive inhibitor, which also effectively decreased systolic blood pressure [92].
4.7. Sustainability Challenges
Extracting chemicals from waste crustacean shells is quite destructive and expensive, requiring fractionation for separating different components. Protein is mostly removed with sodium hydroxide solution while the decomposition of CaCO3 requires hydrochloric acid (both corrosive solvents). To make chitosan, chitin is treated with 40% concentrated sodium hydroxide solution, while the production of 1 kg of chitosan from shrimp shells requires more than 1000 L of water [2]. A sustainable fractionation method to separate proteins, CaCO3 and chitin without hazardous chemicals and to minimize waste needs to be set up. Additionally, it should be noted that the amount of extractable shell pigment decreases with fermentation time, thus in an industrial process it is advisable to carry out pigment removal prior to fermentation [93]. Regarding safety issues, caution is recommended for food, feed and other uses of pulverized shells due to possible traces of heavy metals, as shells have a high bioaccumulation capacity for Mg, Fe, Cu and Zn ions [94].
5. Biomaterials
Shells of both crustaceans and molluscs are indeed functional materials with a plethora of possible applications. Crustacean-derived chitin and chitosan in particular can potentially be used as chemical building blocks for biofuels, flavors and fragrances, bioactive molecules (pharmaceuticals), industrial chemicals, plastics, and a number of biomaterials (Table 2) [87]. Some of these applications are being investigated as very promising, but require additional time until they accomplish full implementation (particularly in clinical cases).

Table 2.
Biomedical applications of chitin and its derivatives. Adapted from Singh et al. [42].
5.1. Bone Tissue Regeneration/Bioceramic
Since the main component of mollusc shells is similar to bones and teeth, their matrix has a potential to be used in bone tissue bioengineering [9]. Nacreous structures in particular appear to be the strongest of all structures found in shells [95]. The powdered oyster nacre stimulates bone cell differentiation and bone formation in vitro and in vivo. It was prepared as an injectable osteogenic biomaterial for treating vertebral bone loss of sheep vertebrae [96]. In 12 weeks, the functional new bone trabeculae were formed and covered with osteoid lined with osteoblasts, indicating continuing bone formation [96]. For bone tissue engineering applications, macroporous bone tissue scaffold using oyster shell with a biphasic structure of hydroxyapatite/beta-tricalcium phosphate was developed. It had an excellent cell biocompatibility, strong plasticity and controllable pore size [97]. Hydroxyapatite also has outstanding bioactivity and osteoconductivity. Hence, microrod hydroxyapatite bundles demonstrated high protein adsorption capability, which facilitates the control of cell attachment and proliferation and is thus beneficial to tissue regeneration [98]. Furthermore, nano-hydroxyapatite ceramics can be applied in load-bearing artificial bones with excellent mechanical properties [7].
5.2. Cosmetics
Clam shell powder has a potential as a new biomaterial with high calcium, phosphate, chitin and protein that gives a great result in preventing UV light [99]. Hydroxyapatite from shells can thus be used as an additive in the formulation of emulsions of sunscreen lotions, by the substitution of oxybenzone, zinc oxide and titanium dioxide [3,99,100]. In particular, avobenzone and oxybenzone act as an absorber of UVA and UVB, while titanium dioxide and zinc oxide act as physical blockers of UV radiation in sunscreen formulation. However, they cause photo-allergic reactions, while others are suspected as estrogen disrupters [100]. Due to this concern, hydroxyapatite derived from clam shell is a potential novel component of sunscreens.
Chitosan has specificities that can be highly applicable in the cosmetic industry, especially for hair, in relation to electrostatic interactions.
5.3. Wound Healing
Crustacean-derived chitin can be processed in the form of powder, sponge, film and fibers [74]. The main application of chitin film and fiber is in medicine as a wound-dressing material. Chitin can be further deacetylated into chitosan, when the degree of deacetylation reaches about 50% [74]. The bacteriostatic and fungistatic properties of chitosan make it an excellent ingredient of topical skin ointments as a wound healing agent [84]. The immunostimulating properties of chitin and chitosan in wound healing are associated with the increase in macrophage production, subsequent release of cytokines including transforming growth factor-beta, platelet-derived growth factor, fibroblast growth factor, tumor necrosis factor-alpha, interleukins needed for healing processes [83].
5.4. Biocomposites/Biofillers
Calcium carbonate is a common filler in plastics and rubber. Calcined, powdered mollusc shells are good additives for polyesters, particularly polylactic acid, endowing antibacterial properties to biocomposites due to the presence of hydroxyl ions and active oxygen species [3,101]. Calcined oyster shell powder can also improve mechanical properties of plastic and paper, when used as biofiller with particle size 30–40 μm [102]. Therefore, composites containing micrometer-range CaCO3 particles are promising materials for the design of fillers for plastics and microstructured coatings with a high surface density of functional groups [103].
Mollusc shells can be successfully turned into mixed-material medical radiation shields. The shielding sheets comprising 0.3 mm thick layers of oyster shell have a shielding efficiency of 37.32% for the low-energy X-rays, typically encountered in medical institutions [104].
5.5. Biopackaging
Biopolymers deriving from waste shells can be used to manufacture eco-friendly bioplastics for biomedical, industrial and household applications, biopackaging being one of them [105]. Petroleum-based plastics in packaging films needs to be replaced with biodegradable materials. To that end, easily degradable chitin biopolymers, having oxygen and nitrogen atoms in their structure, represent natural alternative to obsolete processes [83]. Chitosan-based food packaging includes systems capable of inhibiting microorganisms, which is crucial for food quality. The antimicrobial biopackaging obtained from chitosan is one of the most promising active packaging types developed over the last decade [106].
5.6. Sustainability Challenges
Some of the potential applications for shell waste as biomaterials are developed only as niche applications. Some are tested only in laboratory scale, hence not realized in production. Namely, chitosan is much easier to process than chitin, but the stability of chitosan-based materials is generally lower, due to their more hydrophilic character and pH sensitivity [74]. It is imperative to further investigate the range of bioactivities associated with such bioactive molecules, to develop new separation and enrichment technologies, and better techniques for stabilization of these biomolecules [83] before they can be fully explored and commercialized. These investigations need to take into consideration not only the overall performance of each biomaterial, their chemical stability and reusability, but also their economic and environmental values for maintaining sustainability.
The production of hydroxyapatite is an example of an achievable conscious attempt to utilize waste shells for manufacturing of a superior material. The low temperature process needed has considerable industrial advantage over current hydroxyapatite production methods, which require high temperature and pressure and incur considerable costs [30].
6. Construction
Shells have traditionally been used in houses and walls, as path aggregates, and mortar mixes in various coastal areas around the world [5]. However, over the centuries, the most widely used building material has been concrete. Concrete is a mixture of gravel, sand, cement, water, and mortar formed by cement and sand [3]. The binder component—cement, is the most expensive of concrete components, therefore, its replacement is sought for to reduce production costs, as long as standards for its mechanical properties are met [8]. To that end, mollusc shells can be used as cement additives (the filling material) after crushing and heat treatment. They can also be used as aggregates to replace conventional aggregates in concrete, such as river sand. Incorporating mollusc shell ash/powder for cement partial replacement resulted in reduced early compressive strength of concrete. The strength increased with age due to hydration of calcium oxide. The flexural and splitting tensile strength was improved due to the development of good bonding between the binder matrix and aggregates and this increased elasticity, although some studies reported a decrease in splitting tensile strength [107] (Table 3). Therefore, waste shell powder can possibly supplement the non-renewable limestone powder in cement-based materials [108], which can also be modified to mitigate noise pollution as an acoustically absorbent material [109,110].

Table 3.
The effects on the product performance when using various types of shells as cement additives or concrete aggregates. The presence of waste shells generally reduces the strength of concrete (particularly as the replacement dosage increases) but it remains within the acceptable range. Adapted from Zhan et al. [7].
Mollusk shells can also be used to produce the gypsum plaster or green cement, which is used mainly in interior applications due to its low water resistance and brittleness in matrix. Its thermal and sound insulation, and fire resistance, make it as one of the most widely used construction material [111]. Waste shells can be incorporated as asphalt modifiers, where they improve its consistency, hardness, elasticity, recovery, resistance to deformation of bituminous binders, and a number of other characteristics [112,113].
Sustainability Challenges
The utilization of waste shells in concrete helps in their waste management and in producing the cost-efficient, green concrete. Making a full use of these resources will reduce the environmental pressure, damage and shortages due to excessive quarrying and limestone extraction [114]. Their market value can be promising when the waste-derived biogenic calcium carbonate minerals and construction materials are optimally combined [108]. However, in the pretreatment of the waste shells, washing and calcination is required regardless of the specific construction application, using significant amounts of resources and energy, as mentioned above. Furthermore, high chloride content in an aggregate causes the faster corrosion of steel, and its use should be avoided in reinforced concrete. The cost of shell cleaning thus could be higher than in conventional aggregates, which might drive some producers away from this resource [22]. Besides, the quantity of waste shells on a particular location may or may not be sustainable enough as an aggregate replacement material, and the transportation of additional quantities of shells from further distances may increase its overall price. In locations with widely accessible conventional aggregates and production processes, the utilization of waste shells for concrete may not necessarily be inexpensive, adding to the sustainability issues.
7. Conclusions
Although we have a history of recognizing bivalve and crustacean shells as valuable resources, shells produced nowadays are largely considered as waste, despite the fact that CaCO3 is one of the most highly exploited resources globally. Shell waste is still orders of magnitude less than the yearly CaCO3 demand [115]. Nevertheless, shell waste repurposing and valorization are highly important for the determination of strategies to make them a standard valuable resource for a number of applications. Some of these applications may demonstrate more sustainable modus than already established processes, some may be less sustainable in industrial implementation, but adjustments should be appraised for the sake of the environmental benefit. Furthermore, bivalve aquaculture alone has the potential to act as a carbon sink, sequestering several million metric tons of CO2 per year globally [116]. Unfortunately, most of the aqua-cultured bivalves and crustaceans are traded fresh and their shells are disposed of as domestic waste. Much of that waste is handled in furnaces where high temperatures of incineration release the previously trapped CO2 from CaCO3 back to the atmosphere [116]. Thus, in order to be subject to upgrading, waste shells should be collected in an organized manner and their quantities estimated. For any large-scale applications, a stable supply of shells needs to be ensured, particularly in order to design (at least a partial) substitution of the limestone with the shell-derived CaCO3. Certainly, care should be taken that during the repurposing of waste shells, the environmental impact of the process should be reduced [117], particularly in terms of energy and water demand. To that end, the use of cleaner energy sources could ameliorate the environmental impact of the repurposing operations. However, any shell repurposing operation should be evaluated not only through the environmental footprint, but also in terms of economic value and impact. The reuse and valorization of waste shells must take into consideration the collection, cleaning, storage, transport, and utilization costs. In any case, to gain a full momentum, sustainable waste shell bioeconomy and repurposing needs an integrated support from the legislative standpoints, local governments, and related industry operators.
Author Contributions
Conceptualization, N.T.P. and V.L.; methodology, N.T.P.; visualization, I.S.-P. and R.Č.-R.; writing—original draft preparation, N.T.P. and V.L.; writing—review and editing, N.T.P.; funding acquisition, R.Č.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Scientific Centre of Excellence for Marine Bioprospecting-BioProCro, a project co-financed by the Croatian Government and the European Union through the European Regional Development Fund—the Competitiveness and Cohesion Operational Programme (KK.01.1.1.01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Food and Agriculture Organization of the United Nations. World Food and Agriculture—Statistical Yearbook 2021; Food & Agriculture Organization: Rome, Italy, 2021; ISBN 978-92-5-134332-6. [Google Scholar]
- Yan, N.; Chen, X. Sustainability: Don’t Waste Seafood Waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Summa, D.; Lanzoni, M.; Castaldelli, G.; Fano, E.A.; Tamburini, E. Trends and Opportunities of Bivalve Shells’ Waste Valorization in a Prospect of Circular Blue Bioeconomy. Resources 2022, 11, 48. [Google Scholar] [CrossRef]
- Tokeshi, M.; Ota, N.; Kawai, T. A Comparative Study of Morphometry in Shell-bearing Molluscs. J. Zool. 2000, 251, 31–38. [Google Scholar] [CrossRef]
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from Aquaculture: A Valuable Biomaterial, Not a Nuisance Waste Product. Rev. Aquacult. 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Vélez-Henao, J.A.; Weinland, F.; Reintjes, N. Life Cycle Assessment of Aquaculture Bivalve Shellfish Production—A Critical Review of Methodological Trends. Int. J. Life Cycle Assess. 2021, 26, 1943–1958. [Google Scholar] [CrossRef]
- Zhan, J.; Lu, J.; Wang, D. Review of Shell Waste Reutilization to Promote Sustainable Shellfish Aquaculture. Rev. Aquacult. 2022, 14, 477–488. [Google Scholar] [CrossRef]
- Murphy, J.N.; Kerton, F.M. Characterization and Utilization of Waste Streams from Mollusc Aquaculture and Fishing Industries. In Fuels, Chemicals and Materials from the Oceans and Aquatic Sources; Kerton, F.M., Yan, N., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 189–227. ISBN 978-1-119-11719-3. [Google Scholar]
- Bonnard, M.; Boury, B.; Parrot, I. Key Insights, Tools, and Future Prospects on Oyster Shell End-of-Life: A Critical Analysis of Sustainable Solutions. Environ. Sci. Technol. 2020, 54, 26–38. [Google Scholar] [CrossRef]
- Ishangulyyev, R.; Kim, S.; Lee, S. Understanding Food Loss and Waste—Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef]
- de Alvarenga, R.A.F.; Galindro, B.M.; de Fatima Helpa, C.; Soares, S.R. The Recycling of Oyster Shells: An Environmental Analysis Using Life Cycle Assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef]
- Kobatake, H.; Kirihara, S. Lowering the Incineration Temperature of Fishery Waste to Optimize the Thermal Decomposition of Shells and Spines. Fish. Sci. 2019, 85, 573–579. [Google Scholar] [CrossRef]
- Hardy, B.L.; Moncel, M.-H.; Kerfant, C.; Lebon, M.; Bellot-Gurlet, L.; Mélard, N. Direct Evidence of Neanderthal Fibre Technology and Its Cognitive and Behavioral Implications. Sci. Rep. 2020, 10, 4889. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, S.; Moroni, A.; Tassoni, L.; Boschin, F.; Badino, F.; Bortolini, E.; Boscato, P.; Crezzini, J.; Figus, C.; Forte, M.; et al. Bone Tools, Ornaments and Other Unusual Objects during the Middle to Upper Palaeolithic Transition in Italy. Quat. Int. 2020, 551, 169–187. [Google Scholar] [CrossRef]
- Douka, K.; Spinapolice, E.E. Neanderthal Shell Tool Production: Evidence from Middle Palaeolithic Italy and Greece. J. World Prehist. 2012, 25, 45–79. [Google Scholar] [CrossRef]
- Gamble, L.H. The Origin and Use of Shell Bead Money in California. J. Anthropol. Archaeol. 2020, 60, 101237. [Google Scholar] [CrossRef]
- Maurer, B. Primitive and Nonmetallic Money. In Handbook of the History of Money and Currency; Battilossi, S., Cassis, Y., Yago, K., Eds.; Springer: Singapore, 2020; pp. 87–104. ISBN 9789811305955. [Google Scholar]
- Guo, P. Marriage-Related Exchanges and the Agency of Women among the Langalanga, Solomon Islands. Oceania 2020, 90, 273–291. [Google Scholar] [CrossRef]
- Silva Mota, E.L.; Nóbrega Alves, R.R.; Pereira Dias, T.L. Fishing, Trade, and Local Ecological Knowledge of the Marine Gastropod, Cassis Tuberosa—A Target Species of the International Shell Trade. Ethnobiol. Conserv. 2020, 9, 23. [Google Scholar] [CrossRef]
- Nijman, V.; Spaan, D.; Nekaris, K.A.-I. Large-Scale Trade in Legally Protected Marine Mollusc Shells from Java and Bali, Indonesia. PLoS ONE 2015, 10, e0140593. [Google Scholar] [CrossRef]
- Watson, G.J.; Bonner, A.; Murray, J.M.; Hebblethwaite, Z. Offsetting the Impact of the Marine Ornamental Trade: A Case Study of Two Temperate Top Shells (Osilinus Lineatus and Gibbula Umbilicalis) as Potential Clean-up Crew: TEMPERATE GASTROPOD CLEAN-UP CREW. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 731–742. [Google Scholar] [CrossRef]
- Eziefula, U.G. Developments in Utilisation of Agricultural and Aquaculture By-Products as Aggregate in Concrete—A Review. Environ. Technol. Rev. 2018, 7, 19–45. [Google Scholar] [CrossRef]
- Her, S.; Park, T.; Zalnezhad, E.; Bae, S. Synthesis and Characterization of Cement Clinker Using Recycled Pulverized Oyster and Scallop Shell as Limestone Substitutes. J. Clean. Prod. 2021, 278, 123987. [Google Scholar] [CrossRef]
- Barros, M.C.; Bello, P.M.; Bao, M.; Torrado, J.J. From Waste to Commodity: Transforming Shells into High Purity Calcium Carbonate. J. Clean. Prod. 2009, 17, 400–407. [Google Scholar] [CrossRef]
- Deng, J.-J.; Zhang, M.-S.; Li, Z.-W.; Lu, D.-L.; Mao, H.-H.; Zhu, M.-J.; Li, J.-Z.; Luo, X.-C. One-Step Processing of Shrimp Shell Waste with a Chitinase Fused to a Carbohydrate-Binding Module. Green Chem. 2020, 22, 6862–6873. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, Y.; Ma, M.; Oh, D.-H.; Fu, X. Characterization of Chitinase from Exiguobacterium Antarcticum and Its Bioconversion of Crayfish Shell into Chitin Oligosaccharides. Food Res. Int. 2022, 158, 111517. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, X.; Duan, L.; Liu, D.; Han, Z. Ammonia Nitrogen Removal in a Biological Nitrifying System Using Oyster Shells as Alkalinity-Releasing Filling Materials. In Proceedings of the 2016 ASABE International Meeting; American Society of Agricultural and Biological Engineers, Orlando, FL, USA, 17–20 July 2016. [Google Scholar]
- Chilakala, R.; Thannaree, C.; Shin, E.J.; Thenepalli, T.; Ahn, J.W. Sustainable Solutions for Oyster Shell Waste Recycling in Thailand and the Philippines. Recycling 2019, 4, 35. [Google Scholar] [CrossRef]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Biodiesel Production via Transesterification of Palm Olein Using Waste Mud Crab (Scylla Serrata) Shell as a Heterogeneous Catalyst. Bioresour. Technol. 2009, 100, 6362–6368. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.I.; Barakat, H.; Patterson, D.A. Production of Hydroxyapatite from Waste Mussel Shells. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 192002. [Google Scholar] [CrossRef]
- Mohanraj, J.; Pandi, M.U.; Ranjan, R.; Shunmugara, T. Marine Mollusc Is an Excellent Ornament. Res. J. Appl. Sci. 2011, 6, 92–98. [Google Scholar] [CrossRef][Green Version]
- Illanes, A. (Ed.) Enzyme Biocatalysis: Principles and Applications; Springer: Dordrecht, The Netherlands, 2008; ISBN 978-1-4020-8360-0. [Google Scholar]
- Oladoja, N.A.; Adelagun, R.O.A.; Ahmad, A.L.; Ololade, I.A. Phosphorus Recovery from Aquaculture Wastewater Using Thermally Treated Gastropod Shell. Process Saf. Environ. Prot. 2015, 98, 296–308. [Google Scholar] [CrossRef]
- Jones, M.I.; Wang, L.Y.; Abeynaike, A.; Patterson, D.A. Utilisation of Waste Material for Environmental Applications: Calcination of Mussel Shells for Waste Water Treatment. Adv. Appl. Ceram. 2011, 110, 280–286. [Google Scholar] [CrossRef]
- Morse, G.; Brett, S.; Guy, J.; Lester, J. Review: Phosphorus Removal and Recovery Technologies. Sci. Total Environ. 1998, 212, 69–81. [Google Scholar] [CrossRef]
- Abeynaike, A.; Wang, L.; Jones, M.I.; Patterson, D.A. Pyrolysed Powdered Mussel Shells for Eutrophication Control: Effect of Particle Size and Powder Concentration on the Mechanism and Extent of Phosphate Removal. Asia-Pac. J. Chem. Eng. 2011, 6, 231–243. [Google Scholar] [CrossRef]
- Martins, M.C.; Santos, E.B.H.; Marques, C.R. First Study on Oyster-Shell-Based Phosphorous Removal in Saltwater—A Proxy to Effluent Bioremediation of Marine Aquaculture. Sci. Total Environ. 2017, 574, 605–615. [Google Scholar] [CrossRef] [PubMed]
- von Ahnen, M.; Pedersen, P.B.; Dalsgaard, J. Nitrate Removal from Aquaculture Effluents Using Woodchip Bioreactors Improved by Adding Sulfur Granules and Crushed Seashells. Water Sci. Technol. 2018, 77, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Ergas, S.J.; Lopez-Luna, E.; Sahu, A.K.; Palaniswamy, K. Autotrophic Biological Denitrification for Complete Removal of Nitrogen from Septic System Wastewater. Water Air Soil Pollut. Focus 2006, 6, 111–126. [Google Scholar] [CrossRef]
- Murphy, J.N.; Schneider, C.M.; Hawboldt, K.; Kerton, F.M. Hard to Soft: Biogenic Absorbent Sponge-like Material from Waste Mussel Shells. Matter 2020, 3, 2029–2041. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhan, J.; Costa, N. Use of Shell Chitin Extracted from Seafood Processing Waste in Recycling of Industrial Wastewater; Gupta, S.M., Ed.; SPIE: Boston, MA, USA, 2001; pp. 403–412. [Google Scholar]
- Singh, R.; Upadhyay, S.; Singh, M.; Sharma, I.; Sharma, P.; Kamboj, P.; Saini, A.; Voraha, R.; Sharma, A.; Upadhyay, T.; et al. Chitin, Chitinases and Chitin Derivatives in Biopharmaceutical, Agricultural and Environmental Perspective. Biointerface Res. Appl. Chem. 2020, 11, 9985–10005. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Lo, S.-L.; Kuo, J. Using Pretreated Waste Oyster and Clam Shells and Microwave Hydrothermal Treatment to Recover Boron from Concentrated Wastewater. Bioresour. Technol. 2011, 102, 7802–7806. [Google Scholar] [CrossRef]
- Farm, C. Metal Sorption to Natural Filter Substrates for Storm Water Treatment—Column Studies. Sci. Total Environ. 2002, 298, 17–24. [Google Scholar] [CrossRef]
- Tudor, H.E.A.; Gryte, C.C.; Harris, C.C. Seashells: Detoxifying Agents for Metal-Contaminated Waters. Water Air Soil Pollut. 2006, 173, 209–242. [Google Scholar] [CrossRef]
- Du, Y.; Lian, F.; Zhu, L. Biosorption of Divalent Pb, Cd and Zn on Aragonite and Calcite Mollusk Shells. Environ. Pollut. 2011, 159, 1763–1768. [Google Scholar] [CrossRef]
- Santás-Miguel, V.; Campillo-Cora, C.; Núñez-Delgado, A.; Fernández-Calviño, D.; Arias-Estévez, M. Utilization of Mussel Shell to Remediate Soils Polluted with Heavy Metals. In Biomass-Derived Materials for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 221–242. ISBN 978-0-323-91914-2. [Google Scholar]
- Ok, Y.S.; Oh, S.-E.; Ahmad, M.; Hyun, S.; Kim, K.-R.; Moon, D.H.; Lee, S.S.; Lim, K.J.; Jeon, W.-T.; Yang, J.E. Effects of Natural and Calcined Oyster Shells on Cd and Pb Immobilization in Contaminated Soils. Environ. Earth Sci. 2010, 61, 1301–1308. [Google Scholar] [CrossRef]
- Bi, D.; Yuan, G.; Wei, J.; Xiao, L.; Feng, L. Conversion of Oyster Shell Waste to Amendment for Immobilising Cadmium and Arsenic in Agricultural Soil. Bull. Environ. Contam. Toxicol. 2020, 105, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Remonsellez, F.; Zarrias, N.; Bol, R.; Fuentes, B. Characterization and Low-Cost Treatment of an Industrial Arid Soil Polluted with Lead Sulfide in Northern Chile. Environ. Earth Sci. 2017, 76, 294. [Google Scholar] [CrossRef]
- Garrido-Rodríguez, B.; Fernández-Calviño, D.; Nóvoa Muñoz, J.C.; Arias-Estévez, M.; Díaz-Raviña, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. PH-Dependent Copper Release in Acid Soils Treated with Crushed Mussel Shell. Int. J. Environ. Sci. Technol. 2013, 10, 983–994. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Lapolli, F.R.; Nagel-Hassemer, M.E.; Lobo-Recio, M.Á. Optimization of Acid Mine Drainage Remediation with Central Composite Rotatable Design Model. Energy Procedia 2017, 136, 233–238. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Lapolli, F.R.; Nagel-Hassemer, M.E.; Lobo-Recio, M.Á. Optimization of Fe and Mn Removal from Coal Acid Mine Drainage (AMD) with Waste Biomaterials: Statistical Modeling and Kinetic Study. Waste Biomass Valorization 2020, 11, 1143–1157. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huang, H.; Nguyen, T.A.H.; Soda, S. Recycling Clamshell as Substrate in Lab-Scale Constructed Wetlands for Heavy Metal Removal from Simulated Acid Mine Drainage. Process Saf. Environ. Prot. 2022, 165, 950–958. [Google Scholar] [CrossRef]
- Pat-Espadas, A.; Loredo Portales, R.; Amabilis-Sosa, L.; Gómez, G.; Vidal, G. Review of Constructed Wetlands for Acid Mine Drainage Treatment. Water 2018, 10, 1685. [Google Scholar] [CrossRef]
- Yu, G.; Wang, G.; Chi, T.; Du, C.; Wang, J.; Li, P.; Zhang, Y.; Wang, S.; Yang, K.; Long, Y.; et al. Enhanced Removal of Heavy Metals and Metalloids by Constructed Wetlands: A Review of Approaches and Mechanisms. Sci. Total Environ. 2022, 821, 153516. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Soda, S.; Horiuchi, K. Removal of Heavy Metals from Acid Mine Drainage with Lab-Scale Constructed Wetlands Filled with Oyster Shells. Water 2022, 14, 3325. [Google Scholar] [CrossRef]
- Steckbauer, A.; Klein, S.G.; Duarte, C.M. Additive Impacts of Deoxygenation and Acidification Threaten Marine Biota. Glob. Chang. Biol. 2020, 26, 5602–5612. [Google Scholar] [CrossRef]
- Clements, J.C.; Chopin, T. Ocean Acidification and Marine Aquaculture in North America: Potential Impacts and Mitigation Strategies. Rev. Aquacult. 2017, 9, 326–341. [Google Scholar] [CrossRef]
- Leung, J.Y.S.; Zhang, S.; Connell, S.D. Is Ocean Acidification Really a Threat to Marine Calcifiers? A Systematic Review and Meta-Analysis of 980+ Studies Spanning Two Decades. Small 2022, 18, 2107407. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.A.; Ragg, N.L.C. Effects of Crushed Mussel, Perna Canaliculus, Shell Enrichment on Seawater Carbonate Buffering and Development of Conspecific Larvae Exposed to Near-future Ocean Acidification. J. World Aquac. Soc. 2022, 53, 271–289. [Google Scholar] [CrossRef]
- Bergstrom, E.; Silva, J.; Martins, C.; Horta, P. Seagrass Can Mitigate Negative Ocean Acidification Effects on Calcifying Algae. Sci. Rep. 2019, 9, 1932. [Google Scholar] [CrossRef]
- Blanc, J.M.; Molinet, C.; Subiabre, R.; Díaz, P.A. Cadmium Determination in Chilean Blue Mussels Mytilus Chilensis: Implications for Environmental and Agronomic Interest. Mar. Pollut. Bull. 2018, 129, 913–917. [Google Scholar] [CrossRef]
- Casado-Coy, N.; Martínez-García, E.; Sánchez-Jerez, P.; Sanz-Lázaro, C. Mollusc-Shell Debris Can Mitigate the Deleterious Effects of Organic Pollution on Marine Sediments. J. Appl. Ecol. 2017, 54, 547–556. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as Ecosystem Engineers: The Role of Shell Production in Aquatic Habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Hassoun, A.E.R.; Bantelman, A.; Canu, D.; Comeau, S.; Galdies, C.; Gattuso, J.-P.; Giani, M.; Grelaud, M.; Hendriks, I.E.; Ibello, V.; et al. Ocean Acidification Research in the Mediterranean Sea: Status, Trends and next Steps. Front. Mar. Sci. 2022, 9, 892670. [Google Scholar] [CrossRef]
- Lim, J.; Cho, H.; Kim, J. Optimization of Wet Flue Gas Desulfurization System Using Recycled Waste Oyster Shell as High-Grade Limestone Substitutes. J. Clean. Prod. 2021, 318, 128492. [Google Scholar] [CrossRef]
- Jung, J.H.; Yoo, K.S.; Kim, H.G.; Lee, H.K.; Shon, B.H. Reuse of Waste Oyster Shells as a SO2/NOx Removal Absorbent. J. Ind. Eng. Chem. 2007, 13, 512–517. [Google Scholar]
- Huang, Y.-F.; Lee, Y.-T.; Chiueh, P.-T.; Lo, S.-L. Microwave Calcination of Waste Oyster Shells for CO2 Capture. Energy Procedia 2018, 152, 1242–1247. [Google Scholar] [CrossRef]
- O’Neil, D.D.; Gillikin, D.P. Do Freshwater Mussel Shells Record Road-Salt Pollution? Sci. Rep. 2015, 4, 7168. [Google Scholar] [CrossRef] [PubMed]
- Fay, L.; Shi, X. Laboratory Investigation of Performance and Impacts of Snow and Ice Control Chemicals for Winter Road Service. J. Cold Reg. Eng. 2011, 25, 89–114. [Google Scholar] [CrossRef]
- Schade, J.; Lidelöw, S.; Lönnqvist, J. The Thermal Performance of a Green Roof on a Highly Insulated Building in a Sub-Arctic Climate. Energy Build. 2021, 241, 110961. [Google Scholar] [CrossRef]
- Hanumesh, M.; Claverie, R.; Séré, G. A Roof of Greenery, but a Sky of Unexplored Relations—Meta-Analysis of Factors and Properties That Affect Green Roof Hydrological and Thermal Performances. Sustainability 2021, 13, 10017. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The Potential Use of Oyster Shell Waste in New Value-Added By-Product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- Fujita, T.; Fukase, M.; Miyamoto, H.; Matsumoto, T.; Ohue, T. Increase of Bone Mineral Density by Calcium Supplement with Oyster Shell Electrolysate. Bone Miner. 1990, 11, 85–91. [Google Scholar] [CrossRef]
- Malaweera, B.O.; Wijesundara, W.M.N.M. Use of Seafood Processing By-Products in the Animal Feed Industry. In Seafood Processing By-Products; Kim, S.-K., Ed.; Springer: New York, NY, USA, 2014; pp. 315–339. ISBN 978-1-4614-9589-5. [Google Scholar]
- Amar, B.; Philip, R.; Bright Singh, I.S. Efficacy of Fermented Prawn Shell Waste as a Feed Ingredient for Indian White Prawn, Fenneropenaeus Indicus. Aquac. Nutr. 2006, 12, 433–442. [Google Scholar] [CrossRef]
- No, H.K.; Hur, E.Y. Control of Foam Formation by Antifoam during Demineralization of Crustacean Shell in Preparation of Chitin. J. Agric. Food Chem. 1998, 46, 3844–3846. [Google Scholar] [CrossRef]
- Paul, M.K.; Mini, K.D.; Antony, A.C.; Mathew, J. Deproteinization of Shrimp Shell Waste by Kurthia Gibsonii Mb126 Immobilized Chitinase. J. Pure Appl. Microbiol. 2022, 16, 909–923. [Google Scholar] [CrossRef]
- Coma, V. Recent Developments in Chitin and Chitosan Bio-Based Materials Used for Food Preservation. In Polysaccharide Building Blocks; Habibi, Y., Lucia, L.A., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 143–175. ISBN 978-0-470-87419-6. [Google Scholar]
- Mellegård, H.; From, C.; Christensen, B.E.; Granum, P.E. Inhibition of Bacillus Cereus Spore Outgrowth and Multiplication by Chitosan. Int. J. Food Microbiol. 2011, 149, 218–225. [Google Scholar] [CrossRef]
- Hayes, M.; Carney, B.; Slater, J.; Brück, W. Mining Marine Shellfish Wastes for Bioactive Molecules: Chitin and Chitosan—Part B: Applications. Biotechnol. J. 2008, 3, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.V.; Lele, S.S. Nutraceuticals and Bioactive Compounds from Seafood Processing Waste; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Tran, D.V.; Dang, T.T.; Cao, T.T.T.; Hua, N.T.; Pham, H.Q. Natural Astaxanthin Extracted from Shrimp Waste for Pigment Improvement in the Orange Clownfish, Amphiprion Percula. Aquac. Res. 2022, 53, 4190–4198. [Google Scholar] [CrossRef]
- Skonberg, D.I.; Donahue, D.W.; Bayer, R.C.; Floreto, E.; Riley, J.G. Quality Evaluation of American Lobsters Fed Diets Containing Crab Processing Waste. J. Aquat. Food Prod. Technol. 2001, 10, 17–29. [Google Scholar] [CrossRef]
- Kerton, F.M.; Liu, Y.; Murphy, J.N.; Hawboldt, K. Renewable Resources from the Oceans: Adding Value to the by-Products of the Aquaculture and Fishing Industries. In Proceedings of the 2014 Oceans, St. John’s, NL, Canada, 14–19 September 2014; pp. 1–3. [Google Scholar]
- Preuss, H.; Kaats, G. Chitosan as a Dietary Supplement for Weight Loss: A Review. Curr. Nutr. Food Sci. 2006, 2, 297–311. [Google Scholar] [CrossRef]
- Kim, S.; Rajapakse, N. Enzymatic Production and Biological Activities of Chitosan Oligosaccharides (COS): A Review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Muzzarelli, R. Chitosan-Based Dietary Foods. Carbohydr. Polym. 1996, 29, 309–316. [Google Scholar] [CrossRef]
- Niaz, T.; Hafeez, Z.; Imran, M. Prospectives of Antihypertensive Nano-Ceuticals as Alternative Therapeutics. Curr. Drug Targets 2017, 18, 1269–1280. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kim, B.; Kim, S.-K. Antihypertensive Activity of Chitin Derivatives. Biopolymers 2006, 83, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Healy, M.; Green, A.; Healy, A. Bioprocessing of Marine Crustacean Shell Waste. Acta Biotechnol. 2003, 23, 151–160. [Google Scholar] [CrossRef]
- Palaniappan, M.; Selvarajan, S.; Srinivasamurthy, S.; Chandrasekaran, A. Oyster Shell Calcium Induced Parotid Swelling. J. Pharmacol. Pharmacother. 2014, 5, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Tan, Y.-Q.; Zhang, L.; Zhang, Y.-X.; Song, Y.-H.; Ye, Y.; Xia, M.-S. Bio-Filler from Waste Shellfish Shell: Preparation, Characterization, and Its Effect on the Mechanical Properties on Polypropylene Composites. J. Hazard. Mater. 2012, 217–218, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Lamghari, M.; Almeida, M.J.; Berland, S.; Huet, H.; Laurent, A.; Milet, C.; Lopez, E. Stimulation of Bone Marrow Cells and Bone Formation by Nacre: In Vivo and in Vitro Studies. Bone 1999, 25, 91S–94S. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, Q.; Pu, X.; Hou, Z.; Zhang, Q. Biphasic Calcium Phosphate Macroporous Scaffolds Derived from Oyster Shells for Bone Tissue Engineering. Chem. Eng. J. 2011, 173, 837–845. [Google Scholar] [CrossRef]
- Wu, S.-C.; Kao, Y.-L.; Lu, Y.-C.; Hsu, H.-C.; Ho, W.-F. Preparation and Characterization of Microrod Hydroxyapatite Bundles Obtained from Oyster Shells through Microwave Irradiation. J. Aust. Ceram. Soc. 2021, 57, 1541–1551. [Google Scholar] [CrossRef]
- Ghazali, S.R.; Rosli, N.H.; Hassan, L.S.; Helmi Rozaini, M.Z.; Hamzah, H. Determination of New Biomaterials of Clams as An Active Ingredient in Sunscreen. J. Phys. Conf. Ser. 2021, 1874, 012075. [Google Scholar] [CrossRef]
- Ghazali, S.R.; Rosli, N.H.; Hassan, L.S.; Helmi Rozaini, M.Z.; Hamzah, H. Biocompatibility of Hydroxyapatite (HAp) Derived from Clamshell as Active Ingredients in Sunscreen Product. IOP Conf. Ser. Earth Environ. Sci. 2021, 646, 012059. [Google Scholar] [CrossRef]
- Wu, C.-S.; Wu, D.-Y.; Wang, S.-S. Antibacterial Properties of Biobased Polyester Composites Achieved through Modification with a Thermally Treated Waste Scallop Shell. ACS Appl. Bio Mater. 2019, 2, 2262–2270. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Han, C.; Ahn, J.-W. Synthesis of Aragonite-Precipitated Calcium Carbonate from Oyster Shell Waste via a Carbonation Process and Its Applications. Korean J. Chem. Eng. 2017, 34, 225–230. [Google Scholar] [CrossRef]
- Danilovtseva, E.N.; Palshin, V.A.; Strelova, M.S.; Lopatina, I.N.; Kaneva, E.V.; Zakharova, N.V.; Annenkov, V.V. Functional Polymers for Modeling the Formation of Biogenic Calcium Carbonate and the Design of New Materials. Polym. Adv. Techs 2022, 33, 2984–3001. [Google Scholar] [CrossRef]
- Kim, S.-C. Process Technology for Development and Performance Improvement of Medical Radiation Shield Made of Eco-Friendly Oyster Shell Powder. Appl. Sci. 2022, 12, 968. [Google Scholar] [CrossRef]
- Audrézet, F.; Pochon, X.; Floerl, O.; Le Guen, M.-J.; Trochel, B.; Gambarini, V.; Lear, G.; Zaiko, A. Eco-Plastics in the Sea: Succession of Micro- and Macro-Fouling on a Biodegradable Polymer Augmented With Oyster Shell. Front. Mar. Sci. 2022, 9, 891183. [Google Scholar] [CrossRef]
- Martinez, L.M.T. Handbook of Ecomaterials; Springer: New York, NY, USA, 2019; ISBN 978-3-319-68254-9. [Google Scholar]
- Tayeh, B.A.; Hasaniyah, M.W.; Zeyad, A.M.; Yusuf, M.O. Properties of Concrete Containing Recycled Seashells as Cement Partial Replacement: A Review. J. Clean. Prod. 2019, 237, 117723. [Google Scholar] [CrossRef]
- Wang, J.; Liu, E. Upcycling Waste Seashells with Cement: Rheology and Early-Age Properties of Portland Cement Paste. Resour. Conserv. Recycl. 2020, 155, 104680. [Google Scholar] [CrossRef]
- Peceño, B.; Arenas, C.; Alonso-Fariñas, B.; Leiva, C. Substitution of Coarse Aggregates with Mollusk-Shell Waste in Acoustic-Absorbing Concrete. J. Mater. Civ. Eng. 2019, 31, 04019077. [Google Scholar] [CrossRef]
- Peceño, B.; Leiva, C.; Alonso-Fariñas, B.; Gallego-Schmid, A. Is Recycling Always the Best Option? Environmental Assessment of Recycling of Seashell as Aggregates in Noise Barriers. Processes 2020, 8, 776. [Google Scholar] [CrossRef]
- Sophia, M.; Sakthieswaran, N. Waste Shell Powders as Valuable Bio- Filler in Gypsum Plaster—Efficient Waste Management Technique by Effective Utilization. J. Clean. Prod. 2019, 220, 74–86. [Google Scholar] [CrossRef]
- Stel’makh, S.A.; Shcherban’, E.M.; Beskopylny, A.N.; Mailyan, L.R.; Meskhi, B.; Beskopylny, N.; Dotsenko, N.; Kotenko, M. Nanomodified Concrete with Enhanced Characteristics Based on River Snail Shell Powder. Appl. Sci. 2022, 12, 7839. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Ji, G.; Zhang, Y.; Zhao, J.; Su, H. Effect of Biowaste on the High- and Low-Temperature Rheological Properties of Asphalt Binders. Adv. Civ. Eng. 2021, 2021, 5516546. [Google Scholar] [CrossRef]
- Wang, W.; Wei, W.; Gao, S.; Chen, G.; Yuan, J.; Li, Y. Agricultural and Aquaculture Wastes as Concrete Components: A Review. Front. Mater. 2021, 8, 762568. [Google Scholar] [CrossRef]
- Morris, J.P.; Wang, Y.; Backeljau, T.; Chapelle, G. Biomimetic and Bio-Inspired Uses of Mollusc Shells. Mar. Genom. 2016, 27, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.A.; Álvarez-Salgado, X.A.; Antelo, L.T. Assessing the Impact of Bivalve Aquaculture on the Carbon Circular Economy. J. Clean. Prod. 2021, 279, 123873. [Google Scholar] [CrossRef]
- Vicente, F.A.; Ventura, S.P.M.; Passos, H.; Dias, A.C.R.V.; Torres-Acosta, M.A.; Novak, U.; Likozar, B. Crustacean Waste Biorefinery as a Sustainable Cost-Effective Business Model. Chem. Eng. J. 2022, 442, 135937. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).