Loquat Leaf Extract Inhibits Oxidative Stress-Induced DNA Damage and Apoptosis via AMPK and Nrf2/HO-1 Signaling Pathways in C2C12 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Preparation of the Loquat Extract (LE)
2.4. Cell Culture and Viability Assays
2.5. ROS Quantification
2.6. Comet Assay
2.7. Western Blot Analysis
2.8. Apoptosis Detection
2.9. Nuclear Morphology Analysis
2.10. DNA Fragmentation Assays
2.11. Measurement of Mitochondrial Membrane Potential (MMP)
2.12. Statistical Analysis
3. Results
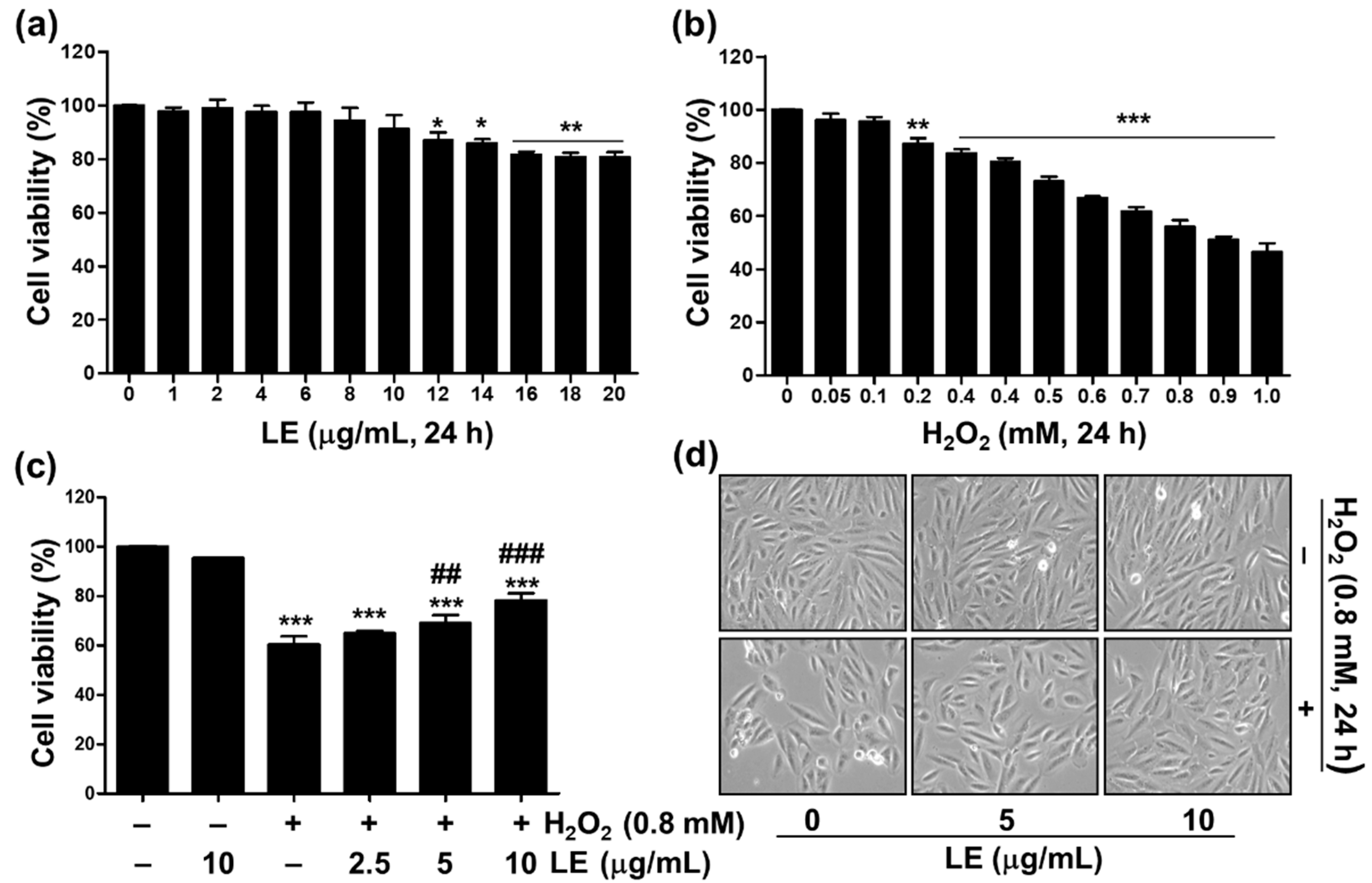
3.1. Effect of LE on Cytotoxicity of H2O2
3.2. Effect of LE on ROS Generation and DNA Damage
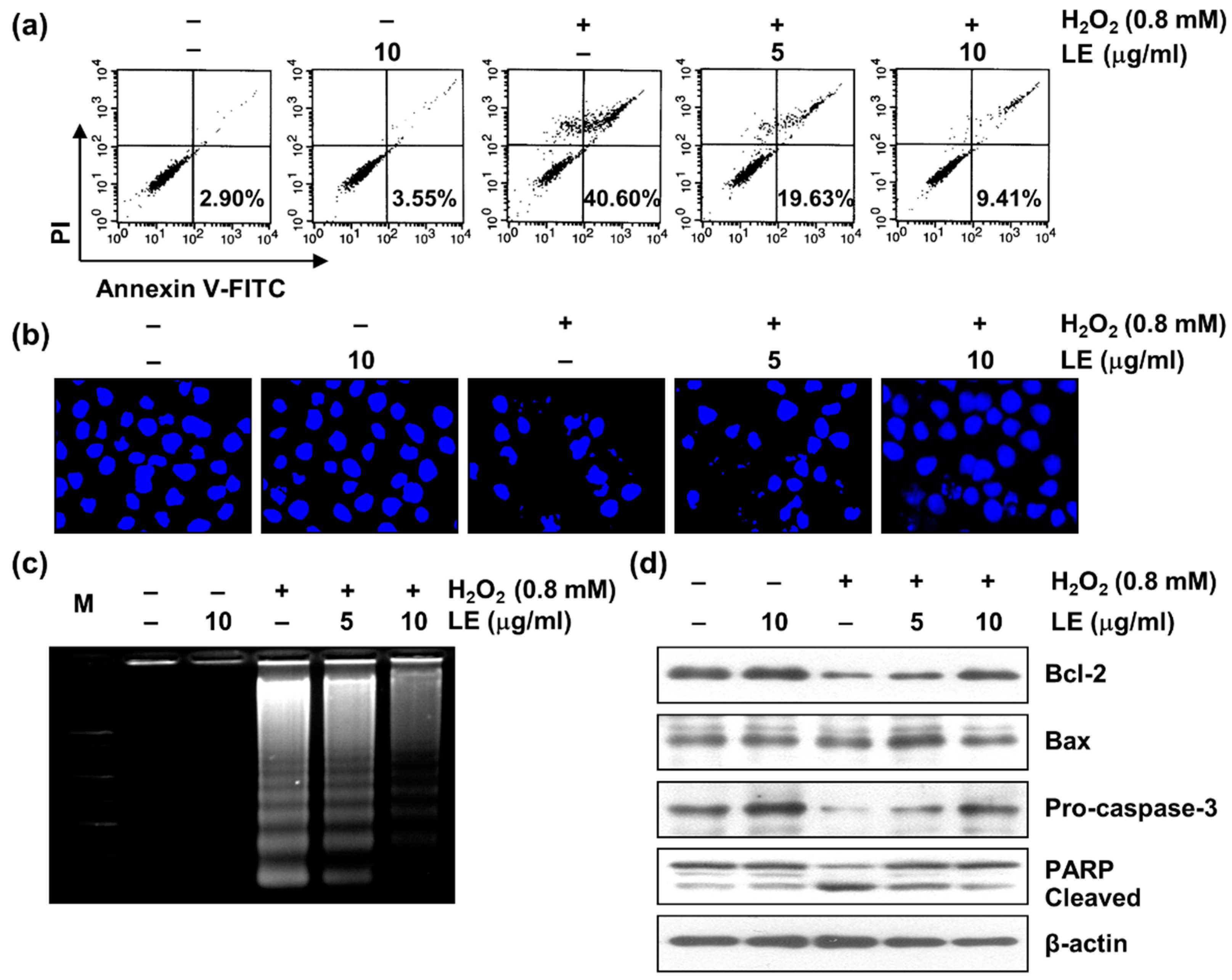
3.3. Effect of LE on Apoptosis
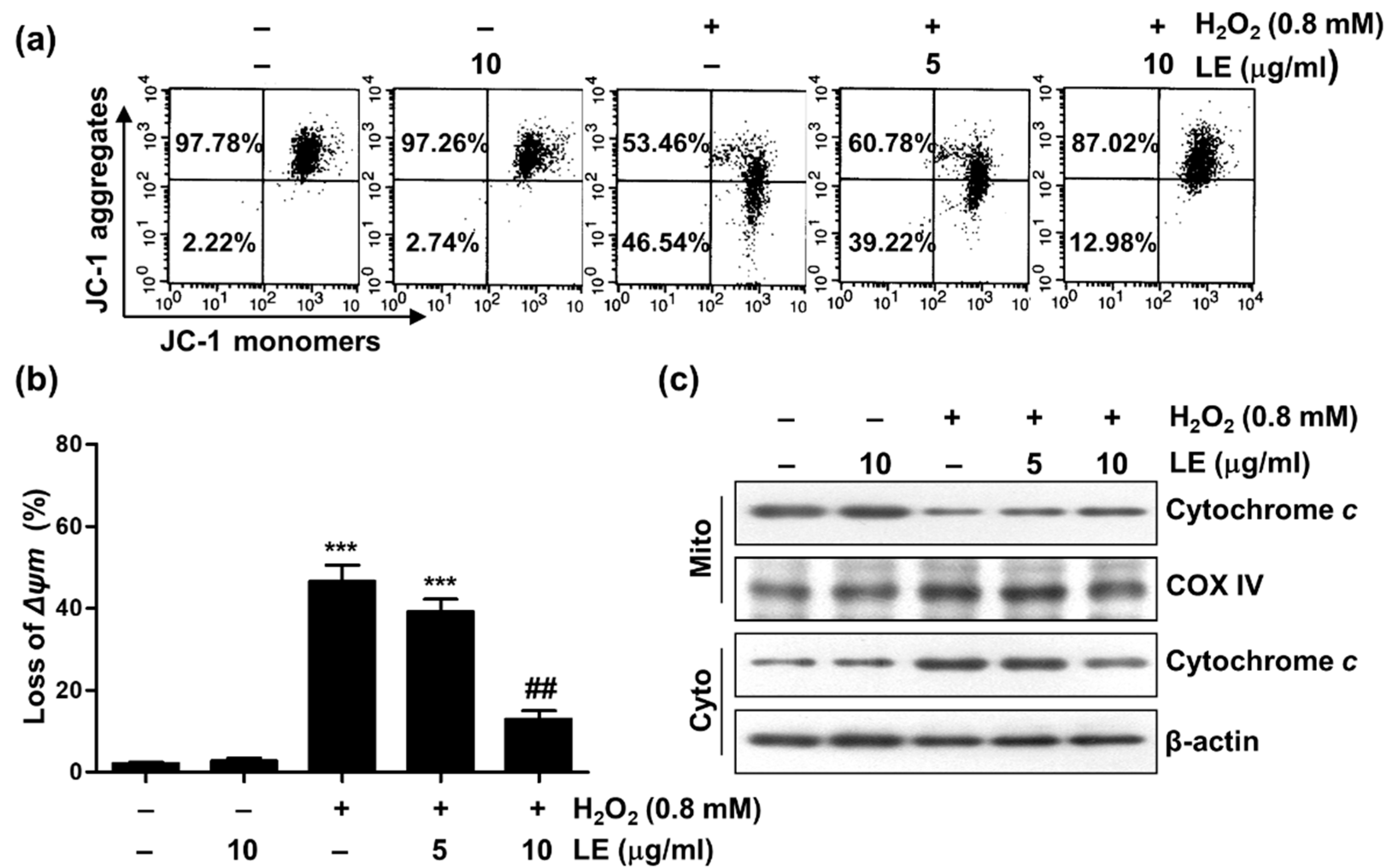
3.4. Effect of LE on Mitochondrial Dysfunction
3.5. Effect of LE on AMPK Signaling
3.6. Effect of LE on AMPK-Dependent ROS Generation and DNA Damage
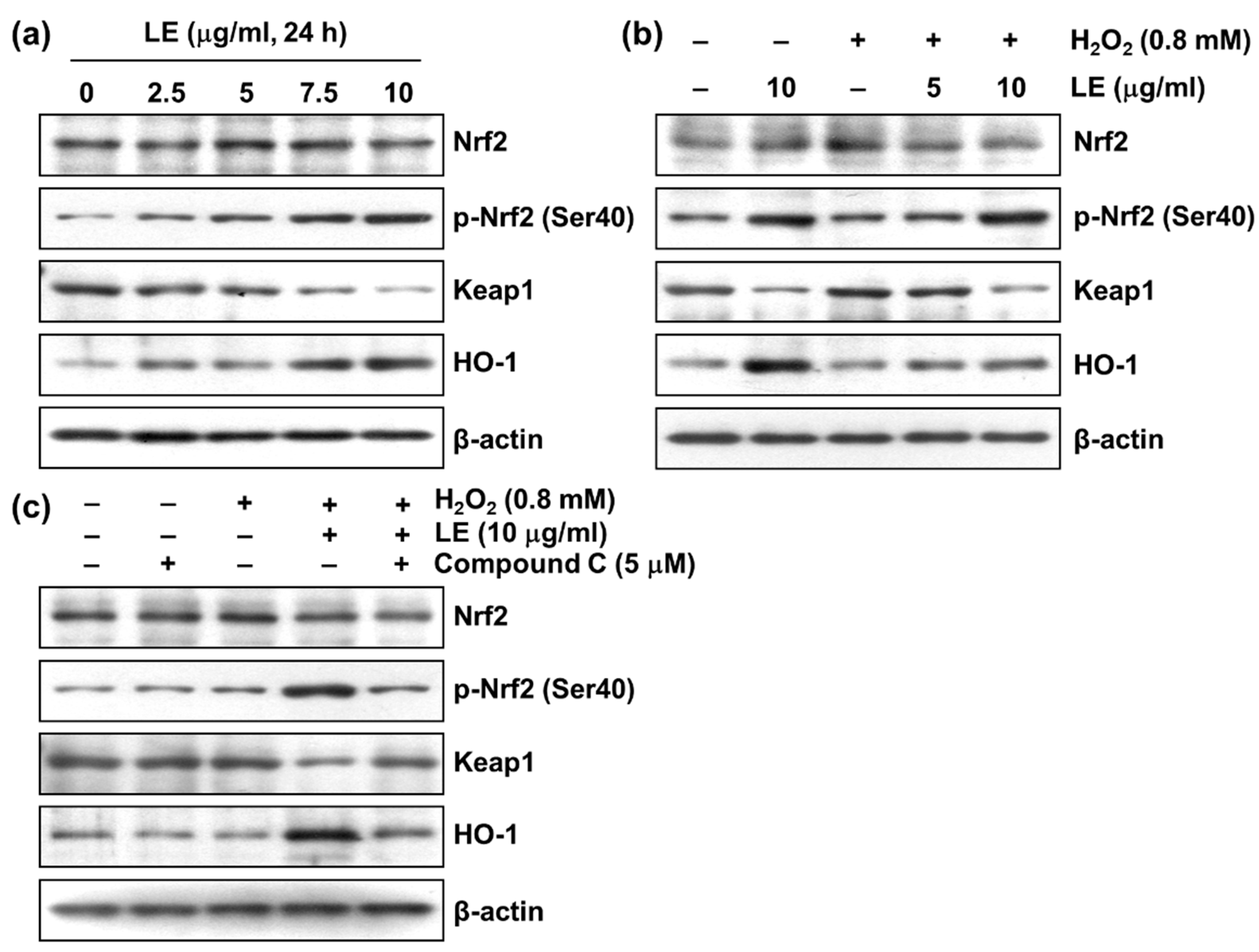
3.7. Effect of LE on Nrf2/HO-1 Signaling
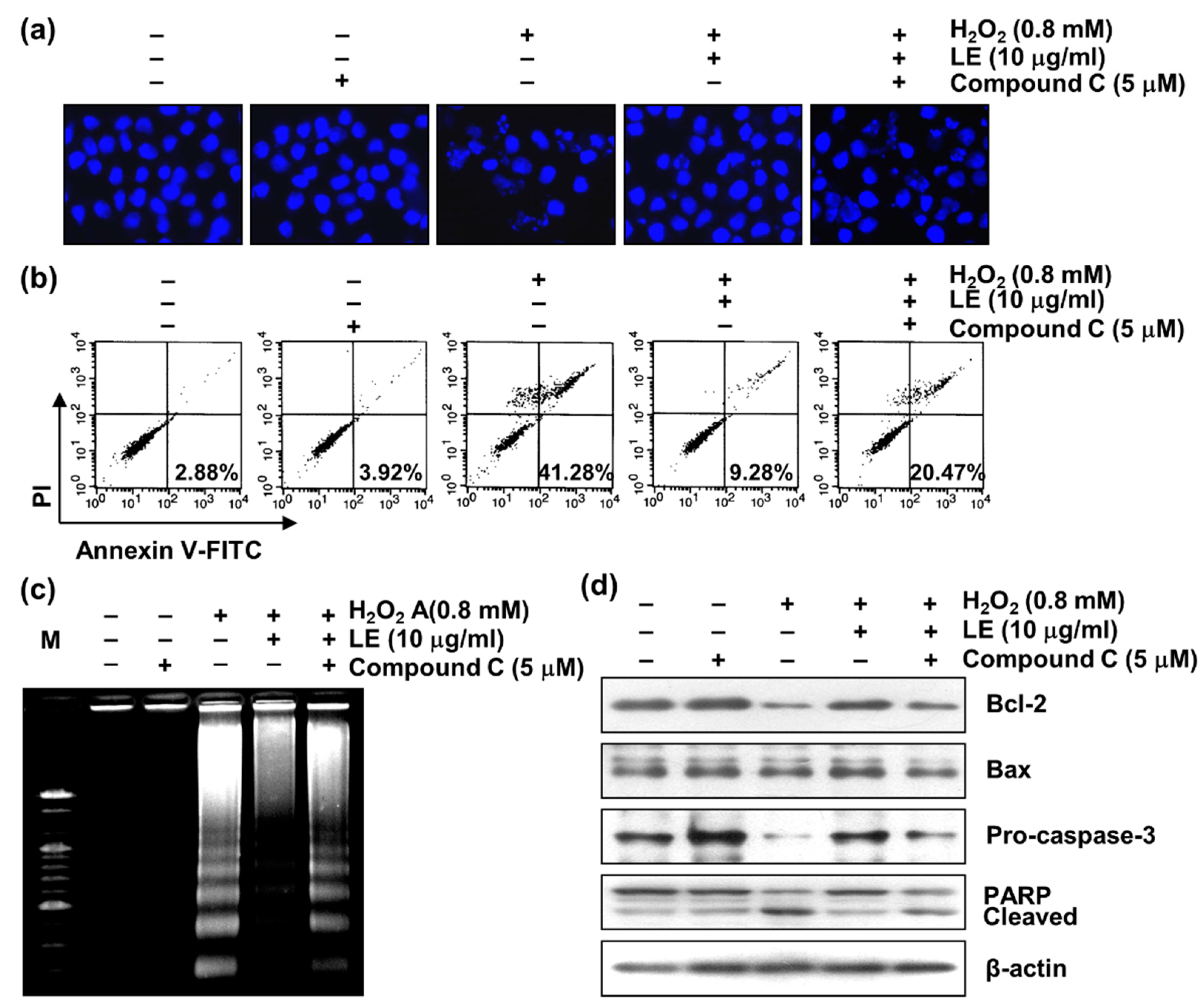
3.8. Role of AMPK in LE-Mediated Apoptosis Inhibition
3.9. Role of AMPK in LE-Mediated Mitochondrial Dysfunction Inhibition
3.10. Effect of LE on Myotube Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Bratton, S.B. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 2013, 19, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumor Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Thirupathi, A.; de Souza, C.T. Multi-regulatory network of ROS: The interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef]
- Lee, E.K.; Kim, J.A.; Park, S.J.; Kim, J.K.; Heo, K.; Yang, K.M.; Son, T.G. Low-dose radiation activates Nrf1/2 through reactive species and the Ca2+/ERK1/2 signaling pathway in human skin fibroblast cells. BMB Rep. 2013, 46, 258–263. [Google Scholar] [CrossRef]
- Chapple, S.J.; Siow, R.C.; Mann, G.E. Crosstalk between Nrf2 and the proteasome: Therapeutic potential of Nrf2 inducers in vascular disease and aging. Int. J. Biochem. Cell Biol. 2012, 44, 1315–1320. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Xu, C.; Li, X. Biological activities of extracts from loquat (Eriobotrya japonica Lindl.): A Review. Int. J. Mol. Sci. 2016, 17, 1983. [Google Scholar] [CrossRef]
- Jabir, M.S.; Hussien, A.A.; Sulaiman, G.M.; Yaseen, N.Y.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A.; Rizwana, H. Green synthesis of silver nanoparticles from Eriobotrya japonica extract: A promising approach against cancer cells proliferation, inflammation, allergic disorders and phagocytosis induction. Artif. Cells Nanomed. Biotechnol. 2021, 49, 48–60. [Google Scholar] [CrossRef]
- OpenComet Scoring Software, Version 1.3.1. Available online: http://cometbio.org (accessed on 27 December 2022).
- Stope, M. Phosphorylation of histone H2A.X as a DNA-associated biomarker (Review). World Acad. Sci. J. 2021, 3, 31. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta 2006, 1757, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Ji, S.Y.; Lee, H.; Choi, S.H.; Kwon, C.Y.; Kim, S.Y.; Lee, E.T.; Choo, S.T.; Kim, G.Y.; Choi, Y.H.; et al. Mori ramulus suppresses hydrogen peroxide-induced oxidative damage in murine myoblast C2C12 cells through activation of AMPK. Int. J. Mol. Sci. 2021, 22, 11729. [Google Scholar] [CrossRef]
- Yuan, J.; Lu, Y.; Wang, H.; Feng, Y.; Jiang, S.; Gao, X.-H.; Qi, R.; Wu, Y.; Chen, H.-D. Paeoniflorin resists H2O2-induced oxidative stress in melanocytes by JNK/Nrf2/HO-1 pathway. Front. Pharmacol. 2020, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Katsuragi, Y.; Ichimura, Y.; Komatsu, M. Regulation of the Keap1–Nrf2 pathway by p62/SQSTM1. Curr. Opin. Toxicol. 2016, 1, 54–61. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007, 17, 422–427. [Google Scholar] [CrossRef]
- Kang, J.S.; Han, M.H.; Kim, G.Y.; Kim, C.M.; Kim, B.W.; Hwang, H.J.; Hyun, Y. Nrf2-mediated HO-1 induction contributes to antioxidant capacity of a Schisandrae Fructus ethanol extract in C2C12 myoblasts. Nutrients 2014, 6, 5667–5678. [Google Scholar] [CrossRef]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and human diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef]
- Yang, H.Y.; Lee, T.H. Antioxidant enzymes as redox-based biomarkers: A brief review. BMB Rep. 2015, 48, 200–208. [Google Scholar] [CrossRef]
- Wang, S.; Song, P.; Zou, M.-H. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin. Sci. (Lond.) 2012, 122, 555–573. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Wang, X.; Wang, G.; Shi, M.; Wu, J. Design of a novel multi channel photonic crystal fiber polarization beam splitter. Opt. Commun. 2017, 400, 79–83. [Google Scholar] [CrossRef]
- Cardaci, S.; Filomeni, G.; Ciriolo, M.R. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J. Cell Sci. 2012, 125, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of platycodin D against oxidative stress-induced DNA damage and apoptosis in C2C12 myoblasts. Gen. Physiol. Biophys. 2020, 39, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Siu, P.M.; Wang, Y.; Alway, S.E. Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci. 2009, 84, 468–481. [Google Scholar] [CrossRef]
- Choi, Y.H. Trans-cinnamaldehyde protects C2C12 myoblasts from DNA damage, mitochondrial dysfunction and apoptosis caused by oxidative stress through inhibiting ROS production. Genes Genom. 2021, 43, 303–312. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski 2020, 48, 124–127. [Google Scholar]
- Dadsena, S.; King, L.E.; García-Sáez, A.J. Apoptosis regulation at the mitochondria membrane level. Biochim. Biophys. Acta. Biomembr. 2021, 1863, 183716. [Google Scholar] [CrossRef]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014, 5, 737–749. [Google Scholar] [CrossRef]
- Hasibuan, F.E.; Syahfitri, W.; Ilyas, S.; Hutahaean, S. Phytochemical screening, antioxidant activity and thin-layer chromatography test of methanol extract and simplicia leaves of loquat (Eriobotrya japonica Lindl). IOP Conf. Ser. Mater. Sci. Eng. 2020, 725, 012069. [Google Scholar] [CrossRef]
- Hyun, M.K.; Kim, D.H.; Park, C.H.; Noh, S.G.; Choi, S.; Lee, J.Y.; Choi, J.H.; Park, D.; Choi, Y.J.; Chung, H.Y. Protective mechanisms of loquat leaf extract and ursolic acid against diabetic pro-inflammation. J. Mol. Med. 2022, 100, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lu, R.; Huang, C.; Lin, D. Taurine Protects C2C12 Myoblasts from impaired cell proliferation and myotube differentiation under cisplatin-induced ROS exposure. Front. Mol. Biosci. 2021, 8, 685362. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Lee, N.H.; Patel, K.D.; Jun, S.K.; Park, J.H.; Knowles, J.C.; Kim, H.W.; Lee, H.H.; Lee, J.H. A Study on myogenesis by regulation of reactive oxygen species and cytotoxic activity by selenium nanoparticles. Antioxidants 2021, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhou, S.; Sakamoto, K. Alpha Mangostin promotes myogenic differentiation of C2C12 mouse myoblast cells. Biochem. Biophys. Res. Commun. 2020, 528, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.X.; Li, S.; Wang, L.; Ko, H.J.; Lee, Y.; Jung, D.Y.; Okutsu, M.; Yan, Z.; Kim, J.K.; Lin, J.D. Baf60c drives glycolytic metabolism in the muscle and improves systemic glucose homeostasis through Deptor-mediated Akt activation. Nat. Med. 2013, 19, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M.; Rigby, P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, F.; Brand-Saberi, B. The eventful somite: Patterning, fate determination and cell division in the somite. Anat. Embryol. 2006, 211 (Suppl. 1), 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Yokoyama, S.; Asahara, H. The myogenic transcriptional network. Cell. Mol. Life Sci. 2011, 68, 1843–1849. [Google Scholar] [CrossRef]
- Berkes, C.A.; Tapscott, S.J. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 2005, 16, 585–595. [Google Scholar] [CrossRef]
- Jin, W.; Peng, J.; Jiang, S. The epigenetic regulation of embryonic myogenesis and adult muscle regeneration by histone methylation modification. Biochem. Biophys. Rep. 2016, 6, 209–219. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, N.; De Simone, F.; Pizza, C.; Mahmood, N.; Moore, P.S.; Conti, C.; Orsi, N.; Stein, M.L. Constituents of Eriobotrya japonica. A study of their antiviral properties. J. Nat. Prod. 1992, 55, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Uemitsu, N.; Shirota, M.; Matsumoto, K.; Tezuka, Y. A new triterpene ester from Eriobotrya japonica. Chem. pharm. Bull. 1996, 44, 2181–2182. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Suneja, M.; Ebert, S.M.; Bongers, K.S.; Fox, D.K.; Malmberg, S.E.; Alipour, F.; Shields, R.K.; Adams, C.M. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 2011, 13, 627–638. [Google Scholar] [CrossRef]
- Noh, K.K.; Chung, K.W.; Sung, B.; Kim, M.J.; Park, C.H.; Yoon, C.; Choi, J.S.; Kim, M.K.; Kim, C.M.; Kim, N.D.; et al. Loquat (Eriobotrya japonica) extract prevents dexamethasone-induced muscle atrophy by inhibiting the muscle degradation pathway in Sprague Dawley rats. Mol. Med. Rep. 2015, 12, 3607–3614. [Google Scholar] [CrossRef][Green Version]
- Cho, Y.H.; Lee, S.Y.; Kim, C.M.; Kim, N.D.; Choe, S.; Lee, C.H.; Shin, J.H. Effect of loquat leaf extract on muscle strength, muscle mass, and muscle function in healthy adults: A randomized, double-blinded, and placebo-controlled trial. Evid. Based Complement. Alternat. Med. 2016, 2016, 4301621. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.H.; Jang, J.Y.; Lee, J.H.; Choi, Y.W.; Choi, Y.H.; Kim, N.D. Loquat Leaf Extract Inhibits Oxidative Stress-Induced DNA Damage and Apoptosis via AMPK and Nrf2/HO-1 Signaling Pathways in C2C12 Cells. Appl. Sci. 2023, 13, 572. https://doi.org/10.3390/app13010572
Kwon YH, Jang JY, Lee JH, Choi YW, Choi YH, Kim ND. Loquat Leaf Extract Inhibits Oxidative Stress-Induced DNA Damage and Apoptosis via AMPK and Nrf2/HO-1 Signaling Pathways in C2C12 Cells. Applied Sciences. 2023; 13(1):572. https://doi.org/10.3390/app13010572
Chicago/Turabian StyleKwon, Young Hoon, Jung Yoon Jang, Jun Ho Lee, Young Whan Choi, Yung Hyun Choi, and Nam Deuk Kim. 2023. "Loquat Leaf Extract Inhibits Oxidative Stress-Induced DNA Damage and Apoptosis via AMPK and Nrf2/HO-1 Signaling Pathways in C2C12 Cells" Applied Sciences 13, no. 1: 572. https://doi.org/10.3390/app13010572
APA StyleKwon, Y. H., Jang, J. Y., Lee, J. H., Choi, Y. W., Choi, Y. H., & Kim, N. D. (2023). Loquat Leaf Extract Inhibits Oxidative Stress-Induced DNA Damage and Apoptosis via AMPK and Nrf2/HO-1 Signaling Pathways in C2C12 Cells. Applied Sciences, 13(1), 572. https://doi.org/10.3390/app13010572







