Effects of Rosemary Extract on C2C12 Myoblast Differentiation and 5-Aminoimidazole-4-carboxamide Ribonucleoside (AICAR)-Induced Muscle Cell Atrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Induction of Differentiation
2.3. Cell Viability Assay
2.4. Measurement of Myotube Diameter
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
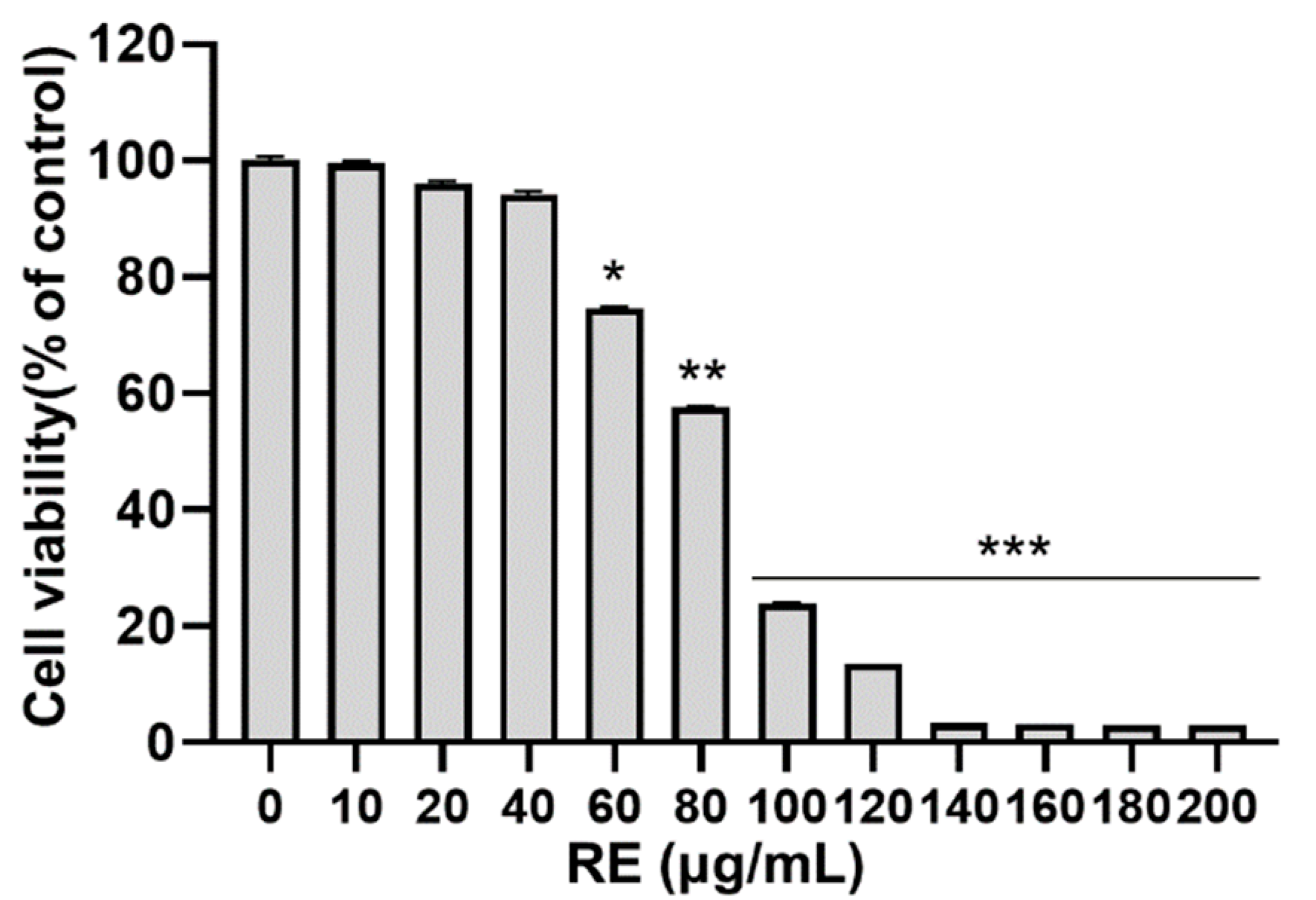
3.1. Effects of RE on Cytotoxicity in C2C12 Myoblasts
3.2. Effects of RE on C2C12 Myotube Differentiation
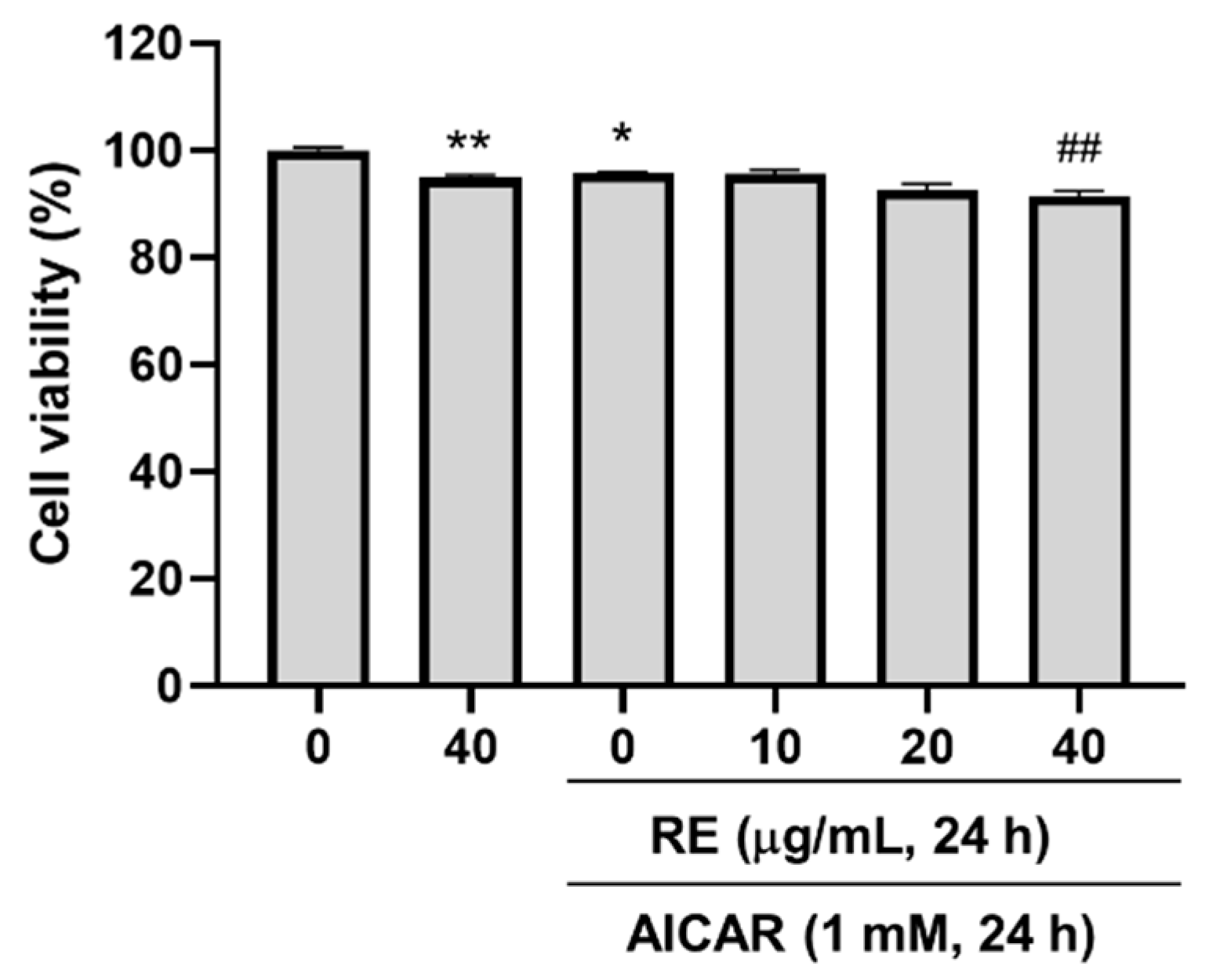
3.3. Effects of AICAR on C2C12 Myotube Atrophy
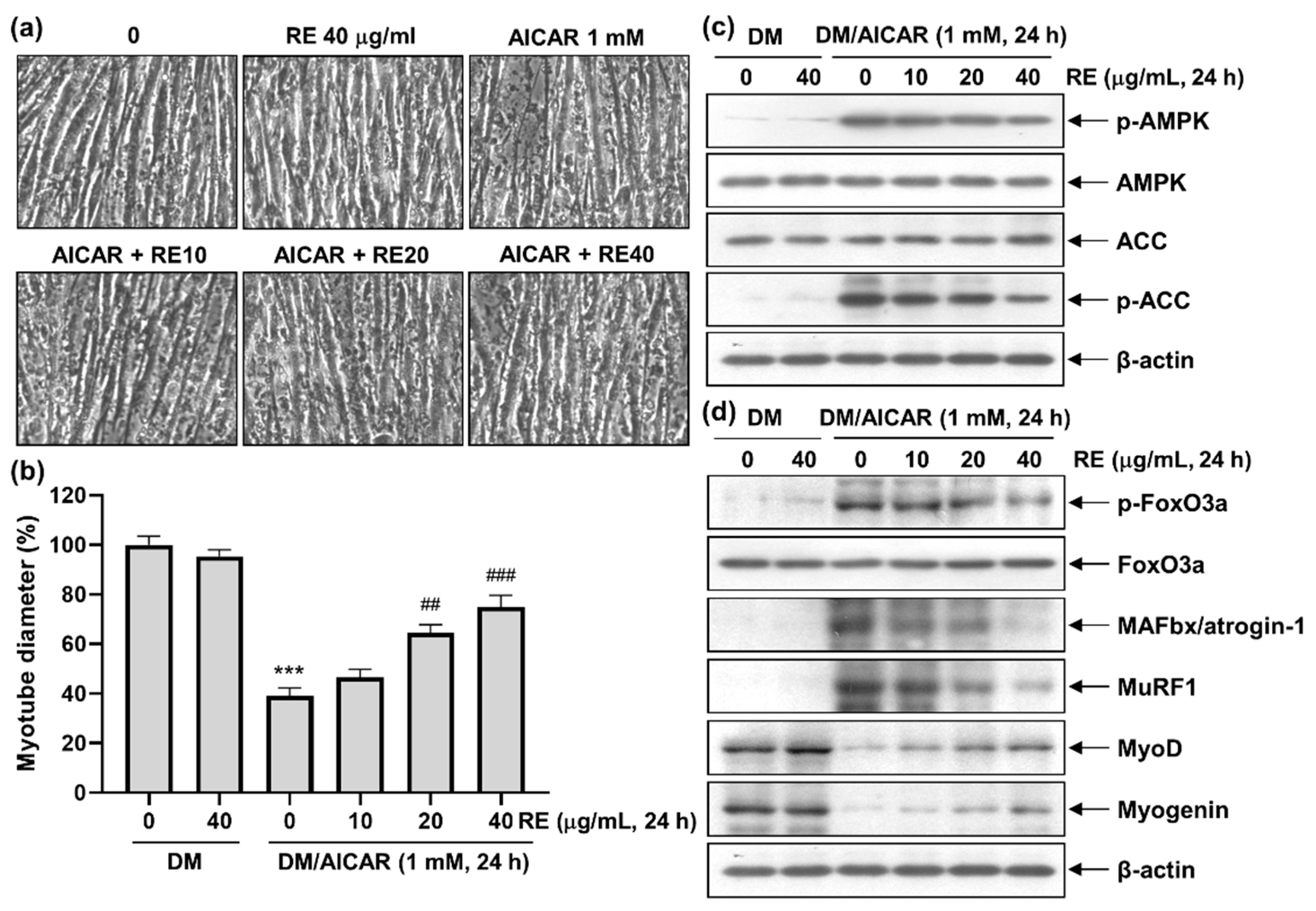
3.4. Effect of RE on AICAR-Induced C2C12 Myotube Atrophy
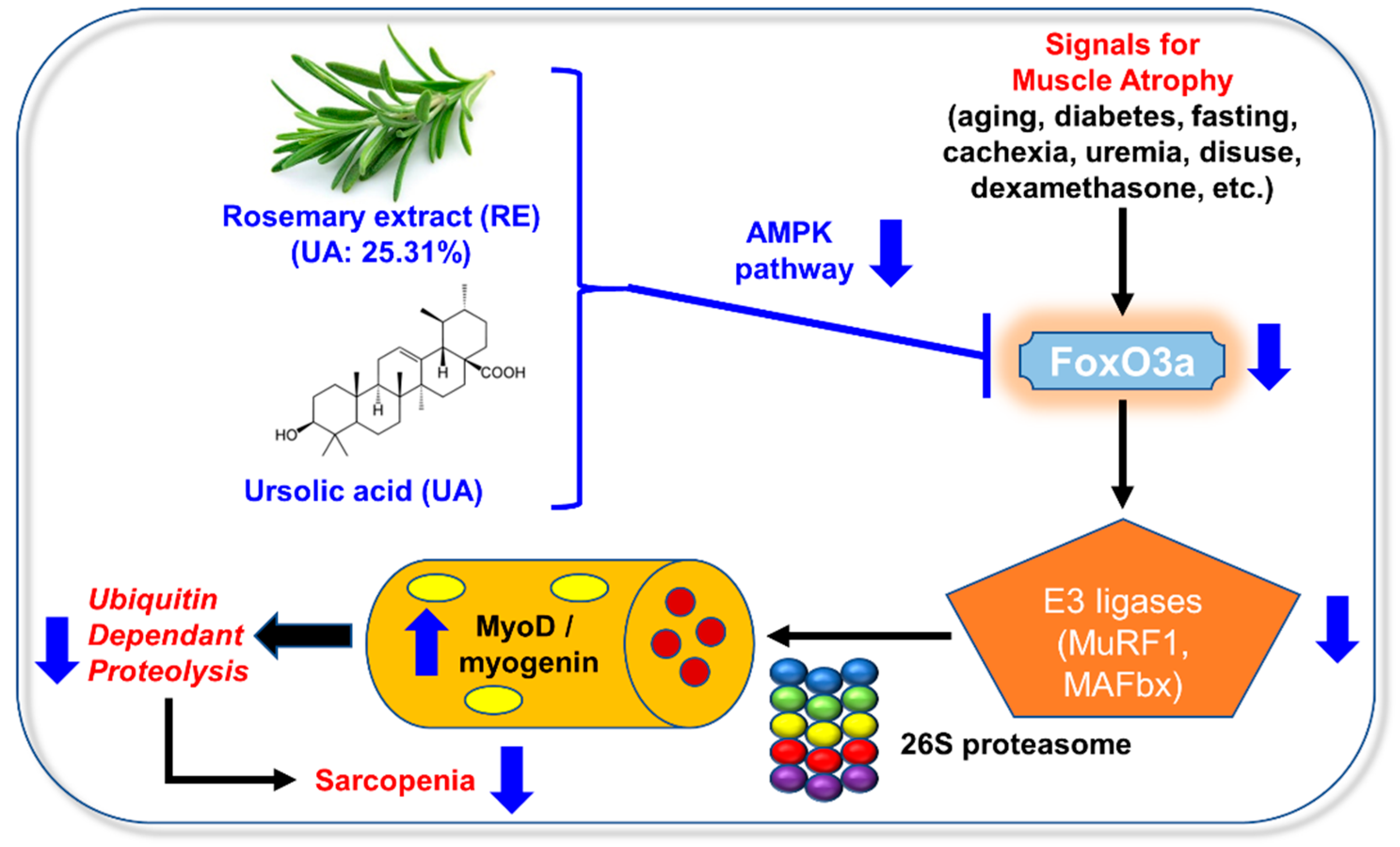
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Yoo, J.I.; Kim, J.T.; Park, C.H.; Cha, Y. Diagnosis and management of sarcopenia after hip fracture surgery: Current concept review. Hip Pelvis 2022, 34, 1–9. [Google Scholar] [CrossRef]
- Dao, T.; Green, A.E.; Kim, Y.A.; Bae, S.J.; Ha, K.T.; Gariani, K.; Lee, M.R.; Menzies, K.J.; Ryu, D. Sarcopenia and muscle aging: A brief overview. Endocrinol. Metab. 2020, 35, 716–732. [Google Scholar] [CrossRef]
- Dhillon, R.J.; Hasni, S. Pathogenesis and management of sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Farr, S.A.; Niehoff, M.L.; Ceddia, M.A.; Herrlinger, K.A.; Lewis, B.J.; Feng, S.; Welleford, A.; Butterfield, D.A.; Morley, J.E. Effect of botanical extracts containing carnosic acid or rosmarinic acid on learning and memory in SAMP8 mice. Physiol. Behav. 2016, 165, 328–338. [Google Scholar] [CrossRef]
- Ho, C.T.; Wang, M.; Wei, G.J.; Huang, T.C.; Huang, M.T. Chemistry and antioxidative factors in rosemary and sage. Biofactors 2000, 13, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Elbahnasawy, A.S.; Valeeva, E.R.; El-Sayed, E.M.; Rakhimov, I.I. The impact of thyme and rosemary on prevention of osteoporosis in Rats. J. Nutr. Metab. 2019, 2019, 1431384. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Dohi, H.; Egashira, Y.; Hirai, S. Erucic acid derived from rosemary regulates differentiation of mesenchymal stem cells into osteoblasts/adipocytes via suppression of peroxisome proliferator-activated receptor gamma transcriptional activity. Phytother. Res. 2020, 34, 1358–1366. [Google Scholar] [CrossRef]
- Morel, S.; Hugon, G.; Vitou, M.; Védère, M.; Fons, F.; Rapior, S.; Saint, N.; Carnac, G. A bioassay-guided fractionation of rosemary leaf extract identifies carnosol as a major hypertrophy inducer in human skeletal muscle cells. Nutrients 2021, 13, 4190. [Google Scholar] [CrossRef]
- Kahnt, M.; Fischer Nee Heller, L.; Al-Harrasi, A.; Csuk, R. Ethylenediamine derived carboxamides of betulinic and ursolic acid as potential cytotoxic agents. Molecules 2018, 23, 2558. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Nath Mishra, B.; Khan, F. 3D-QSAR and docking studies on ursolic acid derivatives for anticancer activity based on bladder cell line T24 targeting NF-kB pathway inhibition. J. Biomol. Struct. Dyn. 2019, 37, 3822–3837. [Google Scholar] [CrossRef] [PubMed]
- Abu-Gharbieh, E.; Shehab, N.G.; Almasri, I.M.; Bustanji, Y. Antihyperuricemic and xanthine oxidase inhibitory activities of Tribulus arabicus and its isolated compound, ursolic acid: In vitro and in vivo investigation and docking simulations. PLoS ONE 2018, 13, e0202572. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Naimi, M.; Murphy, B.; Hudlicky, T.; Tsiani, E. Rosmarinic acid, a rosemary extract polyphenol, increases skeletal muscle cell glucose uptake and activates AMPK. Molecules 2017, 22, 1669. [Google Scholar] [CrossRef]
- Kuang, J.X.; Shen, Q.; Zhang, R.Q.; Fang, Q.Y.; Deng, X.; Fan, M.; Cheng, C.R.; Zhang, X.W.; Liu, X. Carnosol attenuated atrophy of C2C12 myotubes induced by tumour-derived exosomal miR-183-5p through inhibiting Smad3 pathway activation and keeping mitochondrial respiration. Basic Clin. Pharmacol. Toxicol. 2022, 131, 500–513. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Vlavcheski, F.; Giacca, A.; MacPherson, R.E.K.; Tsiani, E. Carnosic acid attenuates the free fatty acid-induced insulin resistance in muscle cells and adipocytes. Cells 2022, 11, 167. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kojima-Yuasa, A.; Tadano, H.; Mizuno, A.; Kon, A.; Norikura, T. Ursolic acid improves the indoxyl sulfate-induced impairment of mitochondrial biogenesis in C2C12 cells. Nutr. Res. Pract. 2022, 16, 147–160. [Google Scholar] [CrossRef]
- Kim, M.; Sung, B.; Kang, Y.J.; Kim, D.H.; Lee, Y.; Hwang, S.Y.; Yoon, J.H.; Yoo, M.A.; Kim, C.M.; Chung, H.Y.; et al. The combination of ursolic acid and leucine potentiates the differentiation of C2C12 murine myoblasts through the mTOR signaling pathway. Int. J. Mol. Med. 2015, 35, 755–762. [Google Scholar] [CrossRef]
- Noh, K.K.; Chung, K.W.; Sung, B.; Kim, M.J.; Park, C.H.; Yoon, C.; Choi, J.S.; Kim, M.K.; Kim, C.M.; Kim, N.D.; et al. Loquat (Eriobotrya japonica) extract prevents dexamethasone-induced muscle atrophy by inhibiting the muscle degradation pathway in Sprague Dawley rats. Mol. Med. Rep. 2015, 12, 3607–3614. [Google Scholar] [CrossRef][Green Version]
- Sung, B.; Hwang, S.Y.; Kim, M.J.; Kim, M.; Jeong, J.W.; Kim, C.M.; Chung, H.Y.; Kim, N.D. Loquat leaf extract enhances myogenic differentiation, improves muscle function and attenuates muscle loss in aged rats. Int. J. Mol. Med. 2015, 36, 792–800. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, S.Y.; Kim, C.M.; Kim, N.D.; Choe, S.; Lee, C.H.; Shin, J.H. Effect of loquat leaf extract on muscle strength, muscle mass, and muscle function in healthy adults: A randomized, double-blinded, and placebo-controlled trial. Evid. Based Complement. Alternat. Med. 2016, 2016, 4301621. [Google Scholar] [CrossRef]
- Ding, M.; Xie, Y.; Wagner, R.J.; Jin, Y.; Carrao, A.C.; Liu, L.S.; Guzman, A.K.; Powell, R.J.; Hwa, J.; Rzucidlo, E.M.; et al. Adiponectin induces vascular smooth muscle cell differentiation via repression of mammalian target of rapamycin complex 1 and FoxO4. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1403–1410. [Google Scholar] [CrossRef]
- Rudnicki, M.A.; Schnegelsberg, P.N.; Stead, R.H.; Braun, T.; Arnold, H.H.; Jaenisch, R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 1993, 75, 1351–1359. [Google Scholar] [CrossRef]
- Kablar, B.; Krastel, K.; Ying, C.; Asakura, A.; Tapscott, S.J.; Rudnicki, M.A. MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development 1997, 124, 4729–4738. [Google Scholar] [CrossRef]
- Ishibashi, J.; Perry, R.L.; Asakura, A.; Rudnicki, M.A. MyoD induces myogenic differentiation through cooperation of its NH2- and COOH-terminal regions. J. Cell Biol. 2005, 171, 471–482. [Google Scholar] [CrossRef]
- Vavvas, D.; Apazidis, A.; Saha, A.K.; Gamble, J.; Patel, A.; Kemp, B.E.; Witters, L.A.; Ruderman, N.B. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J. Biol. Chem. 1997, 272, 13255–13261. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Hayashi, T.; Hirshman, M.F.; Smith, J.T.; Habinowski, S.A.; Kaijser, L.; Mu, J.; Ljungqvist, O.; Birnbaum, M.J.; Witters, L.A.; et al. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem. Biophys. Res. Commun. 2000, 273, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Birk, J.B.; Wojtaszewski, J.F. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J. Physiol. 2006, 577, 1021–1032. [Google Scholar] [CrossRef]
- Merrill, G.F.; Kurth, E.J.; Hardie, D.G.; Winder, W.W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 1997, 273, E1107–E1112. [Google Scholar] [CrossRef]
- Thomson, D.M.; Fick, C.A.; Gordon, S.E. AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signaling responses to high-frequency electrically stimulated skeletal muscle contractions. J. Appl. Physiol. 2008, 104, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Csibi, A.; Raibon, A.; Cornille, K.; Gay, S.; Bernardi, H.; Candau, R. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J. Cell. Biochem. 2012, 113, 695–710. [Google Scholar] [CrossRef]
- Karalaki, M.; Fili, S.; Philippou, A.; Koutsilieris, M. Muscle regeneration: Cellular and molecular events. In Vivo 2009, 23, 779–796. [Google Scholar] [PubMed]
- Ceafalan, L.C.; Popescu, B.O.; Hinescu, M.E. Cellular players in skeletal muscle regeneration. Biomed. Res. Int. 2014, 2014, 957014. [Google Scholar] [CrossRef] [PubMed]
- Grefte, S.; Kuijpers-Jagtman, A.M.; Torensma, R.; Von den Hoff, J.W. Skeletal muscle development and regeneration. Stem Cells Dev. 2007, 16, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Jagoe, R.T.; Goldberg, A.L. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 183–190. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Liu, Y.; Du, M.; Li, X.; Chen, L.; Shen, Q.; Tian, J.; Zhang, D. Role of the ubiquitin-proteasome pathway on proteolytic activity in postmortem proteolysis and tenderisation of sheep skeletal muscle. Int. J. Food Sci. Technol. 2016, 51, 2353–2359. [Google Scholar] [CrossRef]
- Hyatt, J.P.; Roy, R.R.; Baldwin, K.M.; Edgerton, V.R. Nerve activity-independent regulation of skeletal muscle atrophy: Role of MyoD and myogenin in satellite cells and myonuclei. Am. J. Physiol. Cell Physiol. 2003, 285, C1161–C1173. [Google Scholar] [CrossRef][Green Version]
- Tintignac, L.A.; Lagirand, J.; Batonnet, S.; Sirri, V.; Leibovitch, M.P.; Leibovitch, S.A. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J. Biol. Chem. 2005, 280, 2847–2856. [Google Scholar] [CrossRef]
- Jorgensen, S.B.; Wojtaszewski, J.F.; Viollet, B.; Andreelli, F.; Birk, J.B.; Hellsten, Y.; Schjerling, P.; Vaulont, S.; Neufer, P.D.; Richter, E.A.; et al. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005, 19, 1146–1148. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yakabe, Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci. Biotechnol. Biochem. 2007, 71, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V.; Guadagnin, E.; Gomes, L.; Roder, I.; Sandri, C.; Petersen, Y.; Milan, G.; Masiero, E.; Del Piccolo, P.; Foretz, M.; et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010, 29, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, S.; Liu, L. Phosphorylation and acetylation modifications of FOXO3a: Independently or synergistically? Oncol. Lett. 2017, 13, 2867–2872. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Jang, J.Y.; Kwon, Y.H.; Lee, S.H.; Park, C.; Choi, Y.H.; Kim, N.D. Effects of Rosemary Extract on C2C12 Myoblast Differentiation and 5-Aminoimidazole-4-carboxamide Ribonucleoside (AICAR)-Induced Muscle Cell Atrophy. Appl. Sci. 2023, 13, 986. https://doi.org/10.3390/app13020986
Lee JH, Jang JY, Kwon YH, Lee SH, Park C, Choi YH, Kim ND. Effects of Rosemary Extract on C2C12 Myoblast Differentiation and 5-Aminoimidazole-4-carboxamide Ribonucleoside (AICAR)-Induced Muscle Cell Atrophy. Applied Sciences. 2023; 13(2):986. https://doi.org/10.3390/app13020986
Chicago/Turabian StyleLee, Jun Ho, Jung Yoon Jang, Young Hoon Kwon, Seung Ho Lee, Cheol Park, Yung Hyun Choi, and Nam Deuk Kim. 2023. "Effects of Rosemary Extract on C2C12 Myoblast Differentiation and 5-Aminoimidazole-4-carboxamide Ribonucleoside (AICAR)-Induced Muscle Cell Atrophy" Applied Sciences 13, no. 2: 986. https://doi.org/10.3390/app13020986
APA StyleLee, J. H., Jang, J. Y., Kwon, Y. H., Lee, S. H., Park, C., Choi, Y. H., & Kim, N. D. (2023). Effects of Rosemary Extract on C2C12 Myoblast Differentiation and 5-Aminoimidazole-4-carboxamide Ribonucleoside (AICAR)-Induced Muscle Cell Atrophy. Applied Sciences, 13(2), 986. https://doi.org/10.3390/app13020986







