Abstract
In anaerobic digestion (AD), butyrate is degraded by syntrophic consortium, but can accumulate in highly loaded AD systems. The effect of butyrate on the AD process attracts much less attention than propionate or acetate. In this work, an enrichment culture of the thermophilic butyrate-oxidizing syntrophic consortium was obtained by gradually increasing the initial butyrate concentration from 20 to 170 mM. Surprisingly, even the highest butyrate concentration did not significantly inhibit the methanogenic community, and the stage of acetate degradation was the limiting overall rate of the process. At 170 mM butyrate, the bacterial community changed towards the dominance of syntrophic acetate-oxidizing (SAO) bacteria related to Syntrophaceticus (42.9%), Syntrophomonas (26.2%) and Firmicutes (26.2%), while the archaeal community experienced a sharp decrease in the abundance of Methanosarcina thermophila (from 86.0 to 25.0%) and increase in Methanothermobacter thermautotrophicus (from 3.2 to 53.1%) and Methanomassiliicoccus (from 3.2 to 21.9%). Thus, the shift from acetoclastic methanogenesis to SAO coupled to hydrogenotrophic methanogenesis occurred as an adaptive strategy to overcome high acetate (~200 mM) build-up. Bioaugmentation with the obtained enrichment culture was effective in mitigating the butyrate-dominated VFA build-up during the AD of readily biodegradable waste, increasing the methane production rate, methane yield and volatile solids removal by more than 3.5, 6.2 and 2.9 times, respectively. Our study revealed that the thermophilic butyrate-oxidizing consortia as bioaugmented culture could be the potential strategy to alleviate the high organic load and VFA stress of AD.
1. Introduction
Environmental problems associated with the increase in waste volumes and the depletion of natural resources, causing, as a result, the need for an active search for alternative energy sources, sparked a scientific interest in the process of the microbial anaerobic digestion (AD) of organic waste. AD technologies speed up the waste stabilization by hundreds of times, decrease emission of greenhouse gases from landfills and produce renewable energy in the form of biogas. Efficient biogas production depends on balanced microbial activity, which necessarily involves hydrolytic, acidogenic and syntrophic (acetogenic) bacteria, as well as methanogenic archaea. Complex trophic interactions exist between different microbial groups in methanogenic communities [1,2].
Volatile fatty acids (VFAs) are the key intermediates of organic waste transformation into biogas. The degradation of VFAs (along with hydrolysis) determines the overall rate of AD process [3]. In the absence of inorganic electron acceptors, VFAs can be degraded only when an active and balanced consortium of syntrophic bacteria and methanogenic archaea develop in the reactor. Syntrophic bacteria oxidize VFAs anaerobically only when the concentration of the products (especially H2 and formate) is kept low. This leads to the dependence of syntrophic bacteria on their hydrogen/formate-utilizing methanogenic partners [1].
VFAs accumulate rapidly when syntrophic bacteria or methanogenic archaea are suppressed or in the case of the substrate overloading. In the latter case, hydrolytic and acidogenic bacteria develop faster than synthrophs and methanogens, which are unable to oxidize VFAs at the same rate as they are produced. VFA accumulation results in a gradual decrease in pH level, which negatively affects methanogenesis, and the AD process becomes unstable. An increased concentration of VFAs is therefore an important indicator of instability of the AD process [4,5]. VFA (mainly acetate, propionate and butyrate) accumulation often happens during AD of a readily biodegradable feedstock, such as food waste [6], sewage sludge [7] or agroindustrial waste [8]. For example, the acidification and termination of methane production were observed during the thermophilic AD of food waste when the concentrations of VFAs reached 10–14 g/L, corresponding to 4–14 mM propionate, 54–70 mM butyrate and 75–120 mM acetate [9]. The investigation of adaptation mechanisms in microbial communities exposed to increased VFA concentration and searching for syntrophic bacteria and methanogenic archaea, which are resistant to high concentrations of acetate, propionate and butyrate, is of special importance for the practical application of these consortia in the AD process and for the stabilization of biogas production.
Acetate is a pivotal intermediate in the anaerobic decomposition of organic matter, and it contributes to a major part of the produced methane [10]. It has been reported that syntrophic acetate oxidation (SAO) has become the dominant pathway in overcoming acetate accumulation from initially acetoclastic methanogenesis-dominant anaerobic systems under high ammonia stress [11,12]. Additionally, the SAO pathway also played a significant role in the anaerobic digester suppressed through high concentrations of VFAs [13,14] and high temperatures [15] due to the inhibition of acetoclastic methanogens.
Propionate has the most pronounced and the most difficult-to-overcome inhibitory effect on the microorganisms in the methanogenic community. A number of works have therefore focused on syntrophic propionate degradation [16,17]. In the work of Jannat et al. [18], propionate degrading consortia were enriched in sequential mode, from lower (1 g/L or 13.7 mM of propionate) to higher strength phase with 3 g/L (or 41.1 mM) propionate. Four syntrophic microbial groups, Syntrophaceae, Syntrophomonadaceae, Methanobacterium and Methanosaeta were dominant in the lower strength phase. However, the substrate accelerated microbial shifts were observed in the high-strength phase with a significant decrease in Syntrophaceae of up to 26.9% [18]. Syntrophobacter was identified as the most abundant and consistent bacterial partner in syntrophic propionate conversion, regardless of the concentration of propionate, while among methanogen partners, Methanoculleus was specifically enriched at the inhibitory levels of propionate, likely due to the ability to function under unfavorable environmental conditions [19].
Although the amount of butyrate produced during organic waste decomposition can be 5–10 times higher than that of propionate [9], the effect of butyrate on the AD process attracts considerably less attention. In a mesophilic upflow anaerobic sludge blanket reactor fed with high-butyrate (up to 6 g/L or 68 mM) containing palm oil mill effluent, butyrate was β-oxidized to acetate and hydrogen by obligate proton reducers in syntrophic association with H2 utilizing methanogens [20]. The conversion of acetate to methane appeared to be a rate-limiting step but a key microbial community was not identified. Better butyrate-tolerated microbial consortia were domesticated and enriched through adding butyric acid, ranging from 0.2 to 4.4 g/(L⋅d) (i.e., up to 55 mM in the influent) in the mesophilic continuously stirred reactor [21]. The syntrophic consortia mainly included butyrate-oxidizing bacteria (Syntrophomonas), acetate-oxidizing bacteria (Synergistaceae and Mesotoga), interspecies H2 transfer promoted bacteria (DMER64) and methanogen (Methanosarcina). No inhibition of the microbial community was observed [21]. It is well-known that the thermophilic AD system has higher biochemical reaction rates but is more prone to destabilization as a result of the VFA build-up [22,23,24]. Moreover, it would be interesting to study the effect of much higher concentrations of butyrate on the shifts in the microbial community in order to better evaluate patterns of adaptation to inhibition. Such findings could be useful to improve degradation efficiencies, and the obtained enriched culture could be used to recover soured (specifically, butyrate-accumulated) digesters by bioaugmentation.
The goals of the present work were: (1) the gradual adaptation of the thermophilic methanogenic community to extremely high (up to 170 mM) butyrate concentrations; (2) to study the change in the thermophilic microbial community in response to extremely high concentrations of butyrate; and (3) to evaluate the feasibility of bioaugmentation using an enriched syntrophic consortium to stabilize the overloaded AD system.
2. Materials and Methods
2.1. Inoculum
A thermophilically digested mixture of primary and secondary sewage sludge (1:1 ratio by volume) from the Kuryanovo wastewater treatment plant (Moscow, Russia) was used as an inoculum. The inoculum contained 4.5% total solids and 2.96% volatile solids.
2.2. Enrichment of Syntrophic Consortium
The enrichment of syntrophic consortium was conducted at 50 °C in 500 mL serum bottles. Modified Pfennig medium [25] with microelements and vitamins solutions [26,27] was prepared as described in [28]. A total 100 mL of medium was dispensed into the bottles under argon atmosphere by using a bottle-top dispenser. Bottles were sealed with butyl rubber stoppers, closed with aluminum caps and sterilized. Sodium butyrate (98%, Thermo Scientific, Waltham, MA, USA) was used as a substrate. Enrichment was begun at the initial butyrate concentration of 20 mM. The cell biomass (10% vol/vol) was sequentially transferred into fresh media with higher butyrate concentrations (50, 95 and 170 mM) after the butyrate and all metabolite products were consumed and methane production was terminated. The initial pH of the inoculated medium was 6.8–7.0. pH was not controlled during incubation.
2.3. Bioaugmentation Experiment
Enrichment culture adapted to 170 mM butyrate was used to overcome the AD destabilization of simulated, readily biodegradable organic waste (dog food) containing 90.1% total solids, 80.6% volatile solids, 14.5% crude protein, 35.5% carbohydrates, 2.3% crude lipids and 9% crude cellulose (according to the manufacturer’s analysis). Thickened, thermophilically digested sludge was used as an inoculum. Two inoculum-to-substrate ratios (ISR) on VS basis were tested: 50/50 (the optimal value) and 20/80 (unfavorable value, potentially resulting in excessive VFA accumulation and the destabilization of the AD process [29]). The bioaugmentation experiments were carried out in 120 mL vials. Bioaugmentation vials containing 10 g of the inoculum and substrate mixture with ISR of 50/50 or 20/80 were supplemented with 10 mL of the enrichment culture. The control vials were supplemented with 10 mL of distilled water instead of the enrichment culture. The final concentration of VS in all mixtures was 7.5%. The vials were purged with argon, sealed with rubber stoppers and closed with aluminum caps. The incubation was carried out in triplicate at 50 °C for 30 days without agitation. The composition of the gas phase, VFA concentration and pH were monitored regularly. Total solids (TS) and volatile solids (VS) content were determined at the onset and end of the experiment.
2.4. Analytical Methods
Concentrations of CH4, H2 and CO2 in the gas phase and of acetate, propionate, butyrate and iso-butyrate in the liquid phase were analyzed using a Crystal 5000.2 gas chromatograph (GC) (Chromatec, Yoshkar-Ola, Russia) as described previously [30]. Total solids (TS) content was determined after drying the sample to a constant weight at 105 °C. Nonvolatile solids (NVS) content was determined by the combustion of dry sample in a muffle furnace to constant weight at 650 °C. Volatile solids (VS) content was calculated as the difference in weight between the TS and NVS. The pH was determined using an FE20 pH meter equipped with an InLab® microelectrode (both Mettler Toledo, Greifensee, Switzerland). The maximal rates of butyrate degradation, acetate production and consumption and methane production were calculated via the angle of the tangent line at the steepest part of the relevant curve. The average rates were calculated as the amount of substrate consumed or products formed over the whole period of the experiment. The rates were expressed in mmol/(L day).
2.5. Analysis of Microbial Diversity
2.5.1. DNA Extraction
An amount of 2–10 mL of microbial suspension from thoroughly mixed enrichment cultures was placed into tubes using a syringe and centrifuged at 13,000× g for 10 min. The microbial pellets were used for DNA extraction. DNA was extracted using the method based on the modified Birnboim–Doli DNA alkaline extraction method and purified with the Wizard DNA cleanup system (Promega GmbH, Walldorf, Germany) [31].
2.5.2. Amplification, Cloning and Sequencing
The obtained DNA was used in PCR for archaeal and bacterial 16S rRNA genes with primer sets Univ27f/Univ1492r [32] and A8F/A915R [33]. The PCR products were purified by electrophoresis in agarose gel using the Wizard PCR Preps kit (Promega). Purified PCR products were ligated into the pGEM-T EasySystem vector (Promega), according to the manufacturer’s recommendations and cloned into E. coli DH10B. The sequencing of PCR products and cloned fragments was performed on an ABI3730 DNA Analyzer (Applied Biosystems) using the Big Dye Terminator v 3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) according to the manufacturer’s recommendations.
2.5.3. Phylogenetic Analysis
The initial analysis of the obtained sequences was performed using the NCBI BLAST service (http://www.ncbi.nlm.nih.gov/blast, accessed on 1 August 2021). The chimeric sequences were checked and removed using the Bellerophon program [34]. Sequence editing was performed using the BioEdit editor (http://jwbrown.mbio.ncsu.edu/BioEdit/bioedit.html, accessed on 1 August 2021). The phylogenetic dendrogram of bacterial and archaeal 16S rRNA genes was constructed using the neighbor-joining method of MEGA 6.0 [35]. Bootstrap values were calculated after 1000 replications. The GenBank accession numbers of the 16S rRNA gene sequences are MN382163–MN382177.
2.6. Statistical Analysis
The confidence limits of average values of experimental data were calculated using Equation (1) [36]:
where
- —the average value;
- s—the standard deviation;
- n—the number of replicates;
- t—the corresponding t-statistical distribution value, depending on the number of samples and on the degree of confidence (95% in the present study).
3. Results
3.1. Dynamics of Processes Involved in Syntrophic Degradation of Butyrate
A consortium of thermophilic bacteria and archaea adapted to high concentrations of butyrate and acetate was obtained by sequentially transferring the biomass of thermophilically digested sewage sludge into the fresh medium with higher concentrations of butyrate, i.e., 20, 50, 95 and 170 mM, respectively. The dynamics of methane production via syntrophic butyrate degradation followed by the production and degradation of acetate is shown in Figure 1, and the rates of the processes are given in Table 1.
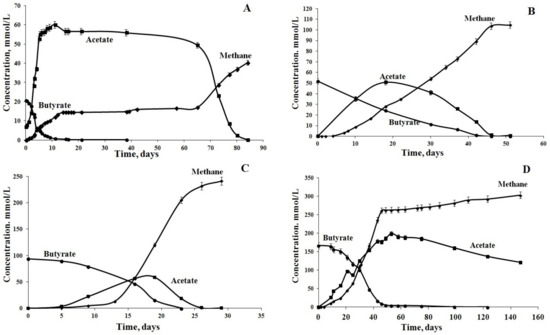
Figure 1.
Dynamics of anaerobic butyrate degradation at different initial concentrations of butyrate: (A) 20 mmol/L, (B) 50 mmol/L, (C) 95 mmol/L and (D) 170 mmol/L.

Table 1.
The rates of processes involved in the syntrophic degradation of butyrate.
Experiments started at an initial butyrate concentration of 20 mM. At this concentration, the complete degradation of butyrate with the production of methane as the final product proceeded in three stages (Figure 1A). During the first stage (10 days), butyrate degradation was accompanied by the rapid accumulation of acetate (up to 60 mM). The amount of acetate produced was not equimolar, i.e., the measured acetate concentration was 1.5 times higher than the one calculated using the Equation (2) [37]:
CH3CH2CH2COO− + 2H2O → 2CH3COO− + H+ + 2H2 ΔGo′ = +48 kJ
The consortium was further transferred to the fresh medium with 50 mM butyrate, after the acetate was consumed completely (Figure 1B). While the rates of butyrate degradation at 50 mM were lower than at 20 mM, the process of methane production was balanced better (Table 1). During the first 20 days of the experiment, 30.2 out of 50 mM butyrate was degraded, and 50.8 mM acetate was accumulated in the medium, which was at least 16% below the maximal theoretically calculated value. This was an indication of coupled processes of syntrophic butyrate degradation and acetoclastic methanogenesis. Hydrogen was detected in the gas phase during the first day of incubation (0.3 mM) and close to the end of the experiment (2.24 mM). No significant effect of H2 on the activity of syntrophic bacteria was observed.
The most efficient butyrate degradation and methane production was observed, when the enrichment culture was further transferred into the medium with 95 mM butyrate (Figure 1C, Table 1). Butyrate decomposition occurred at the highest rate of 10.3 mmol/(L day) and was completed on day 23 of incubation. Hydrogen (up to 0.5 mM) was detected on day 3 and was consumed within 24 h. The highest acetate concentration was 58.8 mM (~47% of the theoretically calculated maximum value), and methane production rate reached the highest value of 21.2 mmol/(L day). This indicated that the microbial community contained active butyrate-degrading syntrophic bacteria, hydrogenotrophic and acetoclastic methanogens and became resistant to high substrate concentrations. Thus, the butyrate in concentrations of up to 100 mM had no inhibitory effect on the enrichment culture.
The subsequent increase in butyrate concentration to 170 mM resulted in a decrease in rates of butyrate degradation and methane production (Figure 1D, Table 1). The highest rate of methane production was 9.4 mmol/(L day), which was 55.7% lower than the value observed when 95 mM butyrate was added (Table 1).
Under these conditions, acetate utilization again became the rate-limiting stage. The highest acetate concentration in the medium was 198.8 mM (58.8% of the theoretically calculated value). Both acetate conversion and methane production decreased sharply after butyrate was consumed on day 50. Acetate concentration decreased only to 78 mM (by 40%) during the following 95 days. Since H2 did not accumulate in the gas phase and pH was maintained at 7.5–8.0, the high concentration of acetate, apparently, was a factor negatively affecting the microbial community.
3.2. Changes in the Composition of the Microbial Community during the Degradation of 170 mM Butyrate
Samples of enrichment culture for DNA extraction were taken twice on days 20 and 120, and it can be seen that at the first stage of butyrate degradation, the microbial community was more diverse than at the end of the experiment (Figure 2, Figure 3 and Figure 4, Table S1).
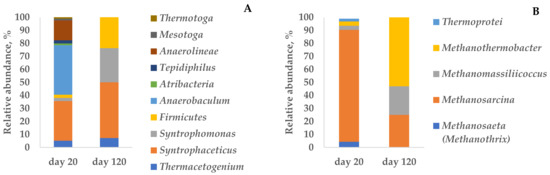
Figure 2.
Relative abundance of bacterial (A) and archaeal (B) community at the genus level in the enrichment culture during the degradation of 170 mM butyrate.
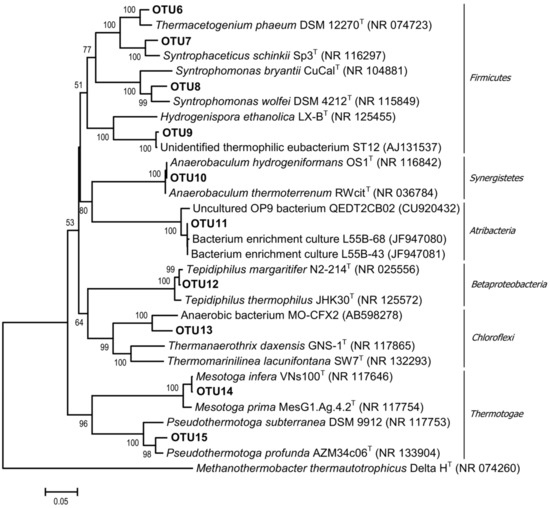
Figure 3.
Phylogenetic tree of bacterial 16S rRNA gene sequence of representative clones of each OTU and related type strains of the most represented bacterial groups of the enrichment culture. The scale bar represents 5% sequence divergence.
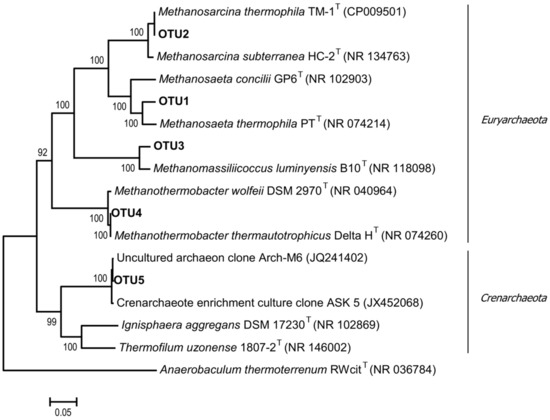
Figure 4.
The phylogenetic tree of archaeal 16S rRNA gene sequence of the representative clones of each OTU and related type strains of the most represented archaeal groups of the enrichment culture. The scale bar represents 5% sequence divergence.
Among methanogenic archaea, Methanosarcina thermophila TM-1 (100% 16S rRNA similarity) dominated (86% of cloned archaeal sequences) on day 20 of the experiment with 170 mM butyrate (Table S1, Figure 4). Acetoclastic methanogens related to Methanothrix thermophila PT (95.8% 16S rRNA similarity) were a minor part of the community responsible for 4.3% of the archaeal sequences. The bacterial community was mainly dominated by Syntrophaceticus (30.4%) and Anaerobaculum (38.0%) with the lower content of Anaerolineae (15.3%).
The composition of the archaeal part of the community changed on day 120. The share of methanogens related to Methanosarcina thermophila decreased to 25%, while the abundance of Methanothermobacter thermautotrophicus and Methanomassiliicoccus luminyensis became more numerous (53.1 and 21.9%, respectively) (Table S1). Similarly, the composition of the bacterial part of the community changed by the end of the experiment (day 120). Syntrophic acetate-oxidizing bacteria (SAOB) related to Syntrophaceticus schinkii and Thermacetogenium phaeum became more abundant (43 and 7% of cloned bacterial sequences, respectively). Over 26% of the sequences belonged to unclassified Firmicutes, in which syntrophic bacteria are common. Oppositely, the abundance of bacteria related to Anaerobaculum hydrogeniformans and Anaerolineae decreased to 14.3 and 7.1%, respectively (Figure 2 and Figure 3).
The results obtained by cloning indicate that high acetate accumulation causes a shift of the major methanogenic pathway from acetoclastic to hydrogenotrophic coupled with syntrophic acetate oxidation. An increase in acetate concentration was the factor driving the changes in the composition of the microbial community.
3.3. Improvement in AD Performance though Bioaugmentation with the Syntrophic Enrichment Culture
The ability of the syntrophic enrichment culture adapted to high VFA concentrations to improve the performance of AD of readily biodegradable waste (dog food) was tested at different ISRs (Table 2).

Table 2.
The performance efficiency of bioaugmentation with enrichment culture to prevent AD failure at different ISRs.
The enrichment culture exhibited high activity both under-favorable (ISR = 50/50) and unfavorable (ISR = 20/80) conditions. Cumulative methane production in the bioaugmented mixtures after 30 days of incubation was maximum both for favorable and unfavorable ISR, reaching 178.5 ± 8.7 and 174.0 ± 8.7 mL/g VS, respectively. In the mixtures with ISR of 50/50 the AD process was stable, with the average methane production rate of 5.19 ± 0.11 mL CH4/(g VS day). The average rate of methane production in the control samples did not exceed 1.50 ± 0.04 mL CH4/(g VS day), i.e., it was 3.5 times lower than in the bioaugmented samples. In the mixtures with ISR of 20/80, a suppression of methanogenesis was observed on days 3–5. Subsequently, however, the AD process stabilized and attained the average methane production rate of 5.11 ± 0.11 mmol CH4/(g VS day). In the control samples (ISR = 20/80), methane production stopped on days 3–5 of incubation (Table 2) due to the inhibition of methanogenic community caused by high VFA build-up (~8.0 g/L), represented mainly by butyrate (53.4 mM), acetate (37.8 mM) and ethanol (20.2 mM).
The maximum concentration of total VFAs was as high as 7.6–7.9 g/L on days 2–3, the main components being butyrate (48.8–52.5 mM), acetate (35.7–38.9 mM) and ethanol (23.1–26.7 mM). In the mixtures supplemented with the enrichment culture, the maximum VFAs concentration was 1.5–2 times higher than in the control, which indicates better substrate decomposition by the microorganisms of the bioaugmented microbial community. This is apparently due to the lack of inhibition by metabolite products, which are rapidly consumed and do not accumulate to inhibitory concentrations. In both bioaugmentation and control mixtures with ISR of 50/50, VFAs were almost completely consumed by the end of incubation (12 mM propionate and 5.5 mM acetate remained). Hydrogen concentration in the headspace did not exceed 0.71 mmol/L for the control and 0.15 mmol/L for the bioaugmentation mixture. While in all control mixtures with ISR of 20/80, H2 concentrations were high (from 15.86 mmol/L at the onset of incubation to 12.14 mmol/L by its end). Such high H2 concentrations indicate a severe imbalance in the AD process. Thus, our findings showed that the syntrophic enrichment culture significantly improved AD performance.
4. Discussion
4.1. Dynamics of Butyrate Degradation
In the AD process, acetate is mainly formed during acetogenesis from other VFAs, such as butyrate (Equation (2)) [23]. The electrons produced during the oxidation of butyrate are transferred to protons to generate hydrogen. It must be mentioned that these oxidation reactions are thermodynamically unfavorable and can only proceed when the produced hydrogen is consumed by methanogens to keep a low partial pressure. Finally, methane is produced in methanogenesis from either the oxidation of acetate (Equation (4)) or the reduction of CO2 by H2 (Equation (5)), which is the most sensitive step to high VFA concentrations compared to other steps. Acetate can be metabolized to methane in two ways: syntrophic acetate oxidation coupled with hydrogenotrophic methanogenesis (Equations (3) and (5)) and acetoclastic methanogenesis (Equation (4)).
CH3COO− + 4H2O → 2HCO3− + H+ + H2 ΔGo′ = +105 kJ
CH3COO− + H2O → CH4 + HCO3− ΔGo′ = −31 kJ
4H2 + 2HCO3− + H+ → CH4 + 3H2O ΔGo′ = −131 kJ
Excessive acetate (Figure 1A) was probably formed due to biomass hydrolysis and the decomposition of yeast extract (a component of the medium, 0.1 g/L). Acetate could also be produced from H2 and CO2 by homoacetogenic bacteria. Since the affinity of homoacetogens for H2 is lower than that of hydrogenotrophic methanogens, they cannot compete and exhibit low activity in balanced methanogenic communities [38]. However, under unfavorable conditions, e.g., in the presence of inhibitors of methanogenic archaea or at high substrate loads, homoacetogenic bacteria gain an advantage for growth [39].
Methanothrix spp. is the most common acetoclastic methanogen in bioreactors with low acetate concentrations, including those for the treatment of mixed sewage sludge [40,41,42]. Methanosarcina spp. is more abundant when acetate concentration in the medium increases, especially in the case of thermophilic communities [43]. Members of the genus Methanosarcina have a high capacity for adaptation to varying environmental conditions, and their increased abundance therefore promotes the stabilization of the community [44]. It was shown previously that the adaptation of the methanogenic community may occur due to the accumulation of Methanothrix spp. [45] and specific aggregation [41], when the concentration of acetate increases gradually. No data are available on the inhibitory concentrations of butyrate used as a sole carbon source. Wang et al. considered the inhibitory effect of butyrate indirect and associated primarily with rapid acetate accumulation in the medium [16].
Our findings (Table 1) are in agreement with the results of Dogan et al. [46], who reported halving the methane production in the presence of 220, 170, and 48 mM of acetate, butyrate, and propionate, respectively. The inhibitory effect of acetate was previously shown to be more pronounced than that of butyrate [16,47].
Literature data show differences in inhibitory concentrations of acetate. Dogan et al. reported a decrease in the activity of acetoclastic methanogens at 68 mM acetate [46]. Illmer and Gstraunthaler found that 150 mM acetate concentration caused the destabilization of a large-scale thermophilic reactor fed with SS and OF-MSW [48]. According to Lins et al., inhibitory acetate concentrations varied from 23 to 81 mM [17]. Nozhevnikova et al. showed that acetate in concentrations from 5 to 100 mM affected the rate of thermophilic methanogenesis: the rate of methane production was higher at lower acetate concentrations [45]. The latter may vary, however, depending on the degree of the community adaptation to experimental conditions, as can be seen from our results. We observed the inhibition of methanogenesis at acetate concentrations above 50 mM in the first transfer. The adaptation of the community to this concentration was found in subsequent transfers. The further accumulation of over 200 mM acetate resulted in the pronounced retardation of acetate conversion to methane. Fotidis et al. found that the maximum growth rate of mesophilic microorganisms was 40% higher than that of thermophiles at acetate concentrations above 120 mM, while at lower acetate concentrations thermophiles had the same growth rate as mesophiles or higher [5]. This may be explained by higher cell permeability at elevated temperatures, so that high substrate concentrations have a more pronounced inhibitory effect [42].
The formation of a small amount of propionate (up to 0.8 mM) was observed in the last experiment with 170 mM butyrate addition. This may also indicate imbalance within the community, i.e., the insufficient activity of H2-utilizing and acetoclastic methanogens [37]. The pH of the medium varied within the range of 6.5–8.0 and was 7.5–8.0 even at high acetate concentrations. This was probably due to the hydrolysis of dead biomass with the release of ammonium, as well as CO2 production and its dissolution in the medium, causing bicarbonate alkalinity.
Thus, butyrate itself in concentrations of up to 170 mM had no significant negative effect on syntrophic bacteria and methanogenic archaea. A conversion of the intermediate metabolite (acetate) was the stage limiting methanogenesis in the course of syntrophic butyrate degradation. To overcome this problem, a dense population of active acetate-utilizing microorganisms with high growth rates should be present in the microbial community.
4.2. Changes in Microbial Community Composition during the Degradation of 170 mM Butyrate
Bacteria most closely related (among cultured strains) to Syntrophomonas wolfei subsp. saponavida DSM 4212 (94.7% similarity of the 16S rRNA gene sequences) were detected in the community on day 20. S. wolfei is capable of syntrophic butyrate degradation [49]. Approximately 30% of the cloned bacterial sequences are related to mesophilic Syntrophaceticus schinkii Sp3 (96.4% 16S rRNA similarity), which are capable of syntrophic acetate oxidation (SAO) [50]. The smaller part of the bacterial community consisted of microorganisms related to other SAOB, including thermophilic ones: 5.1% of the cloned bacterial sequences belonged to Thermacetogenium phaeum DSM 12270 (97.1% 16S rRNA similarity); 1.3% to Pseudothermotoga profunda AZM34c06 (97.4% 16S rRNA similarity); and 2.5% to Tepidiphilus margaritifer N2–214 (99.4% 16S rRNA similarity). Approximately 38% of the sequences belonged to Anaerobaculum hydrogeniformans OS1 (100% 16S rRNA similarity). A. hydrogeniformans is a typical component of microbial communities in thermophilic bioreactors. It ferments sugars, amino acids, and yeast extract, producing H2 [51]. Members of the phylum Chloroflexi, class Anaerolineae, contributed to 15.3% of the bacterial sequences. Anaerolineae is one of the most numerous microbial groups inhabiting anaerobic bioreactors; they are often members of anaerobic syntrophic consortia [52].
In the studied community, Methanosarcina thermophila TM-1 most probably carried out acetoclastic methanogenesis, although it is also able to grow on methanol, methylamines, and H2/CO2 [52]. Members of the genus Methanothrix are sensitive to high acetate concentrations in the medium [53]. However, our data show that these methanogens were able to survive at acetate concentrations of up to 60 mM by aggregation, the formation of a protective external matrix, and existence inside microbial granules. A similar mechanism was described for a mesophilic methanogen Methanothrix (Methanosaeta) harundinacea, which was able to survive at acetate concentrations of ≥100 mM [41]. Hydrogenotrophic methanogens were represented by Methanothermobacter thermautotrophicus Delta H (100% 16S rRNA similarity) and contributed up to 3.2% of archaeal sequences. Microorganisms related to the methyl-reducing methanogens Methanomassiliicoccus luminyensis B10 (95.7% 16S rRNA similarity) were also detected. Members of the genus Methanomassiliicoccus use methanol and methylamines as substrates and exhibit obligate dependence on hydrogen or formate [54].
Obligatory acetoclastic Methanothrix spp., which exhibited high affinity for acetate and can utilize it at very low concentrations [55], dominated in the original inoculum. They were replaced by Methanosarcina spp. with a lower affinity for acetate but higher growth rates and an ability to use other substrates for methanogens along with acetate. Increasing acetate concentrations in the medium were accompanied by the accumulation of Methanosarcina-like microorganisms. When acetate concentration further increased to ≥100 mM, the share of Methanosarcina in the community decreased and hydrogenotrophic Methanothermobacter became the predominant methanogenic archaea. The accumulation of Methanothermobacter in the community was accompanied by the increased abundance of bacteria related to SAOB. Jang et al. reported similar changes in the population of methanogenic archaea: increasing organic loading rate resulted in the decrease in the share of Methanosarcinales from 81.3 to 54%, while the relative abundances of Methanobacteriales and Methanomicrobiales increased from 5 to 17% and from 11 to 25%, respectively [52].
The shift of the key pathway of methanogenesis was the major mechanism for the adaptation of the microbial community to high VFA concentration. Such a shift is probably more important in the case of thermophilic communities, since the SAO process is known to be more efficient under thermophilic conditions (55–60 °C) than under mesophilic ones [56]. Lins et al. and Wang et al. established that adaptive mechanisms acting in the methanogenic community destabilized by excessive VFA accumulation were associated with shifts in the trophic interactions between microbial groups, rather than with changes in microbial abundance [16,17]. However, our results do not agree with the conclusion by Hattori that low acetate concentrations (below 1 mM) are preferable for SAOB, which prevails over acetoclastic methanogens under such conditions [56]. Our results also contradicted the data of Fotidis et al. [5] and Hao [57], who showed that elevated acetate concentrations in the medium under thermophilic conditions promoted the shift from hydrogenotrophic to acetoclastic methanogenesis. However, our results are in agreement with those of Lins et al. [17], who showed predominance of Methanosarcina and Methanoculleus (a genus of hydrogenotrophic methanogens) in the inoculum obtained for preventing acetate destabilization of AD process, together with predominance of members of the genus Clostridium, including C. ultunense (SAOB). Moreover, they showed that inoculum obtained by shock load (150 mM) provided higher methane yield than sequentially adapted ones [17]. This is probably due to the loss of a part of the bacterial community in the course of adaptation. Using labeled acetate, Sasaki et al. showed that the thermophilic digestion of OF-MSW resulted in conversion of ~80% acetate to methane via the SAO pathway with subsequent hydrogenotrophic methanogenesis, while acetoclastic methanogenesis was responsible only for ~20% [58].
4.3. Improvement in AD Performance through Bioaugmentation, Comparison with Recent Research
The accumulation of VFAs caused by impact loadings often leads to the acidification and failure of AD systems. Bioaugmentation is considered to be an effective method to alleviate the VFA inhibition of AD. The most recent research mainly reports the improvement of the mesophilic AD process through bioaugmentation with acetate and propionate-degrading consortiums (Table 3).

Table 3.
Improvements in AD performance through bioaugmentation with syntrophic enrichment cultures.
Cai et al. used the sludge previously acclimated with VFAs for the bioaugmentation of an acidified mesophilic anaerobic digestion system and increased the methane yield by 8.03–9.59 times. Acetoclastic methanogens from the acclimated sludge acted as the main contributors to pH neutralization and methane production during the early phase of bioaugmentation [63]. The bioaugmentation of mesophilic and thermophilic AD under ammonia inhibition with syntrophic acetate and propionate oxidizing consortia was investigated by Li et al. [61]. Their results showed that the bioaugmented reactors recovered earlier than control reactors at 20 and 8 days, respectively, in mesophilic and thermophilic operation mode. Different bioaugmentation mechanism occurred in mesophilic and thermophilic AD, which are derived from the synergetic effects of ammonia tolerance and microbial interactions [61]. Bioaugmentation with a mixture of microorganisms (Bacteria and Archaea) was applied to improve the anaerobic digestion of sewage sludge. The rate constant increased from 0.071 h−1 to 0.087 h−1 as doses of the bioaugmenting mixture were increased, as compared to values of 0.066 h−1 and 0.069 h−1 obtained with sewage sludge alone. Next-generation sequencing revealed that Cytophaga sp. predominated among bacteria in digesters and that the hydrogenotrophic methanogen Methanoculleus sp. was the most abundant genus among archaea [60]. It was also reported that bioaugmentation with propionate-degrading enrichment culture can improve the digestion performance of high C/N ratio feedstock without co-digestion with nitrogen-rich substrate [61]. Bioaugmentation with aero-tolerant propionate enrichment culture, dominated by Spirochaetaceae (30%), Thermovirga (12%), and Methanosaeta (65%), was successful in improving by 11 ± 3% of CH4 production in quasi steady state performing anaerobic digesters. CH4 production increase after bioaugmentation was correlated to increased relative abundance of Methanosaeta and Methanospirillum originating from the bioaugment culture [62]. Amani et al. [47] applied an adapted consortium for the intensification of methanogenesis under high VFAs load. Using the consortium shortened the retention time while the process stability was maintained. It was found that the ratio between active methanogens and syntrophs was one of the key aspects that determined the rate of methanogenesis and the stability of the process [47]. Gagliano et al. showed that active syntrophic interactions promoted increased biogas yield, since they provided for a more complete utilization of the waste substrates [64]. In this study, under thermophilic conditions, both cumulative methane production and methane production rates were significantly (more than 3.5 and 6.2 times, respectively) improved through bioaugmentation with syntrophic butyrate-degrading consortium, which is very competitive with other works (Table 3). Our study revealed the thermophilic butyrate-oxidizing consortia as a bioaugmented culture could be a potential strategy to alleviate the high organic load and VFA stress of AD.
5. Conclusions
This study showed that a gradual increase in butyrate concentration is a viable strategy for the adaptation of the methanogenic microbial community to butyrate concentrations up to 170 mM. The indirect inhibition of the anaerobic degradation of butyrate was clearly demonstrated at the highest concentration of butyrate tested. Based on the dynamics of the conversion of butyrate and the by-product of its syntrophic degradation, namely acetate into methane, it was suggested that the limiting rate of the syntrophic butyrate oxidation is the degradation of acetate due to its accumulation up to ~200 mM. As a response to acetate build-up, a change in the microbial community occurred regarding the enrichment of microbial groups that carry out syntrophic acetate oxidation (Syntrophaceticus and Syntrophomonas) coupled to hydrogenotrophic methanogenesis (Methanothermobacter thermautotrophicus and Methanomassiliicoccus). Thus, acetate inhibition can be overcome. Bioaugmentation with syntrophic butyrate-degrading consortium has been shown to be a viable strategy to alleviate VFA build-up due to organic overload. This finding is of particular practical importance in the case of thermophilic AD, which is more prone to destabilization than the mesophilic process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13010173/s1, Table S1: Phylogenetic affiliation and relative abundance of bacterial and archaeal 16S rRNA gene sequences retrieved from the clone libraries.
Author Contributions
Conceptualization, A.A.N. and A.Y.K.; methodology, T.V.K. and D.S.G.; software, V.P.; validation, D.A.K. and A.A.K.; formal analysis, A.Y.K.; investigation, A.A.N. and A.Y.K.; resources, A.N.N.; data curation, A.Y.K.; writing—original draft preparation, A.A.N.; writing—review and editing, A.Y.K., Y.V.L., and I.Z.; visualization, A.A.N.; supervision, A.N.N.; project administration, A.N.N.; funding acquisition, Y.V.L. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Ministry of Science and Higher Education of the Russian Federation. Chemical analyses were partially supported by grant N 075-15-2022-318 date 20 April 2022, provided for state support for the creation and development of a world-class scientific center “Agrotechnologies for the Future”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stams, A.J.M.; Sousa, D.Z.; Kleerebezem, R.; Plugge, C.M. Role of Syntrophic Microbial Communities in High-Rate Methanogenic Bioreactors. Water Sci. Technol. 2012, 66, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, A. Biogas Production: Microbiology and Technology. In Anaerobes in Biotechnology; Hatti-Kaul, R., Mamo, G., Mattiasson, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 195–234. [Google Scholar] [CrossRef]
- Mcinerney, M.; Sieber, J.; Gunsalus, R. Syntrophy in Anaerobic Global Carbon Cycles. Curr. Opin. Biotechnol. 2009, 20, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, A.; Jarvis, Ä. Microbiological Handbook for Biogas Plants. Swedish Waste Management U. 2009. Available online: https://www.researchgate.net/profile/Anoop-Srivastava/post/Suggestion-on-Research-gaps-in-microbiology-of-biogas-production-and-optimization-Ideas-suggestions-and-links-to-relevant-works-are-welcome/attachment/59d6522779197b80779aa77d/AS%3A510890374070272%401498817165514/download/Microbiological_handbook_for_biogas_plants.pdf (accessed on 1 August 2021).
- Fotidis, I.A.; Karakashev, D.; Angelidaki, I. Bioaugmentation with an Acetate-Oxidising Consortium as a Tool to Tackle Ammonia Inhibition of Anaerobic Digestion. Bioresour. Technol. 2013, 146, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, L.; Xing, W.; Chen, B.; Zhang, L.; Li, A.; Li, R.; Yang, T. Dynamic Behaviors of Batch Anaerobic Systems of Food Waste for Methane Production under Different Organic Loads, Substrate to Inoculum Ratios and Initial PH. J. Biosci. Bioeng. 2019, 128, 733–743. [Google Scholar] [CrossRef]
- Duan, N.; Dong, B.; Wu, B.; Dai, X. High-Solid Anaerobic Digestion of Sewage Sludge under Mesophilic Conditions: Feasibility Study. Bioresour. Technol. 2012, 104, 150–156. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Zhen, F.; Liu, H.; Xiao, F.; Sun, Y.; Peng, X.; Li, Y.; Wang, X. Thermodynamics of Volatile Fatty Acid Degradation during Anaerobic Digestion under Organic Overload Stress: The Potential to Better Identify Process Stability. Water Res. 2022, 214, 118187. [Google Scholar] [CrossRef]
- Nikitina, A.A.; Kevbrina, M.V.; Kallistova, A.Y.; Nekrasova, V.K.; Litti, Y.V.; Nozhevnikova, A.N. Intensification of Microbial Decomposition of Organic Fraction of Municipal Waste: Laboratory and Field Experiments. Appl. Biochem. Microbiol. 2015, 51, 393–401. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, L.; Li, C.; Angelidaki, I.; Lv, N.; Ning, J.; Cai, G.; Zhu, G. Deep Insights into the Network of Acetate Metabolism in Anaerobic Digestion: Focusing on Syntrophic Acetate Oxidation and Homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Li, J.; Yi, Y.; Nobu, M.K.; Narihiro, T.; Tang, Y.-Q. Response to Inhibitory Conditions of Acetate-Degrading Methanogenic Microbial Community. J. Biosci. Bioeng. 2020, 129, 476–485. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, J.; Guivernau, M.; Fernández, B.; Vila, J.; Viñas, M.; Riau, V.; Prenafeta-Boldú, F.X. Functional Biodiversity and Plasticity of Methanogenic Biomass from a Full-Scale Mesophilic Anaerobic Digester Treating Nitrogen-Rich Agricultural Wastes. Sci. Total Environ. 2019, 649, 760–769. [Google Scholar] [CrossRef]
- Westerholm, M.; Müller, B.; Singh, A.; Karlsson Lindsjö, O.; Schnürer, A. Detection of Novel Syntrophic Acetate-Oxidizing Bacteria from Biogas Processes by Continuous Acetate Enrichment Approaches. Microb. Biotechnol. 2018, 11, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Z.; Gou, M.; Yi, Y.; Xia, Z.-Y.; Tang, Y.-Q. Identification of Novel Potential Acetate-Oxidizing Bacteria in an Acetate-Fed Methanogenic Chemostat Based on DNA Stable Isotope Probing. J. Gen. Appl. Microbiol. 2018, 64, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Pap, B.; Györkei, Á.; Boboescu, I.Z.; Nagy, I.K.; Bíró, T.; Kondorosi, É.; Maróti, G. Temperature-Dependent Transformation of Biogas-Producing Microbial Communities Points to the Increased Importance of Hydrogenotrophic Methanogenesis under Thermophilic Operation. Bioresour. Technol. 2015, 177, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of Volatile Fatty Acid Concentrations on Methane Yield and Methanogenic Bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Lins, P.; Malin, C.; Wagner, A.O.; Illmer, P. Reduction of Accumulated Volatile Fatty Acids by an Acetate-Degrading Enrichment Culture. FEMS Microbiol. Ecol. 2010, 71, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Jannat, A.; Lee, J.; Shin, S.G.; Hwang, S. Long-Term Enrichment of Anaerobic Propionate-Oxidizing Consortia: Syntrophic Culture Development and Growth Optimization. J. Hazard. Mater. 2020, 401, 123230. [Google Scholar] [CrossRef]
- Cao, L.; Cox, C.D.; He, Q. Patterns of Syntrophic Interactions in Methanogenic Conversion of Propionate. Appl. Microbiol. Biotechnol. 2021, 105, 8937–8949. [Google Scholar] [CrossRef]
- Ahmad, A.; Ghufran, R.; Wahid, Z. Effect of Cod Loading Rate on an Upflow Anaerobic Sludge Blanket Reactor during Anaerobic Digestion of Palm Oil Mill Effluent with POME. J. Environ. Eng. Landsc. Manag. 2012, 20, 256–264. [Google Scholar] [CrossRef]
- Meng, X.; Cao, Q.; Sun, Y.; Huang, S.; Liu, X.; Li, D. 16S RRNA Genes- and Metagenome-Based Confirmation of Syntrophic Butyrate-Oxidizing Methanogenesis Enriched in High Butyrate Loading. Bioresour. Technol. 2022, 345, 126483. [Google Scholar] [CrossRef]
- Ryue, J.; Lin, L.; Kakar, F.L.; Elbeshbishy, E.; Al-Mamun, A.; Dhar, B.R. A Critical Review of Conventional and Emerging Methods for Improving Process Stability in Thermophilic Anaerobic Digestion. Energy Sustain. Dev. 2020, 54, 72–84. [Google Scholar] [CrossRef]
- Nozhevnikova, A.N.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.N.; Zhuravleva, E.A.; Nikitina, A.A. Syntrophy and Interspecies Electron Transfer in Methanogenic Microbial Communities. Microbiology 2020, 89, 129–147. [Google Scholar] [CrossRef]
- Litti, Y.; Nikitina, A.; Kovalev, D.; Ermoshin, A.; Mahajan, R.; Goel, G.; Nozhevnikova, A. Influence of Cationic Polyacrilamide Flocculant on High-Solids Anaerobic Digestion of Sewage Sludge under Thermophilic Conditions. Environ. Technol. 2019, 40, 1146–1155. [Google Scholar] [CrossRef]
- Pfennig, N. Anreicherungskulturen Für Röte und Grune Schwefelbakteria. Zentralbl. Bacterial. Farasiten. Infektionskr. Hyg. Abt. 1965, 1, 503–504. [Google Scholar]
- Pfennig, N.; Lippert, K.D. Über Das Vitamin B12-Bedürfnis Phototropher Schwefelbakterien. Arch. Mikrobiol. 1966, 55, 245–256. [Google Scholar] [CrossRef]
- Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Formation of Methane by Bacterial Extracts. J. Biol. Chem. 1963, 238, 2882–2886. [Google Scholar] [CrossRef]
- Kotsyurbenko, O.; Glagolev, M. Protocols for Measuring Methanogenesis. In Hydrocarbon and Lipid Microbiology Protocols; Springer: Berlin/Heidelberg, Germany, 2015; pp. 227–244. [Google Scholar] [CrossRef]
- Ali Shah, F.; Mahmood, Q.; Maroof Shah, M.; Pervez, A.; Ahmad Asad, S. Microbial Ecology of Anaerobic Digesters: The Key Players of Anaerobiosis. Sci. World J. 2014, 2014, 183752. [Google Scholar] [CrossRef]
- Kovalev, A.A.; Kovalev, D.A.; Zhuravleva, E.A.; Katraeva, I.V.; Panchenko, V.; Fiore, U.; Litti, Y.V. Two-Stage Anaerobic Digestion with Direct Electric Stimulation of Methanogenesis: The Effect of a Physical Barrier to Retain Biomass on the Surface of a Carbon Cloth-Based Biocathode. Renew. Energy 2022, 181, 966–977. [Google Scholar] [CrossRef]
- Boulygina, E.S.; Kuznetsov, B.B.; Marusina, A.I.; Tourova, T.P.; Kravchenko, I.K.; Bykova, S.A.; Kolganova, T.V.; Galchenko, V.F. A Study of Nucleotide Sequences of NifH Genes of Some Methanotrophic Bacteria. Microbiology 2002, 71, 425–432. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Kolganova, T.; Kuznetsov, B.; Tourova, T. Designing and Testing Oligonucleotide Primers for Amplification and Sequencing of Archaeal 16S RRNA Genes. Mikrobiologiia 2002, 71, 283–286. [Google Scholar] [CrossRef]
- Huber, T.; Faulkner, G.; Philip, H. Bellerophon: A Program to Detect Chimeric Sequences in Multiple Sequence Alignments. Bioinformatics 2004, 20, 2317–2319. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Nikitina, A.A.; Ermoshin, A.A.; Zhuravleva, E.A.; Kovalev, A.A.; Kovalev, D.A.; Panchenko, V.; Litti, Y.V. Application of polyacrylamide flocculant for stabilization of anaerobic digestion under conditions of excessive accumulation of volatile fatty acids. Appl. Sci. 2020, 11, 100. [Google Scholar] [CrossRef]
- Xu, K.; Liu, H.; Li, X.; Chen, J.; Wang, A. Typical Methanogenic Inhibitors Can Considerably Alter Bacterial Populations and Affect the Interaction between Fatty Acid Degraders and Homoacetogens. Appl. Microbiol. Biotechnol. 2010, 87, 2267–2279. [Google Scholar] [CrossRef]
- Li, L.; He, Q.; Ma, Y.; Wang, X.; Peng, X. A Mesophilic Anaerobic Digester for Treating Food Waste: Process Stability and Microbial Community Analysis Using Pyrosequencing. Microbial Cell Factories 2016, 15, 65. [Google Scholar] [CrossRef]
- Win, T.T.; Kim, H.; Cho, K.; Song, K.G.; Park, J. Monitoring the Microbial Community Shift throughout the Shock Changes of Hydraulic Retention Time in an Anaerobic Moving Bed Membrane Bioreactor. Bioresour. Technol. 2016, 202, 125–132. [Google Scholar] [CrossRef]
- Zheng, D.; Raskin, L. Quantification of Methanosaeta Species in Anaerobic Bioreactors Using Genus- and Species-Specific Hybridization Probes. Microb. Ecol. 2000, 39, 246–262. [Google Scholar] [CrossRef]
- Ma, K.; Liu, X.; Dong, X. Methanosaeta Harundinacea Sp. Nov., a Novel Acetate-Scavenging Methanogen Isolated from a UASB Reactor. Int. J. Syst. Evol. Microbiol. 2006, 56, 127–131. [Google Scholar] [CrossRef]
- Vavilin, V.; Qu, X.; Mazeas, L.; Lemunier, M.; Duquennoi, C.; He, P.; Bouchez, T. Methanosarcina as the Dominant Aceticlastic Methanogens during Mesophilic Anaerobic Digestion of Putrescible Waste. Antonie Leeuwenhoek 2008, 94, 593–605. [Google Scholar] [CrossRef]
- Ho, D.P.; Jensen, P.D.; Batstone, D.J. Methanosarcinaceae and Acetate-Oxidizing Pathways Dominate in High-Rate Thermophilic Anaerobic Digestion of Waste-Activated Sludge. Appl. Environ. Microbiol. 2013, 79, 6491–6500. [Google Scholar] [CrossRef]
- Wagner, D.; Schirmack, J.; Ganzert, L.; Morozova, D.; Mangelsdorf, K. Methanosarcina Soligelidi Sp Nov., a Desiccation- and Freeze-Thaw-Resistant Methanogenic Archaeon from a Siberian Permafrost-Affected Soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 2986–2991. [Google Scholar] [CrossRef]
- Nozhevnikova, A.N.; Nekrasova, V.; Ammann, A.; Zehnder, A.J.B.; Wehrli, B.; Holliger, C. Influence of Temperature and High Acetate Concentrations on Methanogenensis in Lake Sediment Slurries. FEMS Microbiol. Ecol. 2007, 62, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Dogan, T.; Ince, O.; Oz, N.A.; Ince, B.K. Inhibition of Volatile Fatty Acid Production in Granular Sludge from a UASB Reactor. J. Environ. Sci. Health Part A 2005, 40, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Amani, T.; Nosrati, M.; Mousavi, S.M. Using Enriched Cultures for Elevation of Anaerobic Syntrophic Interactions between Acetogens and Methanogens in a High-Load Continuous Digester. Bioresour. Technol. 2011, 102, 3716–3723. [Google Scholar] [CrossRef] [PubMed]
- Illmer, P.; Gstraunthaler, G. Effect of Seasonal Changes in Quantities of Biowaste on Full Scale Anaerobic Digester Performance. Waste Manag. 2008, 29, 162–167. [Google Scholar] [CrossRef]
- Mcinerney, M.; Bryant, M.P.; Hespell, R.B.; Costerton, J.W. Syntrophomonas Wolfei Gen. Nov. Sp. Nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl. Environ. Microbiol. 1981, 41, 1029–1039. [Google Scholar] [CrossRef]
- Westerholm, M.; Roos, S.; Schnürer, A. Syntrophaceticus Schinkii Gen. Nov., Sp. Nov., an Anaerobic, Syntrophic Acetate-Oxidizing Bacterium Isolated from a Mesophilic Anaerobic Filter. FEMS Microbiol. Lett. 2010, 309, 100–104. [Google Scholar] [CrossRef]
- Maune, M.; Tanner, R. Description of Anaerobaculum Hydrogeniformans Sp. Nov., an Anaerobe That Produces Hydrogen from Glucose, and Emended Description of the Genus Anaerobaculum. Int. J. Syst. Evol. Microbiol. 2011, 62, 832–838. [Google Scholar] [CrossRef]
- Jang, H.M.; Kim, J.H.; Ha, J.H.; Park, J.M. Bacterial and Methanogenic Archaeal Communities during the Single-Stage Anaerobic Digestion of High-Strength Food Wastewater. Bioresour. Technol. 2014, 165, 174–182. [Google Scholar] [CrossRef]
- Welte, C.; Deppenmeier, U. Bioenergetics and Anaerobic Respiratory Chains of Aceticlastic Methanogens. Biochim. Biophys. Acta 2013, 1837, 1130–1147. [Google Scholar] [CrossRef]
- Dridi, B.; Fardeau, M.-L.; Ollivier, B.; Raoult, D.; Drancourt, M. Methanomassiliicoccus Luminyensis Gen. Nov., Sp. Nov., a Methanogenic Archaeon Isolated from Human Faeces. Int. J. Syst. Evol. Microbiol. 2012, 62, 1902–1907. [Google Scholar] [CrossRef]
- Jetten, M.S.M.; Stams, A.J.M.; Zehnder, A.J.B. Methanogenesis from acetate: A comparison of the acetate metabolism in Methanothrix soehngenii and Methanosarcina spp. FEMS Microbiol. Rev. 1992, 88, 181–198. [Google Scholar] [CrossRef]
- Hattori, S. Syntrophic Acetate-Oxidizing Microbes in Methanogenic Environments. Microbes Environ./JSME 2008, 23, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.-P.; Lü, F.; He, P.-J.; Li, L.; Shao, L.-M. Predominant Contribution of Syntrophic Acetate Oxidation to Thermophilic Methane Formation at High Acetate Concentrations. Environ. Sci. Technol. 2011, 45, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Haruta, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Methanogenic Pathway and Community Structure in a Thermophilic Anaerobic Digestion Process of Organic Solid Waste. J. Biosci. Bioeng. 2010, 111, 41–46. [Google Scholar] [CrossRef]
- Li, M.-T.; Rao, L.; Wang, L.; Gou, M.; Sun, Z.-Y.; Xia, Z.-Y.; Song, W.-F.; Tang, Y.-Q. Bioaugmentation with Syntrophic Volatile Fatty Acids-Oxidizing Consortia to Alleviate the Ammonia Inhibition in Continuously Anaerobic Digestion of Municipal Sludge. Chemosphere 2022, 288, 132389. [Google Scholar] [CrossRef]
- Lebiocka, M.; Montusiewicz, A.; Cydzik-Kwiatkowska, A. Effect of Bioaugmentation on Biogas Yields and Kinetics in Anaerobic Digestion of Sewage Sludge. Int. J. Environ. Res. Public Health 2018, 15, 1717. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Sun, Y.; Yuan, Z. Bioaugmentation Strategy for Enhancing Anaerobic Digestion of High C/N Ratio Feedstock with Methanogenic Enrichment Culture. Bioresour. Technol. 2018, 261, 188–195. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; Milferstedt, K.; Hamelin, J.; Zitomer, D.H. Anaerobic Digester Bioaugmentation Influences Quasi Steady State Performance and Microbial Community. Water Res. 2016, 104, 128–136. [Google Scholar] [CrossRef]
- Cai, G.; Zhao, L.; Wang, T.; Lv, N.; Li, J.; Ning, J.; Pan, X.; Zhu, G. Variation of Volatile Fatty Acid Oxidation and Methane Production during the Bioaugmentation of Anaerobic Digestion System: Microbial Community Analysis Revealing the Influence of Microbial Interactions on Metabolic Pathways. Sci. Total Environ. 2021, 754, 142425. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, M.C.; Ismail, S.B.; Stams, A.J.M.; Plugge, C.M.; Temmink, H.; Van Lier, J.B. Biofilm Formation and Granule Properties in Anaerobic Digestion at High Salinity. Water Res. 2017, 121, 61–71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).