A Review of Apta-POF-Sensors: The Successful Coupling between Aptamers and Plastic Optical Fibers for Biosensing Applications
Abstract
:1. Introduction
2. Aptamers as Biorecognition Element
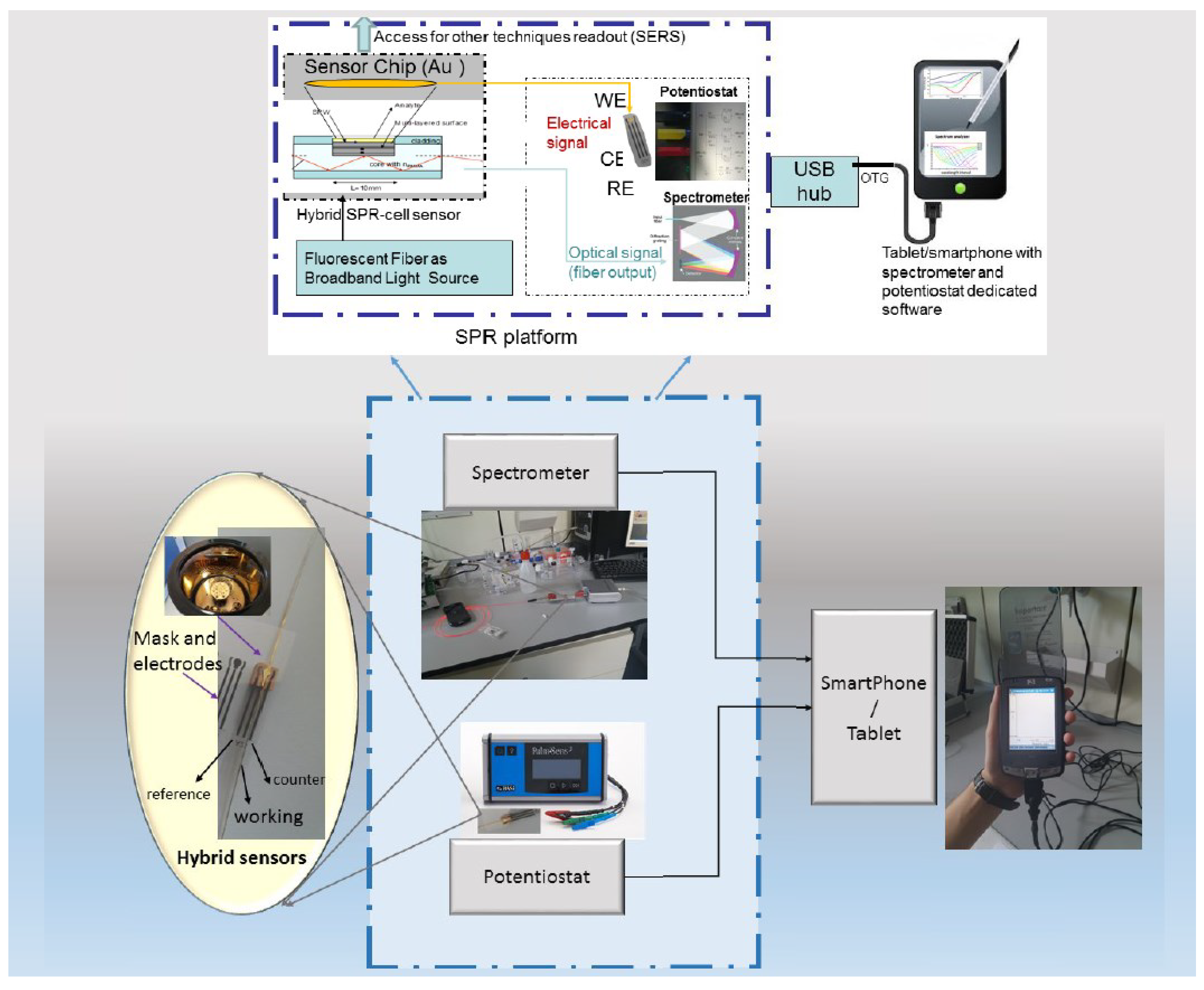
3. Plasmonic Optical Fiber Aptasensors
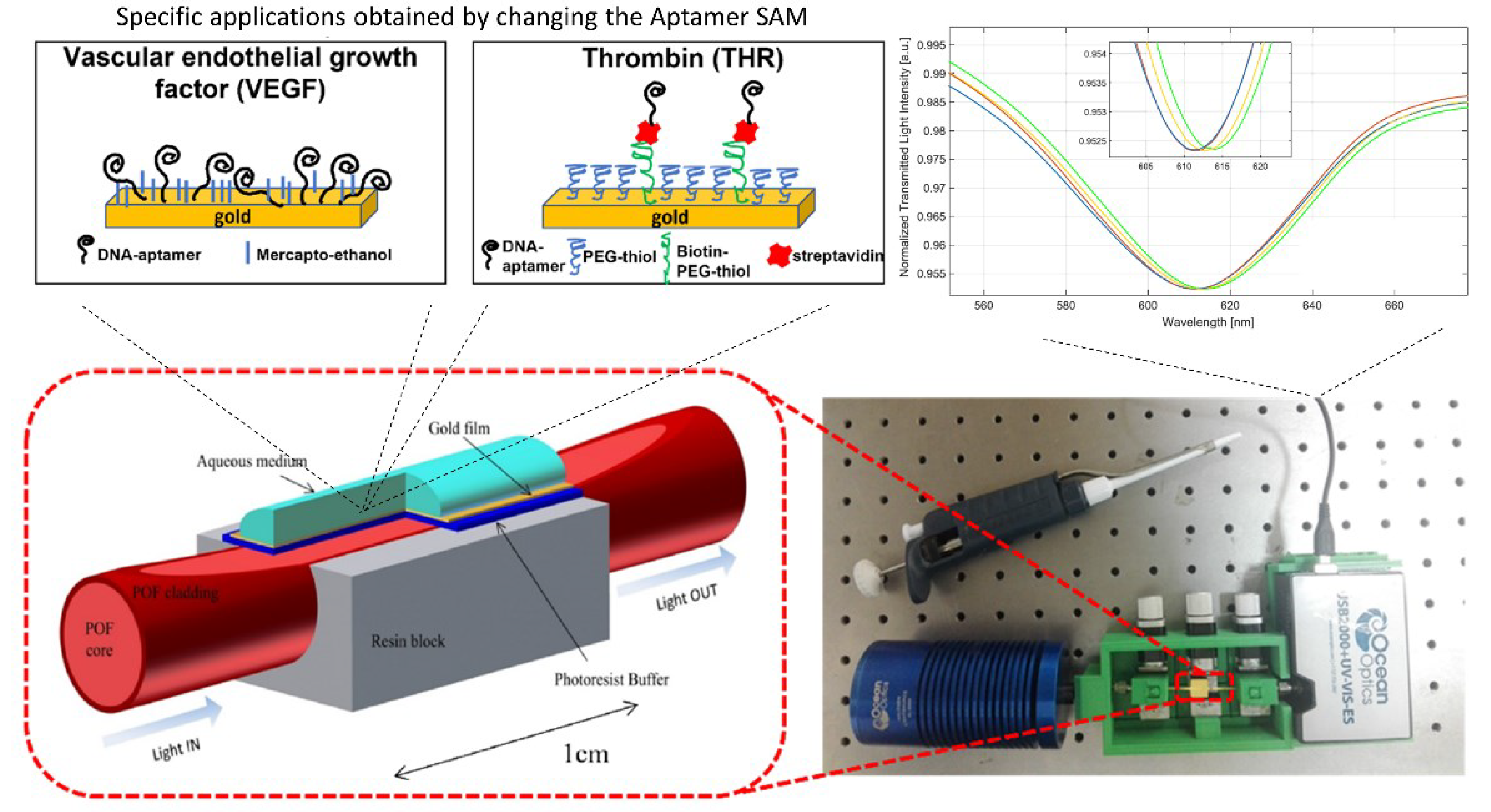
4. A Focus on Relevant Applications of Apta-POF-SPR Sensors
4.1. Antibiotics Detection
4.2. Disease Biomarkers Detection
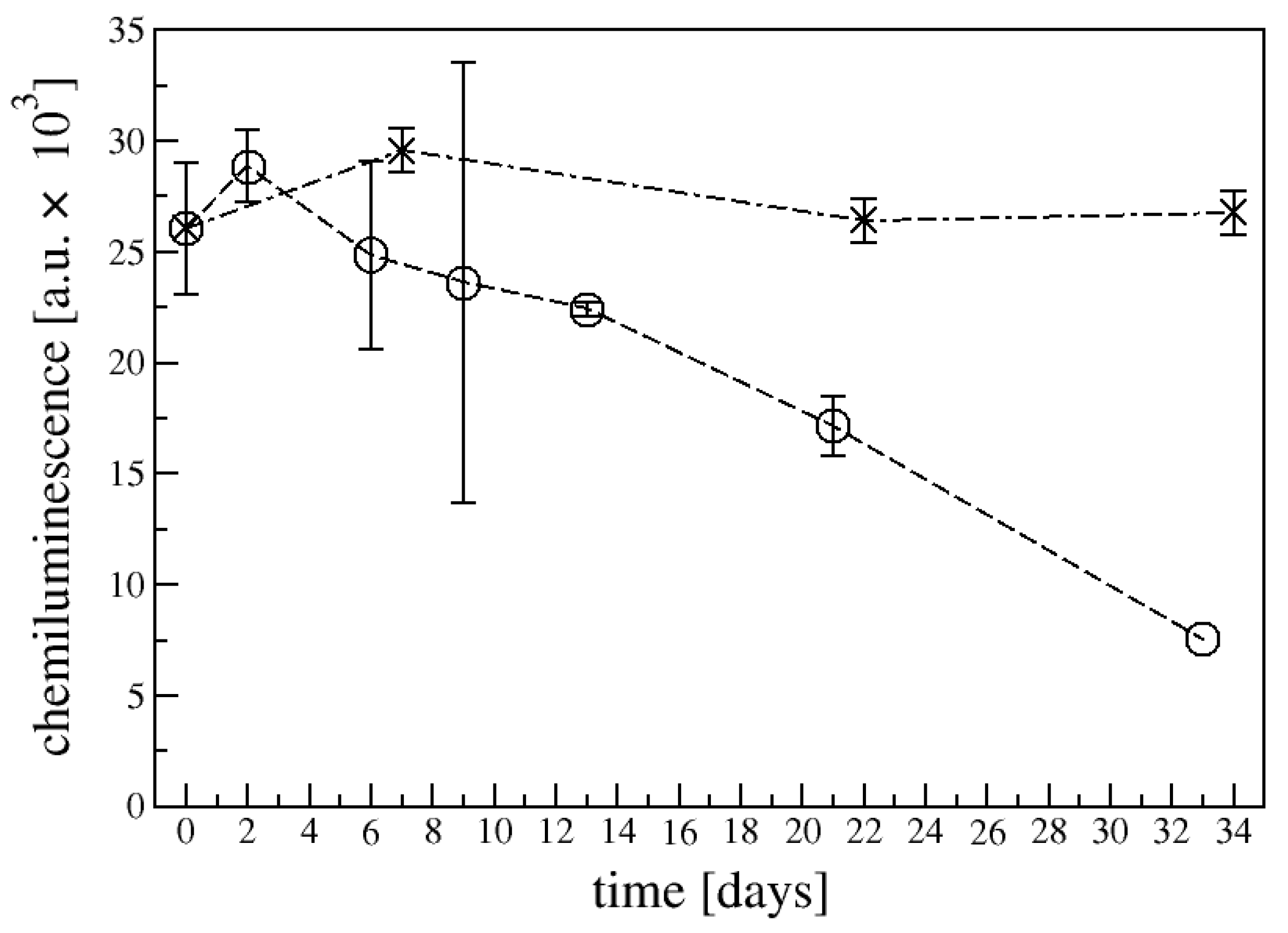
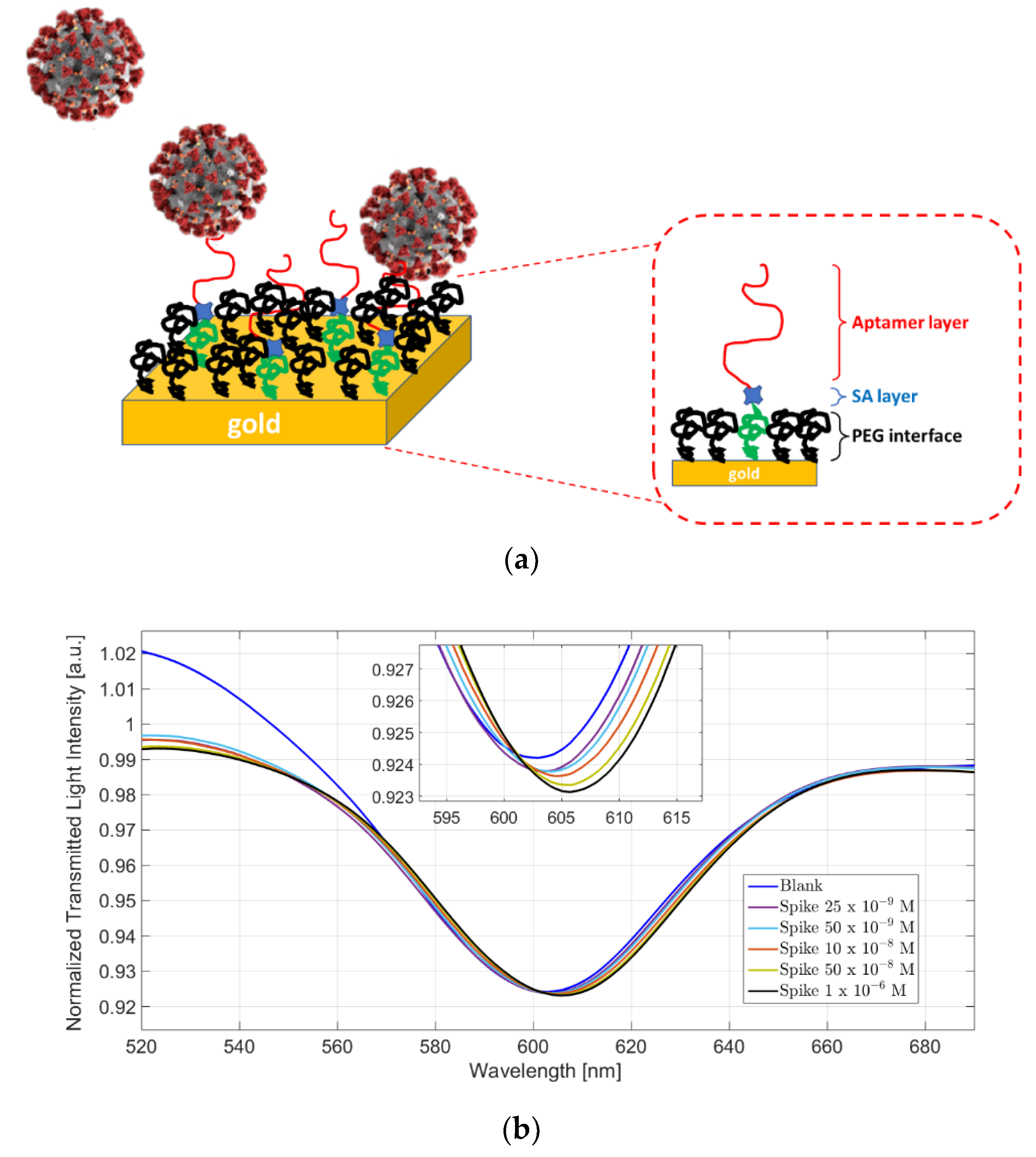
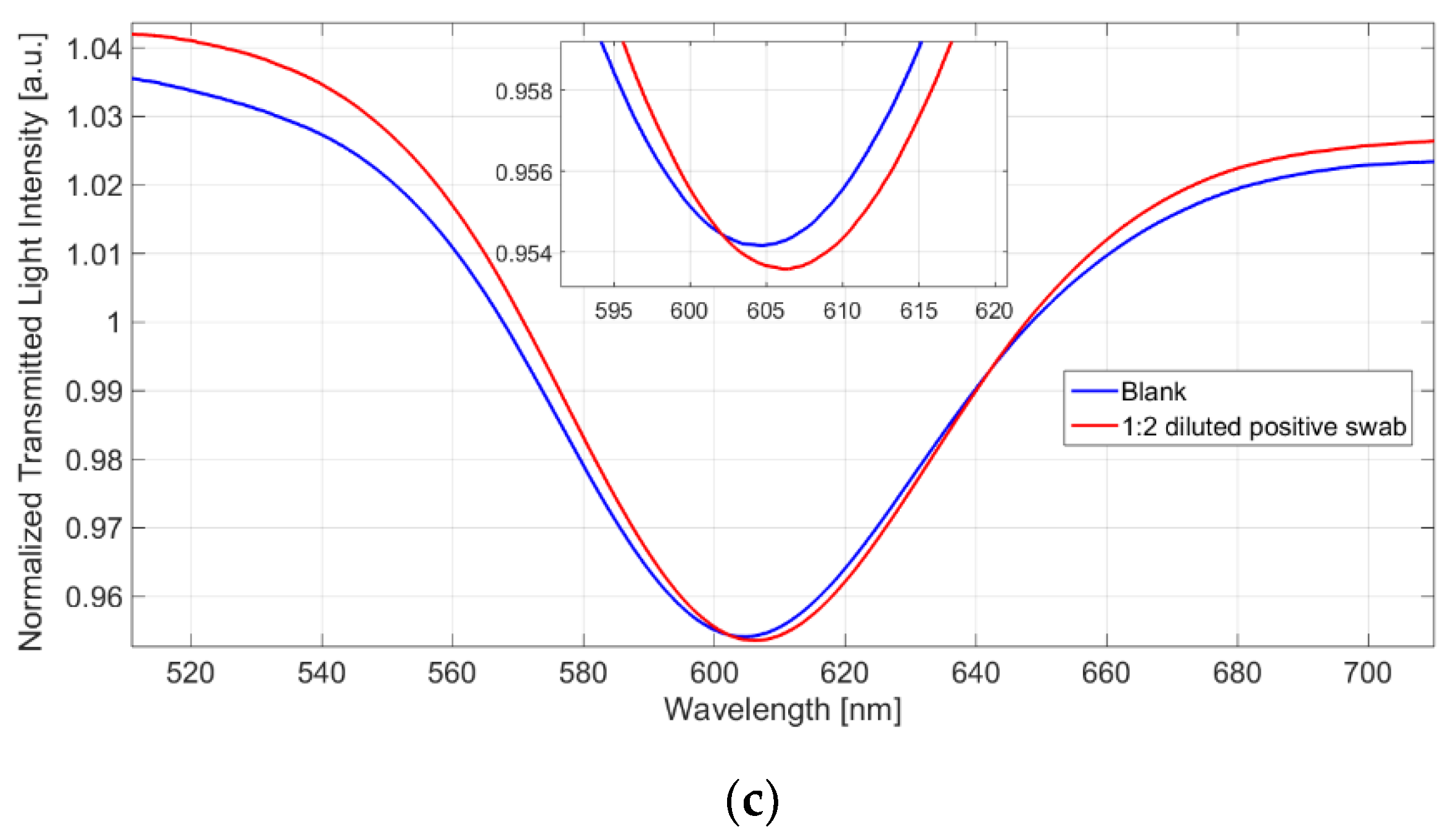
4.3. Virus Detection
5. Pros and Cons of Apta-POF SPR Sensors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Y.; Granville, A.M. Polymer Fiber Optic Sensors—A Mini Review of their Synthesis and Applications. J. Biosens. Bioelectron. 2016, 7, 194. [Google Scholar] [CrossRef]
- Cennamo, N.; Pesavento, M.; Zeni, L. A review on simple and highly sensitive plastic optical fiber probes for bio-chemical sensing. Sens. Actuators B Chem. 2021, 331, 129393. [Google Scholar] [CrossRef]
- Kadhim, R.A.; Abdul, A.K.K.; Yuan, L. Advances in Surface Plasmon Resonance-Based Plastic Optical Fiber Sensors. IETE Tech. Rev. 2020. In preprint. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Pasquardini, L.; Arcadio, F.; Perri, C.; Coppola, N.; Angelillo, I.F.; Altucci, L.; Di Marzo, F.; Parisio, E.M.; et al. (INVITED)Quantitative detection of SARS-CoV-2 virions in aqueous mediums by IoT optical fiber sensors. Results Opt. 2021, 5, 100177. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ștefan, G.; Hosu, O.; De Wael, K.; Lobo-Castañón, M.J.; Cristea, C. Aptamers in biomedicine: Selection strategies and recent advances. Electrochim. Acta 2021, 376, 137994. [Google Scholar] [CrossRef]
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef]
- Navien, T.N.; Thevendran, R.; Hamdani, H.Y.; Tang, T.-H.; Citartan, M. In silico molecular docking in DNA aptamer development. Biochimie 2021, 180, 54–67. [Google Scholar] [CrossRef]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Al Bawab, A.; Ismail, S.I. Aptamers Chemistry: Chemical Modifications and Conjugation Strategies. Molecules 2019, 25, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshavsky-Graham, S.; Urmann, K.; Salama, R.; Massad-Ivanir, N.; Walter, J.-G.; Scheper, T.; Segal, E. Aptamers vs. antibodies as capture probes in optical porous silicon biosensors. Analyst 2020, 145, 4991–5003. [Google Scholar] [CrossRef] [PubMed]
- Hianik, T. Affinity Biosensors for Detection Immunoglobulin E and Cellular Prions. Antibodies vs. DNA Aptamers. Electroanalysis 2016, 28, 1764–1776. [Google Scholar] [CrossRef]
- Liu, L.S.; Wang, F.; Ge, Y.; Lo, P.K. Recent Developments in Aptasensors for Diagnostic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9329–9358. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Rangel, P.X.M.; Bui, B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.; Halpern, J.M. Guide to Selecting a Biorecognition Element for Biosensors. Bioconjug. Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Chiavaioli, F.; Gouveia, C.A.J.; Jorge, P.A.S.; Baldini, F. Towards a Uniform Metrological Assessment of Grating-Based Optical Fiber Sensors: From Refractometers to Biosensors. Biosensors 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.-R.; Foroughi, M.M.; Safaei, M.; Jahani, S.; Ebrahimpour, N.; Borhani, F.; Baravati, N.R.Z.; Aramesh-Boroujeni, Z.; Foong, L.K. A review: Recent advances in ultrasensitive and highly specific recognition aptasensors with various detection strategies. Int. J. Biol. Macromol. 2020, 155, 184–207. [Google Scholar] [CrossRef]
- Feng, C.; Dai, S.; Wang, L. Optical aptasensors for quantitative detection of small biomolecules: A review. Biosens. Bioelectron. 2014, 59, 64–74. [Google Scholar] [CrossRef]
- Gaudin, V. The Growing Interest in Development of Innovative Optical Aptasensors for the Detection of Antimicrobial Residues in Food Products. Biosensors 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majdinasab, M.; Daneshi, M.; Marty, J.L. Recent developments in non-enzymatic (bio)sensors for detection of pesticide residues: Focusing on antibody, aptamer and molecularly imprinted polymer. Talanta 2021, 232, 122397. [Google Scholar] [CrossRef] [PubMed]
- Khedri, M.; Ramezani, M.; Rafatpanah, H.; Abnous, K. Detection of food-born allergens with aptamer-based biosensors. TrAC Trends Anal. Chem. 2018, 103, 126–136. [Google Scholar] [CrossRef]
- Cunha, I.; Biltes, R.; Sales, M.; Vasconcelos, V. Aptamer-Based Biosensors to Detect Aquatic Phycotoxins and Cyanotoxins. Sensors 2018, 18, 2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Tang, D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Hassan, E.M.; DeRosa, M.C. Recent advances in cancer early detection and diagnosis: Role of nucleic acid based aptasensors. TrAC Trends Anal. Chem. 2020, 124, 115806. [Google Scholar] [CrossRef]
- Citartan, M.; Tang, T.-H. Recent developments of aptasensors expedient for point-of-care (POC) diagnostics. Talanta 2019, 199, 556–566. [Google Scholar] [CrossRef]
- Zahra, Q.U.A.; Khan, Q.A.; Luo, Z. Advances in Optical Aptasensors for Early Detection and Diagnosis of Various Cancer Types. Front. Oncol. 2021, 11, 632165. [Google Scholar] [CrossRef]
- Majdinasab, M.; Hayat, A.; Marty, J.L. Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trends Anal. Chem. 2018, 107, 60–77. [Google Scholar] [CrossRef]
- Sharifi, S.; Vahed, S.Z.; Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Khalilov, R.; Ahmadi, M.; Hamidi-Asl, E.; Labib, M. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens. Bioelectron. 2020, 150, 111933. [Google Scholar] [CrossRef]
- Safarpour, H.; Dehghani, S.; Nosrati, R.; Zebardast, N.; Alibolandi, M.; Mokhtarzadeh, A.; Ramezani, M. Optical and electrochemical-based nano-aptasensing approaches for the detection of circulating tumor cells (CTCs). Biosens. Bioelectron. 2020, 148, 111833. [Google Scholar] [CrossRef] [PubMed]
- Drolet, D.W.; Moon-McDermott, L.; Romig, T.S. An enzyme-linked oligonucleotide assay. Nat. Biotechnol. 1996, 14, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.A.; Abrams, B.; Lin, Y.; Jayasena, S.D. Use of a High Affinity DNA Ligand in Flow Cytometry. Nucleic Acids Res. 1996, 24, 702–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aptamer Science Inc. Available online: https://www.aptsci.com/en/technology/aptamer.php (accessed on 15 April 2022).
- Neoventures Biotechnologies. Available online: https://neoaptamers.com/ (accessed on 15 April 2022).
- Somalogic. Available online: https://somalogic.com/technology/ (accessed on 15 April 2022).
- Bashir, A.; Yang, Q.; Wang, J.; Hoyer, S.; Chou, W.; McLean, C.; Davis, G.; Gong, Q.; Armstrong, Z.; Jang, J.; et al. Machine learning guided aptamer refinement and discovery. Nat. Commun. 2021, 12, 2366. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Maniglio, D.; Tatti, R.; Zeni, L.; Bossi, A.M. Deformable molecularly imprinted nanogels permit sensitivity-gain in plasmonic sensing. Biosens. Bioelectron. 2020, 156, 112126. [Google Scholar] [CrossRef]
- Bilro, L.; Alberto, N.; Pinto, J.L.; Nogueira, R. Optical Sensors Based on Plastic Fibers. Sensors 2012, 12, 12184–12207. [Google Scholar] [CrossRef] [Green Version]
- Peters, K. Polymer optical fiber sensors—A review. Smart Mater. Struct. 2010, 20, 13002. [Google Scholar] [CrossRef]
- Loyez, M.; DeRosa, M.C.; Caucheteur, C.; Wattiez, R. Overview and emerging trends in optical fiber aptasensing. Biosens. Bioelectron. 2022, 196, 113694. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Donà, A.; Dacarro, G.; Pallavicini, P.; Pesavento, M.; Zeni, L. Localized Surface Plasmon Resonance with Five-Branched Gold Nanostars in a Plastic Optical Fiber for Bio-Chemical Sensor Implementation. Sensors 2013, 13, 14676–14686. [Google Scholar] [CrossRef]
- Gowri, A.; Sai, V.V.R. Development of LSPR based U-bent plastic optical fiber sensors. Sens. Actuators B Chem. 2016, 230, 536–543. [Google Scholar] [CrossRef]
- Pollet, J.; Delport, F.; Janssen, K.P.; Jans, K.; Maes, G.; Pfeiffer, H.; Wevers, M.; Lammertyn, J. Fiber optic SPR biosensing of DNA hybridization and DNA–protein interactions. Biosens. Bioelectron. 2009, 25, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Bekmurzayeva, A.; Dukenbayev, K.; Shaimerdenova, M.; Bekniyazov, I.; Ayupova, T.; Sypabekova, M.; Molardi, C.; Tosi, D. Etched Fiber Bragg Grating Biosensor Functionalized with Aptamers for Detection of Thrombin. Sensors 2018, 18, 4298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, J.; Lepinay, S.; Caucheteur, C.; DeRosa, M.C. High resolution grating-assisted surface plasmon resonance fiber optic aptasensor. Methods 2013, 63, 239–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Huang, Y.; Chen, C.; Liu, Y.; Guo, T.; Guan, B.-O. Highly sensitive detection of dopamine using a graphene functionalized plasmonic fiber-optic sensor with aptamer conformational amplification. Sens. Actuators B Chem. 2018, 264, 440–447. [Google Scholar] [CrossRef]
- Cennamo, N.; Pesavento, M.; Lunelli, L.; Vanzetti, L.; Pederzolli, C.; Zeni, L.; Pasquardini, L. An easy way to realize SPR aptasensor: A multimode plastic optical fiber platform for cancer biomarkers detection. Talanta 2015, 140, 88–95. [Google Scholar] [CrossRef]
- Cennamo, N.; Pasquardini, L.; Arcadio, F.; Vanzetti, L.E.; Bossi, A.M.; Zeni, L. D-shaped plastic optical fibre aptasensor for fast thrombin detection in nanomolar range. Sci. Rep. 2019, 9, 18740. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, S.; Xu, J.; Li, Z.; Li, C.; Jing, Y.; Zhao, X.; Pan, J.; Zhang, C.; Man, B.Y. Sensitive and selective surface plasmon resonance sensor employing a gold-supported graphene composite film/D-shaped fiber for dopamine detection. J. Phys. D Appl. Phys. 2019, 52, 195402. [Google Scholar] [CrossRef]
- Lao, J.; Han, L.; Wu, Z.; Zhang, X.; Huang, Y.; Tang, Y.; Guo, T. Gold Nanoparticle-Functionalized Surface Plasmon Resonance Optical Fiber Biosensor: In Situ Detection of Thrombin With 1 n·M Detection Limit. J. Light. Technol. 2019, 37, 2748–2755. [Google Scholar] [CrossRef]
- Sypabekova, M.; Korganbayev, S.; González-Vila, Á.; Caucheteur, C.; Shaimerdenova, M.; Ayupova, T.; Bekmurzayeva, A.; Vangelista, L.; Tosi, D. Functionalized etched tilted fiber Bragg grating aptasensor for label-free protein detection. Biosens. Bioelectron. 2019, 146, 111765. [Google Scholar] [CrossRef]
- Sun, D.; Sun, L.-P.; Guo, T.; Guan, B.-O. Label-Free Thrombin Detection Using a Tapered Fiber-Optic Interferometric Aptasensor. J. Light. Technol. 2018, 37, 2756–2761. [Google Scholar] [CrossRef]
- Dillen, A.; Mohrbacher, A.; Lammertyn, J. A Versatile One-Step Competitive Fiber Optic Surface Plasmon Resonance Bioassay Enabled by DNA Nanotechnology. ACS Sens. 2021, 6, 3677–3684. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, M.; Singh, N.K.; Ngashangva, L.; Goswami, P. A smartphone-based fiber-optic aptasensor for label-free detection of Plasmodium falciparum glutamate dehydrogenase. Anal. Methods 2020, 12, 1333–1341. [Google Scholar] [CrossRef]
- Shevchenko, Y.; Francis, T.J.; Blair, D.A.D.; Walsh, R.; DeRosa, M.C.; Albert, J. In Situ Biosensing with a Surface Plasmon Resonance Fiber Grating Aptasensor. Anal. Chem. 2011, 83, 7027–7034. [Google Scholar] [CrossRef] [PubMed]
- Loyez, M.; Hassan, E.M.; Lobry, M.; Liu, F.; Caucheteur, C.; Wattiez, R.; DeRosa, M.C.; Willmore, W.G.; Albert, J. Rapid Detection of Circulating Breast Cancer Cells Using a Multiresonant Optical Fiber Aptasensor with Plasmonic Amplification. ACS Sens. 2020, 5, 454–463. [Google Scholar] [CrossRef]
- Daems, D.; Pfeifer, W.; Rutten, I.; Saccà, B.; Spasic, D.; Lammertyn, J. Three-Dimensional DNA Origami as Programmable Anchoring Points for Bioreceptors in Fiber Optic Surface Plasmon Resonance Biosensing. ACS Appl. Mater. Interfaces 2018, 10, 23539–23547. [Google Scholar] [CrossRef]
- Allsop, T.; Mou, C.; Neal, R.; Mariani, S.; Nagel, D.; Tombelli, S.; Poole, A.; Kalli, K.; Hine, A.; Webb, D.; et al. Real-time kinetic binding studies at attomolar concentrations in solution phase using a single-stage opto-biosensing platform based upon infrared surface plasmons. Opt. Express 2017, 25, 39. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Huang, Y.; Duan, X.; Wei, X.; Fan, Y.; Gan, D.; Yue, S.; Cheng, W.; Chen, T. Fiber optic surface plasmon resonance biosensor for detection of PDGF-BB in serum based on self-assembled aptamer and antifouling peptide monolayer. Biosens. Bioelectron. 2019, 140, 111350. [Google Scholar] [CrossRef]
- Coelho, L.; De Almeida, J.M.M.M.; Santos, J.L.; da Silva Jorge, P.A.; Martins, M.C.L.; Viegas, D.; Queirós, R.B. Aptamer-based fiber sensor for thrombin detection. J. Biomed. Opt. 2016, 21, 087005. [Google Scholar] [CrossRef]
- Arghir, I.; Spasic, D.; Verlinden, B.E.; Delport, F.; Lammertyn, J. Improved surface plasmon resonance biosensing using silanized optical fibers. Sens. Actuators B Chem. 2015, 216, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Zubiate, P.; Zamarreño, C.; Sánchez, P.; Matias, I.; Arregui, F. High sensitive and selective C-reactive protein detection by means of lossy mode resonance based optical fiber devices. Biosens. Bioelectron. 2017, 93, 176–181. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.-H.; Byun, J.-Y.; Kim, J.H.; Kim, M.-G. An optical fiber-based LSPR aptasensor for simple and rapid in-situ detection of ochratoxin A. Biosens. Bioelectron. 2018, 102, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Allsop, T.D.; Neal, R.; Wang, C.; Nagel, D.A.; Hine, A.V.; Culverhouse, P.; Castañón, J.D.A.; Webb, D.J.; Scarano, S.; Minunni, M. An ultra-sensitive aptasensor on optical fibre for the direct detection of bisphenol A. Biosens. Bioelectron. 2019, 135, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiong, M.; Yan, H. A portable optical fiber biosensor for the detection of zearalenone based on the localized surface plasmon resonance. Sens. Actuators B Chem. 2021, 336, 129752. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, J.; Wang, Y.; Chen, J.; Li, Y.; Duan, Y. An aptamer based method for small molecules detection through monitoring salt-induced AuNPs aggregation and surface plasmon resonance (SPR) detection. Sens. Actuators B Chem. 2016, 236, 474–479. [Google Scholar] [CrossRef]
- Tran, D.T.; Knez, K.; Janssen, K.P.F.; Pollet, J.; Spasic, D.; Lammertyn, J. Selection of aptamers against Ara h 1 protein for FO-SPR biosensing of peanut allergens in food matrices. Biosens. Bioelectron. 2013, 43, 245–251. [Google Scholar] [CrossRef]
- Antohe, I.; Schouteden, K.; Goos, P.; Delport, F.; Spasic, D.; Lammertyn, J. Thermal annealing of gold coated fiber optic surfaces for improved plasmonic biosensing. Sens. Actuators B Chem. 2016, 229, 678–685. [Google Scholar] [CrossRef]
- Galatus, R.M.; Cristea, C.; Feier, B.; Cennamo, N.; Zeni, L. SPR based hybrid electro-optic biosensor for β-lactam antibiotics determination in water. Proc. SPIE 2017, 10405, 104050C. [Google Scholar] [CrossRef]
- Cennamo, N.; Pasquardini, L.; Arcadio, F.; Lunelli, L.; Vanzetti, L.; Carafa, V.; Altucci, L.; Zeni, L. SARS-CoV-2 spike protein detection through a plasmonic D-shaped plastic optical fiber aptasensor. Talanta 2021, 233, 122532. [Google Scholar] [CrossRef]
- Lobry, M.; Loyez, M.; Hassan, E.M.; Chah, K.; DeRosa, M.C.; Goormaghtigh, E.; Wattiez, R.; Caucheteur, C. Multimodal plasmonic optical fiber grating aptasensor. Opt. Express 2020, 28, 7539–7551. [Google Scholar] [CrossRef]
- Loyez, M.; Lobry, M.; Hassan, E.M.; DeRosa, M.C.; Caucheteur, C.; Wattiez, R. HER2 breast cancer biomarker detection using a sandwich optical fiber assay. Talanta 2021, 221, 121452. [Google Scholar] [CrossRef]
- Tripathi, S.M.; Dandapat, K.; Bock, W.J.; Mikulic, P.; Perreault, J.; Sellamuthu, B. Gold coated dual-resonance long-period fiber gratings (DR-LPFG) based aptasensor for cyanobacterial toxin detection. Sens. Bio-Sens. Res. 2019, 25, 100289. [Google Scholar] [CrossRef]
- Aptamer Group. Available online: https://aptamergroup.com/ (accessed on 15 April 2022).
- Zahra, Q.; Luo, Z.; Ali, R.; Khan, M.; Li, F.; Qiu, B. Advances in Gold Nanoparticles-Based Colorimetric Aptasensors for the Detection of Antibiotics: An Overview of the Past Decade. Nanomaterials 2021, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-Based Biosensors for Antibiotic Detection: A Review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Yin, S.; Li, Y.; Lu, D.; Zhang, J.; Sun, C. Application of aptamers in detection and chromatographic purification of antibiotics in different matrices. TrAC Trends Anal. Chem. 2017, 95, 1–22. [Google Scholar] [CrossRef]
- Zahra, Q.A.; Fang, X.; Luo, Z.; Ullah, S.; Fatima, S.; Batool, S.; Qiu, B.; Shahzad, F. Graphene Based Nanohybrid Aptasensors in Environmental Monitoring: Concepts, Design and Future Outlook. Crit. Rev. Anal. Chem. 2022, 95, 1–22. [Google Scholar] [CrossRef]
- Varty, K.; O’Brien, C.; Ignaszak, A. Breast Cancer Aptamers: Current Sensing Targets, Available Aptamers, and Their Evaluation for Clinical Use in Diagnostics. Cancers 2021, 13, 3984. [Google Scholar] [CrossRef]
- Negahdary, M. Aptamers in nanostructure-based electrochemical biosensors for cardiac biomarkers and cancer biomarkers: A review. Biosens. Bioelectron. 2020, 152, 112018. [Google Scholar] [CrossRef]
- Huang, J.; Chen, X.; Fu, X.; Li, Z.; Huang, Y.; Liang, C. Advances in Aptamer-Based Biomarker Discovery. Front. Cell Dev. Biol. 2021, 9, 571. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Krüger, A.; de Jesus Santos, A.; de Sá, V.; Ulrich, H.; Wrenger, C. Aptamer Applications in Emerging Viral Diseases. Pharmaceuticals 2021, 14, 622. [Google Scholar] [CrossRef]
- Sánchez-Báscones, E.; Parra, F.; Lobo-Castañón, M.J. Aptamers against viruses: Selection strategies and bioanalytical applications. TrAC Trends Anal. Chem. 2021, 143, 116349. [Google Scholar] [CrossRef]
- Zou, X.; Wu, J.; Gu, J.; Shen, L.; Mao, L. Application of Aptamers in Virus Detection and Antiviral Therapy. Front. Microbiol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irawan, R.; Tjin, S.C.; Fang, X.; Fu, C.Y. Integration of optical fiber light guide, fluorescence detection system, and multichannel disposable microfluidic chip. Biomed. Microdevices 2007, 9, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Kelb, C.; Körner, M.; Prucker, O.; Rühe, J.; Reithmeier, E.; Roth, B. PDMAA Hydrogel Coated U-Bend Humidity Sensor Suited for Mass-Production. Sensors 2017, 17, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitkulov, A.; Tosi, D. Optical Fiber Sensor Based on Plastic Optical Fiber and Smartphone for Measurement of the Breathing Rate. IEEE Sens. J. 2019, 19, 3282–3287. [Google Scholar] [CrossRef]
- Uyor, U.O.; Popoola, A.P.I.; Popoola, O.M.; Aigbodion, V.S. Polymeric cladding materials under high temperature from optical fibre perspective: A review. Polym. Bull. 2020, 77, 2155–2177. [Google Scholar] [CrossRef]
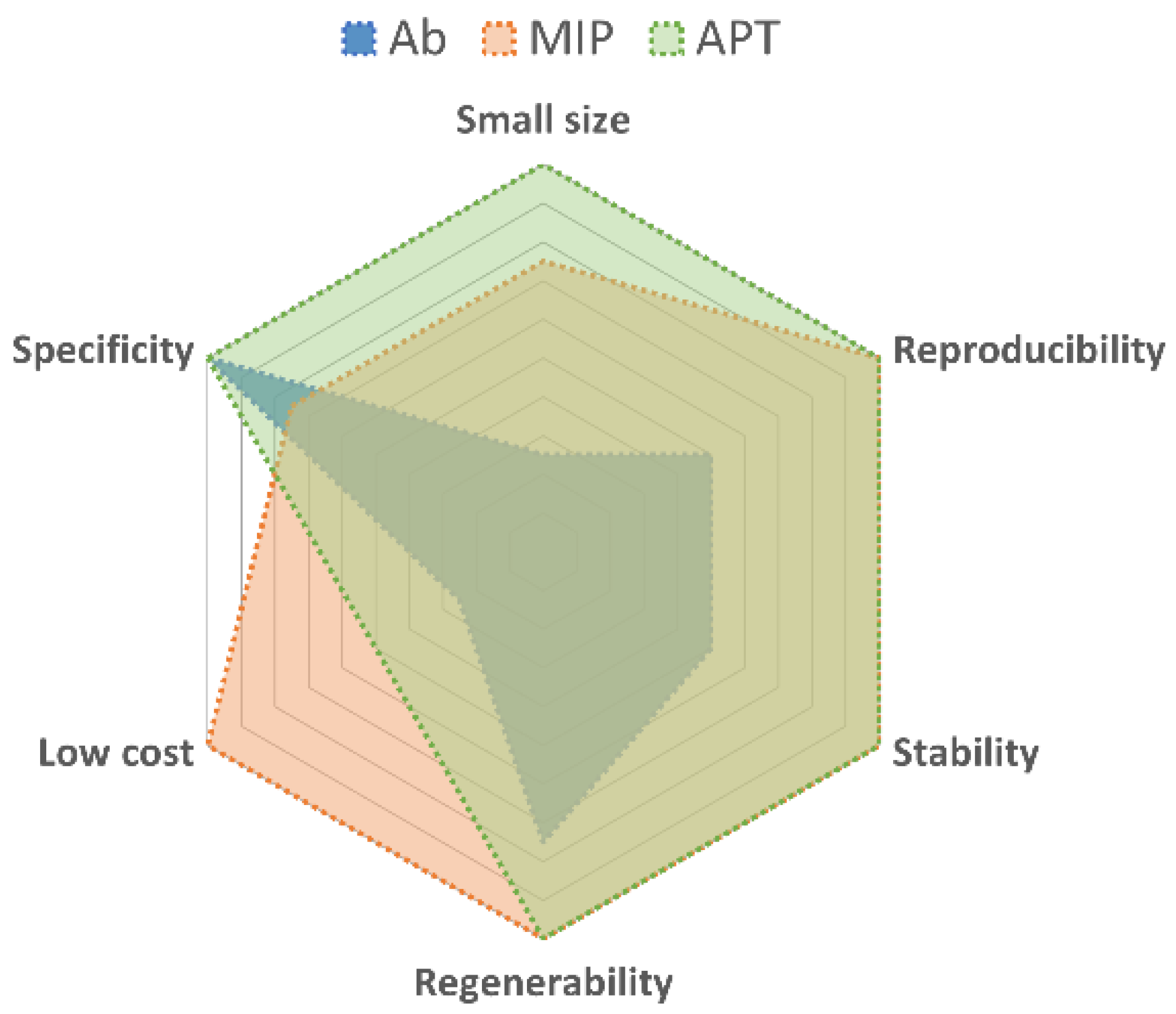
| Aptamers | Antibodies | |
|---|---|---|
| Molecular basis | Nucleic acids | Proteins |
| Molecular modifications | Many examples (significant benefits for clinical applications) | Rare case (reduced affinity to antigens) |
| Size | Small—10/15 KDa | Big—150 KDa |
| Stability | Stable even in high temperature and non-physiological pH environment Susceptible to nucleases and rapid clearance rate in vivo | Susceptible to denaturation in high temperature and non-physiological pH environment Less susceptible to nucleases and low clearance rate in vivo |
| Targets | Wide range, from ions to whole living cells | Only immunogenic molecules |
| Immobilization | By physical adsorption or chemical bonds | By random or oriented or chemical bonds |
| Immunotoxicity | Non-toxic | May cause a serious response in some patients |
| Specificity | High | High |
| Biochemical reactions | Not limited to physiological conditions | Limited to physiological conditions |
| Cost | Less expensive (solid-phase synthesis) | More expensive (animals extraction) |
| Production | Chemical modifications or SELEX | Animals |
| Reproducibility | High batch to batch reproducibility | Low batch to batch reproducibility |
| Ethical concerns | None | Production and development depend on living animals |
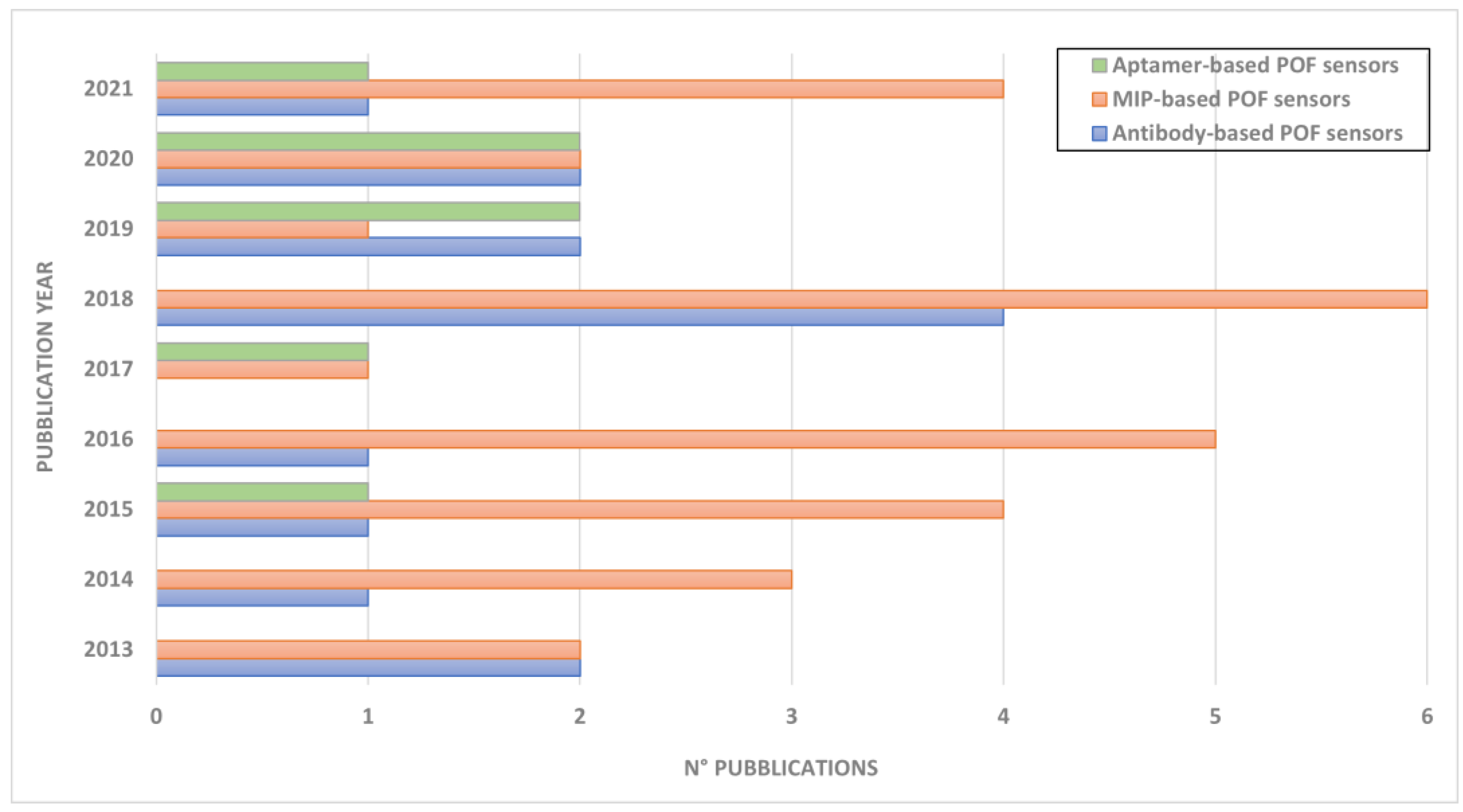
| Fiber Type | Analyte | Optical Detection | Sensitivity [nm/RIU] | LoD [nM] | Reference |
|---|---|---|---|---|---|
| Silica fiber | |||||
| Bragg grating imprinted on silica fiber coated with gold (50 nm) | Thrombin | SPR | - | 22 | Albert et al. 2013 [46] |
| Silica fiber coated with Au (50 nm) | IgE | SPR | ~1500 | 2 ± 1 | Pollet et al. 2009 [44] |
| Silica fiber coated with Au (50 nm) | Ara h1 | SPR | - | 75 | Tran et al. 2013 [68] |
| Bragg grating imprinted on silica fiber coated with gold (50 nm) | Thrombin | SPR | 400 ÷ 1000 | 22.6 | Shevchenko et al. 2011 [56] |
| Tilted silica fiber Bragg gratings coated with gold (35 nm) | HER2 | SPR | 102.03 | ~8.6 × 10−6 | Lobry et al. 2020 [72] |
| Tilted silica fiber Bragg gratings coated with gold (50 nm) | Thrombin | SPR and LSPR | - | 1 | Lao et al. 2019 [51] |
| Bragg grating imprinted on silica fiber coated with gold (50 nm) | CTC (circulating tumor cells) | SPR | - | 49 cells/mL or 10 cells/mL (with amplification) | Loyez et al. 2020 [57] |
| Tilted silica fiber Bragg gratings | Thrombin | SPR | 23.38 | 0.075–0.11 | Sypabekova et al. 2019 [52] |
| Silica fiber with annealed gold layer (50 nm) | Ara h1 | SPR | >1000 | 1.6 | Antohe et al. 2016 [69] |
| Silica fiber with gold coating (45 nm) | HER2 | SPR | 1573.9 | 0.077 | Loyez et al. 2021 [73] |
| Gold-coated silica fiber | Thrombin | SPR | - | 6.1 | Daems et al. 2018 [58] |
| Silica fiber with gold coating (50 nm) | ATP/Thombin/DNA | SPR | - | 72,000/36/30 | Dillen et al. 2021 [54] |
| Silica fiber in gold nanoantennas configuration (Ge/SiO2/Au 36/24/22 nm) | Thrombin | LSPR | 3.4 × 104 | 5 × 10−7 | Allsop et al. 2017 [59] |
| Gold nanorods on silica fiber | Ochratoxin A | LSPR | 601.05 | 0.012 | Lee et al. 2018 [64] |
| Silica fiber in gold nanoantennas configuration (Ge/SiO2/Au 36/24/22 nm) | Bisphenol A | LSPR | ~7 × 103 | 3.3 ± 0.7 × 10−7 | Allsop et al. 2019 [65] |
| Gold nanoparticles on silica fiber | Zearalenone | LSPR | - | 0.3 | Xu et al. 2021 [66] |
| Silica U-bend fiber coated with silver (68 nm) | Bisphenol A | LSPR | 1717 | 0.02 | Luo et al. 2016 [67] |
| Gold coated silica fiber | PDGF-BB (Platelet-derived growth factor) | SPR | 2294.50 | 3.5 × 10−4 | Qian et al. 2019 [60] |
| Silica fiber coating (Cr/Au-/TiO2 2/16/100 nm) | Thrombin | SPR | 3143 | 10 | Coehlo et al. 2016 [61] |
| Silanized silica fiber coated with gold (50 nm) | Thrombin | SPR | 1931 | >34 | Arghir et al. 2015 [62] |
| Long-period fiber grating coated with gold layer (10 nm) | Microcystin-LR | SPR | 3891.5 | ~5 | Tripathi et al. 2019 [74] |
| Bragg grating imprinted on silica fiber coated with gold (32 nm) | Dopamine | SPR | - | 1.7 × 10−4 | Hu et al. 2018 [47] |
| Plastic fiber | |||||
| Plastic optical fiber coated with gold (60 nm) | VEGF | SPR | - | 0.8 | Cennamo et al. 2015 [48] |
| Plastic optical fiber coated with gold (50 nm) | Ampicillin | SPR | 1627.9 | - | Galatus et al. 2017 [70] |
| Plastic optical fiber coated with gold (60 nm) | Thrombin | SPR | - | 1.6 | Cennamo et al. 2019 [49] |
| Plastic optical fiber coated with gold (50 nm) | Dopamine | SPR | 1539 | >0.1 | Sun et al. 2019 [50] |
| Plastic optical fiber coated with gold (60 nm) | SARS-CoV-2 spike protein | SPR | - | 36.7 | Cennamo et al. 2021 [71] |
| Plastic U-bend optical fiber coated with gold | PfGDH (Plasmodium falciparum glutamate dehydrogenase) | SPR | - | 0.264 | Sanjay et al. 2020 [55] |
| Advantages | Disadvantages |
|---|---|
| Highly flexible (easily integrable in small device) | Moderate cost due to the aptamers |
| Highly specific due to the aptamers | Possible susceptible to environmental modification (humidity, temperature) |
| Different configurations allowed (D-shaped, U-bend, etc.) | Wide value of the SPR spectra width at half maximum (FWHM) |
| Easy to produce and manipulate | Working efficiency in a limited range (visible) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquardini, L.; Cennamo, N.; Arcadio, F.; Zeni, L. A Review of Apta-POF-Sensors: The Successful Coupling between Aptamers and Plastic Optical Fibers for Biosensing Applications. Appl. Sci. 2022, 12, 4584. https://doi.org/10.3390/app12094584
Pasquardini L, Cennamo N, Arcadio F, Zeni L. A Review of Apta-POF-Sensors: The Successful Coupling between Aptamers and Plastic Optical Fibers for Biosensing Applications. Applied Sciences. 2022; 12(9):4584. https://doi.org/10.3390/app12094584
Chicago/Turabian StylePasquardini, Laura, Nunzio Cennamo, Francesco Arcadio, and Luigi Zeni. 2022. "A Review of Apta-POF-Sensors: The Successful Coupling between Aptamers and Plastic Optical Fibers for Biosensing Applications" Applied Sciences 12, no. 9: 4584. https://doi.org/10.3390/app12094584
APA StylePasquardini, L., Cennamo, N., Arcadio, F., & Zeni, L. (2022). A Review of Apta-POF-Sensors: The Successful Coupling between Aptamers and Plastic Optical Fibers for Biosensing Applications. Applied Sciences, 12(9), 4584. https://doi.org/10.3390/app12094584









