Spray-Drying Hen Eggs: Effects of the Egg Yolk to Egg White Ratio and Sucrose Addition on the Physicochemical, Functional, and Nutritional Properties of Dried Products and on Their Amino Acid Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Liquid Mixtures with Egg Components
2.2. Characteristics of the Spray-Drying System
2.3. Drying Process Conditions
2.4. Physicochemical Analysis of Products
2.5. Functional Properties Analysis
2.6. Amino Acid Analysis by Gas Chromatography Coupled to a Mass Detector (GC/MS)
2.7. Experimental Design and Statistical Analysis
3. Results
3.1. Effect of Egg Component Mixtures and Sucrose Addition on the Flow of Fresh Egg Products and on the Physicochemical Characteristics of the Dried Powders
| Liquid Egg Components (Factor X1) | Sucrose Content (%, w/w) (Factor X2) | MC (%, w/w) | WSI (%, w/w) | Aw | Color |
|---|---|---|---|---|---|
| Mixture of 100% egg white, [W] | 0.0 | 3.76 ± 0.09 A | 88.63 ± 0.27 B | 0.196 ± 0.002 B | L* A = 95.6 ± 0.2, a* C = −0.6 ± 0.2, b* B = 8.0 ± 1.2 |
| 5.0 | 3.42 ± 0.40 A | 88.46 ± 0.12 AB | 0.290 ± 0.008 A | L* A = 95.5 ± 0.4, a* C = −0.5 ± 0.1, b* B = 6.1 ± 0.5 | |
| 7.5 | 2.11 ± 0.42 A | 88.20 ± 0.54 B | 0.235 ± 0.034 B | L* A = 95.5 ± 0.5, a* C = −0.6 ± 0.0, b* B = 6.8 ± 0.7 | |
| Mixture of egg yolk: egg white (1:3) [M1:3] | 0.0 | 2.03 ± 0.97 B | 92.96 ± 1.63 A | 0.231 ± 0.006 B | L* B = 88.6 ± 0.1, a* B = 5.6 ± 0.4, b* A = 21.7 ± 0.6 |
| 5.0 | 2.72± 0.54 B | 90.91 ± 1.64 AB | 0.277 ± 0.008 A | L* B = 89.4 ± 0.1, a* B = 4.9 ± 0.4, b* A = 18.6 ± 0.8 | |
| 7.5 | 2.15 ± 0.63 B | 90.88 ± 1.13 AB | 0.247 ± 0.028 B | L* B = 90.4 ± 1.7, a* B = 5.4 ± 1.7, b* A = 18.7 ± 2.1 | |
| Mixture of liquid components in a whole egg [WE] | 0.0 | 2.22 ± 0.24 AB | 83.15 ± 0.94 C | 0.248 ± 0.002 B | L* B = 86.7 ± 1.8, a* A = 7.8 ± 1.0, b* A = 26.3 ± 5.4 |
| 5.0 | 2.53 ± 0.28 AB | 90.38 ± 0.68 AB | 0.271 ± 0.001 A | L* B = 86.9 ± 0.3, a* A = 6.0 ± 0.0, b* A = 22.9 ± 3.0 | |
| 7.5 | 2.21 ± 0.26 AB | 88.47 ± 0.36 B | 0.238 ± 0.013 B | L* B = 89.3 ± 0.3, a* A = 5.8 ± 0.2, b* A = 20.7 ± 1.0 |
| Model | X1 | X2 | X1*X2 | Error | Total | ||
|---|---|---|---|---|---|---|---|
| Variable | DF | 8 | 2 | 2 | 4 | 9 | 17 |
| MC [%] | SS | 6.21 | 2.47 | 1.73 | 2.02 | 2.17 | 8.38 |
| p-value | 0.05 A | 0.03 A | 0.07 | 0.17 | |||
| Aw | SS | 0.01 A | 0.00 A | 0.01 A | 0.00 A | 0.00 A | 0.02 A |
| p-value | 0.01 A | 0.41 | 0.00 A | 0.10 | |||
| WSI [%] | SS | 119.45 | 56.04 | 12.04 | 51.38 | 8.47 | 127.92 |
| p-value | 0.00 A | 0.00 A | 0.02 A | 0.00 A | |||
| pH | SS | 3.34 | 3.00 | 0.18 | 0.17 | 0.16 | 3.51 |
| p-value | 0.00 | 0.00 | 0.04 | 0.14 | |||
| Lightness (L) | SS | 193.43 | 182.54 | 6.10 | 4.78 | 7.02 | 200.45 |
| p-value | 0.00 | 0.00 | 0.06 | 0.27 | |||
| Chromaticity coordinate a* | SS | 176.39 | 171.08 | 2.31 | 3.01 | 4.37 | 180.76 |
| p-value | 0.00 | 0.00 | 0.15 | 0.27 | |||
| Chromaticity coordinate b* | SS | 930.07 | 882.44 | 37.01 | 10.62 | 46.20 | 976.27 |
| p-value | 0.00 | 0.00 | 0.07 | 0.73 | |||
3.2. Drying Yields and Nutritional Compositions of Selected Egg Component Powder
3.3. Functional Properties of Powdered Egg Products W, M1:3, and WE5%
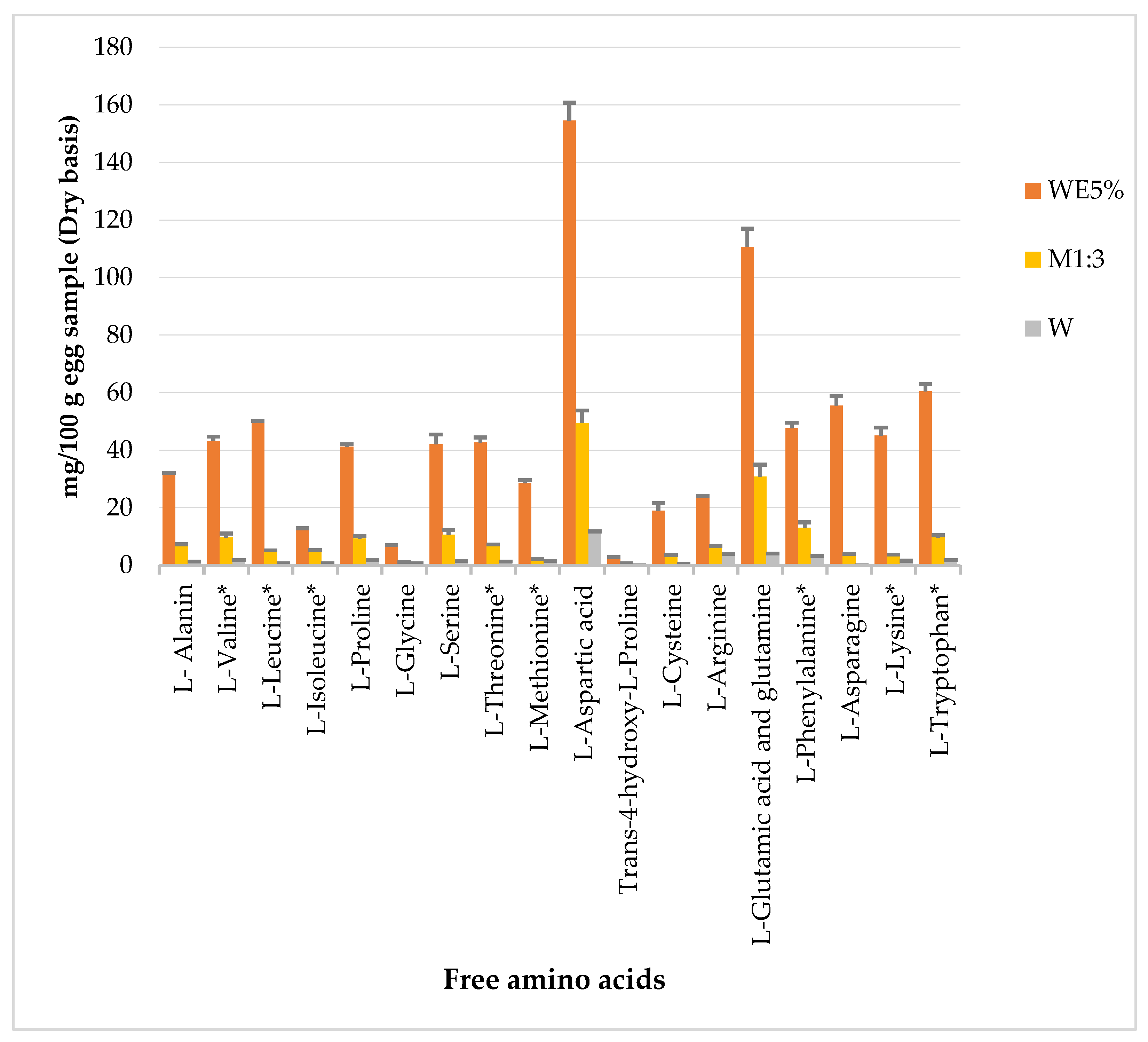
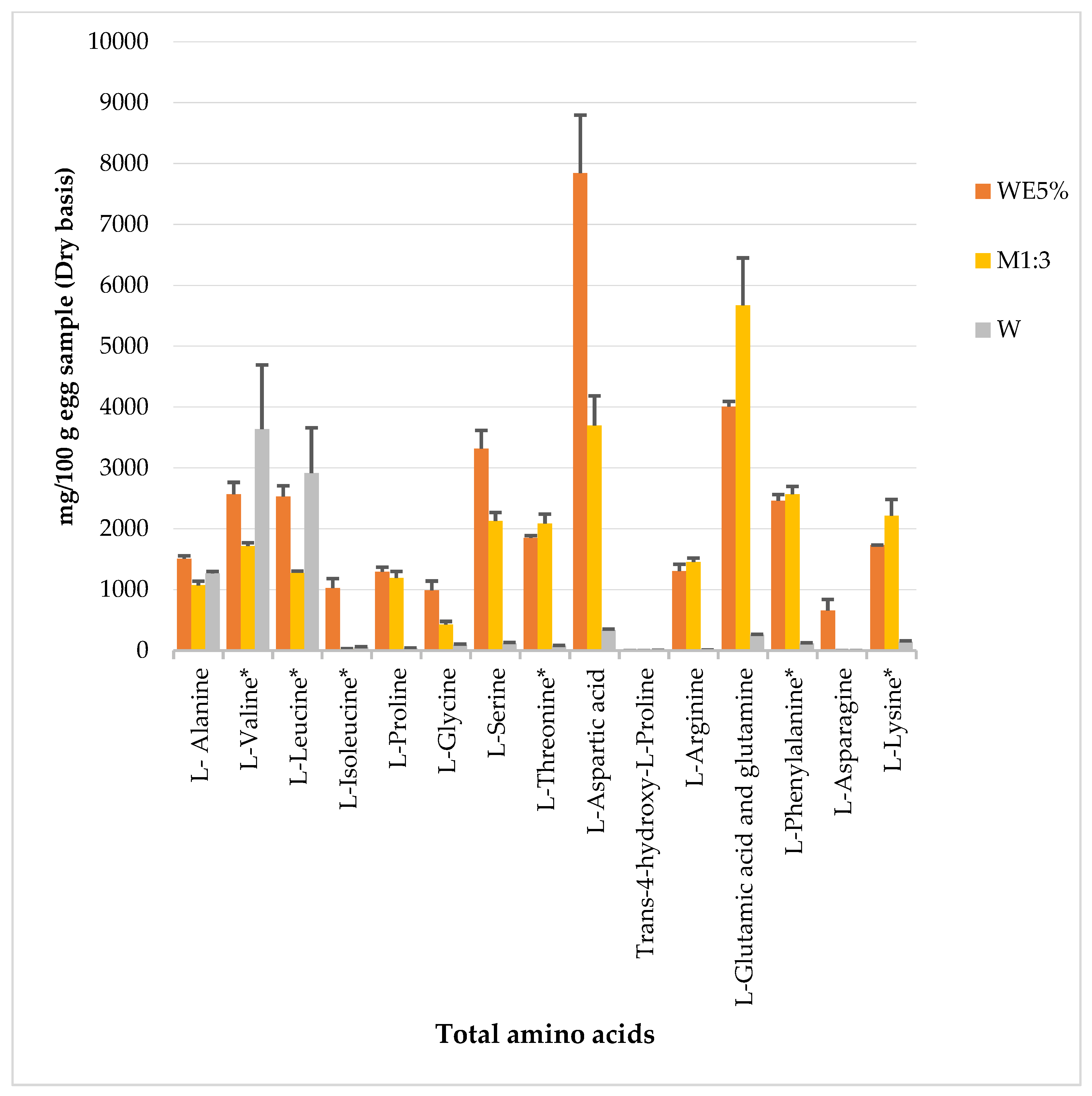
3.4. Amino Acid Profile of Powdered Egg Products WE5%, M1:3, and W
4. Discussion
4.1. The Physicochemical Properties of Egg Powders
4.2. The Nutritional Composition of Dried Egg Components W, M1:3, and WE5%
4.3. Functional Properties, as Affected by Types of Egg Components in W, M1:3, and WE5%
4.4. pH Behavior and the Amino Acid Profile of Egg Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callejas-Garzón, A.M.; Ramírez-Gamboa, J.N. Aprovechamiento de Huevo Deshidratado en la Elaboración de un Producto Cárnico Emulsionado. Bachelor’s Thesis, Universidad de la Salle, Bogotá, Colombia, 2018. [Google Scholar]
- Abreha, E.; Getachew, P.; Laillou, A.; Chitekwe, S.; Baye, K. Physico-chemical and Functionality of Air and Spray Dried Egg Powder: Implications to Improving Diets. Int. J. Food Prop. 2021, 24, 152–162. [Google Scholar] [CrossRef]
- Asghar, A.; Abbas, M. Effect of Spray Dried Whole Egg Powder on Physicochemical and Sensory Properties of Cake. Am. J. Sci. Ind. Res. 2015, 6, 97–102. [Google Scholar] [CrossRef]
- Muñoz, E.D. Elaboración de Clara de Huevo Deshidratada Pasteurizada. Bachelor’s Thesis, Universidad Nacional Agraria La Molina, Lima, Perú, 2017. [Google Scholar]
- Miranda, J.M.; Anton, X.; Redondo-Valbuena, C.; Roca-Saavedra, P.; Rodriguez, J.A.; Lamas, A.; Franco, C.M.; Cepeda, A. Egg and Egg-derived Foods: Effects on Human Health and Use as Functional Foods. Nutrients 2015, 7, 706–729. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.K.; Saleem, M.; Javed, K. Food Materials Science in Egg Powder Industry. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 505–537. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Bansal, N.; Zhang, M.; Schuck, P. Handbook of Food Powders: Processes and Properties, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2013; p. 688. [Google Scholar] [CrossRef]
- Ayadi, M.A.; Khemakhem, M.; Belgith, H.; Attia, H. Effect of Moderate Spray Drying Conditions on Functionality of Dried Egg White and Whole Egg. J. Food Sci. 2008, 73, E281–E287. [Google Scholar] [CrossRef]
- Koc, M.; Koc, B.; Susyal, G.; Yilmazer, M.S.; Ertekin, F.K.; Bağdatlıoğlu, N. Functional and Physicochemical Properties of Whole Egg Powder: Effect of Spray Drying Conditions. J. Food Sci. Technol. 2010, 48, 141–149. [Google Scholar] [CrossRef]
- García-Figueroa, A. Mezclas en Polvo a Base de Huevo Entero Obtenidas Mediante Secado por Aspersion: Efecto de la Temperatura de Secado y Formulación del Alimento. Master’s Thesis, Universidad del Valle, Cali, Colombia, 2020. [Google Scholar]
- Koç, M.; Koç, B.; Yilmazer, M.S.; Ertekin, F.K.; Susyal, G.; Bağdatlıoğlu, N. Physicochemical Characterization of Whole Egg Powder Microencapsulated by Spray Drying. Dry. Technol. 2011, 29, 780–788. [Google Scholar] [CrossRef]
- Bergquist, D.H. Egg Dehydration. In Egg Science and Technology, 4th ed.; Stadelman, W.J., Cotterill, O.J., Eds.; Routledge & CRC Press Login: Boca Ratón, FL, USA, 1995; pp. 335–376. [Google Scholar] [CrossRef]
- Campbell, L.; Raikos, V.; Euston, S. Heat Stability and Emulsifying Ability of Whole Egg and Egg Yolk as Related to Heat Treatment. Food Hydrocoll. 2005, 19, 533–539. [Google Scholar] [CrossRef]
- Lechevalier, V.; Jeantet, R.; Arhaliass, A.; Legrand, J.; Nau, F. Egg White Drying: Influence of Industrial Processing Steps on Protein Structure and Functionalities. J. Food Eng. 2007, 83, 404–413. [Google Scholar] [CrossRef]
- Lai, C.C.; Gilbert, S.G.; Mannheim, C.H. Effect of Composition on the Flow Properties of Egg Powders. J. Food Sci. 1985, 50, 1618–1620. [Google Scholar] [CrossRef]
- Colombian Institute of Technical Standards and Certification. Food Industries. Egg Products. NTC 6116: 2015. Available online: https://tienda.icontec.org/gp-industrias-alimentarias-ovoproductos-ntc6116-2015.html (accessed on 11 February 2020).
- Katekhong, W.; Charoenrein, S. Influence of Spray Drying Temperatures and Storage Conditions on Physical and Functional Properties of Dried Egg White. Dry. Technol. 2017, 36, 169–177. [Google Scholar] [CrossRef]
- Koç, M.; Koç, B.; Güngör, Ö.; Ertekin, F.K. The Effects of Moisture on Physical Properties of Spray-Dried Egg Powder. Dry. Technol. 2012, 30, 567–573. [Google Scholar] [CrossRef]
- Ignário, R.M.; Lannes, S. Preparation of Powdered Egg Yolk Using a Mini Spray Dryer. Ciência Tecnol. Aliment. 2007, 27, 729–732. [Google Scholar] [CrossRef][Green Version]
- Martínez-Morales, M.V. Obtención de Clara de Huevo en Polvo por Medio de la Técnica de Secado Utilizando un Equipo que Opera por Aspersión. Bachelor’s Thesis, Universidad de Costa Rica, San José, Costa Rica, 2016. [Google Scholar]
- Diniz, M.; Martin, A.M. Effects of the Extent of Enzymatic Hydrolysis on Functional Properties of Shark Protein Hydrolysate. LWT Food Sci. Technol. 1997, 30, 266–272. [Google Scholar] [CrossRef]
- Sze, W.K.; Huda, N.; Dewi, M.; Hashim, H. Physicochemical Properties of Egg White Powder from Eggs of Different Types of Bird. Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 384–389. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, Y.; Yang, Y. Study on Process Conditions of Spray-drying Whole Egg Powder. J. Anhui Agric. Sci. 2015, 243–246. Available online: https://caod.oriprobe.com/articles/44708776/Study_on_Process_Conditions_of_Spray_drying_Whole_.htm (accessed on 3 March 2022).
- Orishagbemi, O.C.; Atadoga, J.O.; David, E.K.; Ocheni, J. Functional, Organoleptic Properties and Nutrients Composition of Imitation Egg Powder Produced from Suitable Natural Raw Materials. Int. J. Innov. Res. Sci. Eng. Technol. 2017, 6, 11089–11095. [Google Scholar] [CrossRef]
- Anderson, R.A.; Conway, V.F.P.; Griffin, E.L. Gelatinization of Corn Grits by Roll- and Extrusion-Cooking. Cereal Sci. Today 1969, 14, 4–7. [Google Scholar]
- Restrepo-Osorio, J. Validación de un método cromatográfico para evaluar la calidad proteica de alimentos y su impacto en los niveles plasmáticos. Rev. Cienc. 2019, 23, 43–62. [Google Scholar]
- Llames, C.R.; Fontane, J. Determination of Amino Acids in Feeds: Collaborative Study. J. AOAC Int. 1994, 77, 1362–1402. [Google Scholar] [CrossRef]
- Muzaffar, K.; Nayik, G.A.; Kumar, P. Stickiness Problem Associated with Spray Drying of Sugar and Acid Rich Foods: A Mini Review. J. Nutr. Food Sci. 2015, 5, 1–3. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Roos, Y.H. (Eds.) Non-Equilibrium States and Glass Transitions in Foods: Processing Effects and Product-Specific Implications, 1st ed.; Elsevier: Kidlington, UK, 2016; p. 514. [Google Scholar] [CrossRef]
- Schuck, P.; Dolivet, A.; Jeantet, R. Analytical Methods for Food and Dairy Powders, 1st ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2012; p. 228. [Google Scholar] [CrossRef]
- Froning, G.W.; Peters, D.; Muriana, P.; Eskridge, K.; Travnicek, D.; Sumner, S.S. International Egg Pasteurization Manual; American Egg Board: Park Ridge, IL, USA, 2002; p. 61. [Google Scholar]
- OVODAN. Standard & Customized Egg Products. Available online: https://ovodan.com/egg-powder-products/whole-egg-powder-products/ (accessed on 6 February 2021).
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Hatta, H.; Kapoor, M.P.; Juneja, L.R. Bioactive Components in Egg Yolk. In Egg Bioscience and Biotechnology, 1st ed.; Mine, Y., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 185–237. [Google Scholar] [CrossRef]
- Froning, G.W. Egg Products Industry and Future Perspectives. In Egg Bioscience and Biotechnology, 1st ed.; Mine, Y., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 307–325. [Google Scholar]
- Sugano, M.; Matsuoka, R. Nutritional Viewpoints on Eggs and Cholesterol. Foods 2021, 10, 494. [Google Scholar] [CrossRef]
- Kiczorowska, B.; Samolińska, W.; Kwiecień, M.; Winiarska-Mieczan, A.; Rusinek-Prystupa, E.; Al-Yasiry, A.R.M. Nutritional Value and the Content of Minerals in Eggs Produced in Large-scale, Courtyard and Organic Systems. J. Elem. 2014, 20, 887–895. [Google Scholar] [CrossRef]
- Kanterewicz, R.; Elizalde, B.; Pilosof, A.; Bartholomai, G. Water-Oil Absorption Index (WOAI): A Simple Method for Predicting the Emulsifying Capacity of Food Proteins. J. Food Sci. 1987, 52, 1381–1383. [Google Scholar] [CrossRef]
- Wong, P.; Kitts, D. A Comparison of the Buttermilk Solids Functional Properties to Nonfat Dried Milk, Soy Protein Isolate, Dried Egg White, and Egg Yolk Powders. J. Dairy Sci. 2003, 86, 746–754. [Google Scholar] [CrossRef]
- Pokora, M.; Eckert, E.; Zambrowicz, A.; Bobak, L.; Szołtysik, M.; Dązbrowska, A.; Chrzanowska, J.; Polanowski, A.; Trziszka, T. Biological and Functional Properties of Proteolytic Enzyme-modified Egg Protein by Products. Food Sci. Nutr. 2013, 1, 184–195. [Google Scholar] [CrossRef]
- Gomes, M.T.M.S.; Pelegrine, D.H.G. Solubility of Egg White Proteins: Effect of pH and Temperature. Int. J. Food Eng. 2012, 8, 1–6. [Google Scholar] [CrossRef]
- Zeidler, G. Further-Processing Eggs and Egg Products. In Commercial Chicken Meat and Egg Production; Bell, D.D., Weaver, W.D., Eds.; Springer: Boston, MA, USA, 2002; pp. 1163–1197. [Google Scholar] [CrossRef]
- Heath, J. Chemical and related osmotic changes in egg albumen during storage. Poult. Sci. 1977, 56, 822–828. [Google Scholar] [CrossRef]
- Aftabuddin, M.; Kundu, S. Hydrophobic, hydrophilic, and charged amino acid networks within protein. Biophys. J. 2007, 93, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-C.; Lee, C.-Y.; Weng, W.-L.; Shiah, I.-M. Solubilities of Amino Acids in Water at Various pH Values Under 298.15 K. Fluid Phase Equilibria 2009, 285, 90–95. [Google Scholar] [CrossRef]
- Li, P.; Jin, Y.; Sheng, L. Impact of Microwave Assisted Phosphorylation on the Physicochemistry and Rehydration Behaviour of Egg White Powder. Food Hydrocoll. 2019, 100, 105380. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chen, J.-T.; Chang, W.-T.; Shiah, I.-M. Effect of pH on the solubilities of divalent and trivalent amino acids in water at 298.15 K. Fluid Phase Equilibria 2013, 343, 30–35. [Google Scholar] [CrossRef]
- Goto, T.; Shimamoto, S.; Ohtsuka, A.; Ijiri, D. Analyses of Free Amino Acid and Taste Sensor Traits in Egg Albumen and Yolk Revealed Potential of Value-added Eggs in Chickens. Anim. Sci. J. 2021, 92, e13510. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Free Aromatic Amino Acids in Egg Yolk Show Antioxidant Properties. Food Chem. 2011, 129, 155–161. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.H. Protein and Amino Acid Content in Four Brands of Commercial Table Eggs in Retail Markets in Relation to Human Requirements. Animals 2020, 10, 406. [Google Scholar] [CrossRef]
| Liquid Egg Components (Factor X1) | Sucrose Content (%) (Factor X2) | Total Solids (%, w/w) | Liquid Flows (mL/min) |
|---|---|---|---|
| Mixture of 100% egg white, [W] | 0.0 | 10.60 ± 0.03 F | 3.25 ± 0.07 |
| 5.0 | 14.35 ± 0.02 E | 3.13 ± 0.08 | |
| 7.5 | 16.37 ± 0.01 D | 3.00 ± 0.03 | |
| Mixture of egg yolk: egg white (1:3), [M1:3] | 0.0 | 13.69 ± 0.04 E | 3.20 ± 0.04 |
| 5.0 | 19.20 ± 0.40 C | 3.11 ± 0.01 | |
| 7.5 | 21.70 ± 0.30 B | 3.00 ± 0.03 | |
| Mixture of components in a fresh whole egg, [WE] | 0.0 | 18.70 ± 0.20 C | 3.36 ± 0.02 |
| 5.0 | 22.11 ± 0.02 B | 3.26 ± 0.02 | |
| 7.5 | 22.92 ± 0.00 A | 3.09 ± 0.01 |
| Sample | Sucrose Content in Fresh Liquid Sample (%) | pH in Fresh Liquid Sample | Adjusted pH in Fresh Liquid Sample | pH in Rehydrated Powder |
|---|---|---|---|---|
| Mixture of 100% egg white, [W] | 0.0 | 9.23 ± 0.06 | 8.06 ± 0.08 | 9.78 ± 0.08 A |
| 5.0 | 9.21 ± 0.06 | 8.08 ± 0.03 | 9.29 ± 0.05 AB | |
| 7.5 | 9.15 ± 0.02 | 8.10 ± 0.10 | 9.29 ± 0.27 AB | |
| Mixture of egg yolk: egg white (1:3), [M1:3] | 0.0 | 8.30 ± 0.20 | 8.20 ± 0.01 | 9.25 ± 0.20 AB |
| 5.0 | 8.30 ± 0.20 | 8.05 ± 0.03 | 9.09 ± 0.08 B | |
| 7.5 | 8.30 ± 0.10 | 8.25 ± 0.01 | 9.24 ± 0.09 B | |
| Mixture of liquid components in a whole egg, [WE] | 0.0 | 7.81 ± 0.04 | 7.20 ± 0.09 | 8.51 ± 0.12 C |
| 5.0 | 7.80 ± 0.10 | 7.22 ± 0.06 | 8.44 ± 0.09 C | |
| 7.5 | 7.70 ± 0.00 | 7.21 ± 0.03 | 8.50 ± 0.07 C |
| Analysis | W | M1:3 | WE5% |
|---|---|---|---|
| Moisture and volatile materials (g/100 g) | 4.51 | 3.22 | 4.79 |
| Total protein (g/100 g) | 80.48 | 58.55 | 39.92 |
| Total fat (g/100 g) | 1.21 | 29.61 | 13.18 |
| Total carbohydrates(g/100 g) | 8.19 | 4.45 | 39.42 |
| Ash (g/100 g) | 5.61 | 4.17 | 2.69 |
| Cholesterol (mg/100 g) | 5.62 | 553.41 | 518.36 |
| Total calories (kCal/100 g) | 365.57 | 518.49 | 435.98 |
| Functional Property | Temperature (°C) | W | M1:3 | WE5% |
|---|---|---|---|---|
| WHC (mL water/g wet sample) | 25 | 3.03 ± 0.00 | 0.91 ± 0.00 | 0.30 ± 0.00 |
| WSI (%) | 25 | 88.63 ± 0.27 | 92.96 ± 1.63 | 90.38 ± 0.68 |
| 50 | 76.64 ± 1.05 | 90.97 ± 0.93 | 82.76 ± 0.35 | |
| Dispersibility (s) | 25 | 3440 ± 183 | 530 ± 1 | 360 ± 4 |
| 50 | 372 ± 2 | 163 ± 4 | 84 ± 4 | |
| Wettability (s) | 25 | 4700 ± 92 | 5443 ± 41 | 474 ± 7 |
| 50 | 7478 ± 21 | 3869 ± 40 | 118 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-del-Río, L.M.; García-Figueroa, A.; Fernández-Quintero, A.; Rodríguez-Stouvenel, A. Spray-Drying Hen Eggs: Effects of the Egg Yolk to Egg White Ratio and Sucrose Addition on the Physicochemical, Functional, and Nutritional Properties of Dried Products and on Their Amino Acid Profiles. Appl. Sci. 2022, 12, 4516. https://doi.org/10.3390/app12094516
Vargas-del-Río LM, García-Figueroa A, Fernández-Quintero A, Rodríguez-Stouvenel A. Spray-Drying Hen Eggs: Effects of the Egg Yolk to Egg White Ratio and Sucrose Addition on the Physicochemical, Functional, and Nutritional Properties of Dried Products and on Their Amino Acid Profiles. Applied Sciences. 2022; 12(9):4516. https://doi.org/10.3390/app12094516
Chicago/Turabian StyleVargas-del-Río, Liliana M., Alexis García-Figueroa, Alejandro Fernández-Quintero, and Aida Rodríguez-Stouvenel. 2022. "Spray-Drying Hen Eggs: Effects of the Egg Yolk to Egg White Ratio and Sucrose Addition on the Physicochemical, Functional, and Nutritional Properties of Dried Products and on Their Amino Acid Profiles" Applied Sciences 12, no. 9: 4516. https://doi.org/10.3390/app12094516
APA StyleVargas-del-Río, L. M., García-Figueroa, A., Fernández-Quintero, A., & Rodríguez-Stouvenel, A. (2022). Spray-Drying Hen Eggs: Effects of the Egg Yolk to Egg White Ratio and Sucrose Addition on the Physicochemical, Functional, and Nutritional Properties of Dried Products and on Their Amino Acid Profiles. Applied Sciences, 12(9), 4516. https://doi.org/10.3390/app12094516






