1. Introduction
Liquid–gas ejectors, in which the secondary fluid is gas, have the potential to be used in carbon capture and storage. Ejectors are used to draw more fluid from wells in the oil and gas sector, helping to increase output and reduce costs. Ejectors are also used for post-combustion carbon capture in power plants, to improve external waste heat to reduce the quantity of turbine steam needed for solvent regeneration. In addition to gas compression, ejectors may also be used for refrigeration and CO
2 transportation and storage, and numerous studies have been done to analyze and improve their effectiveness [
1].
One of the challenging issues that has always attracted the attention of the experts is how to control and reduce greenhouse gas emissions because of their vast negative environmental impacts [
2]. Furthermore, due to the district regulations related to greenhouse gas emissions, experts and scholars have recently focused on the more efficient and clear processes in which the amount and type of the released gas are under control [
3]. Due to the demand for hydrocarbon-based fuels, the oil and gas sectors play a pivotal role in the current situation. During the extraction process of gas and oil, some co-products are created that must be discharged into the surrounding environment. Burning the mentioned gaseous material in flare systems is a common method that is followed in gas and oil fields. Although burning the hazardous gaseous products in the flare systems boosts the safety of gas and oil fields and diminishes the internal pressure of the extraction systems, it has a catastrophic impact on the surrounding environment [
4]. From economic and environmental standpoints, utilizing flare gas recovery units in the gas and oil fields has many merits, such as boosting production efficiency, decreasing operating costs and maintenance, and reducing noise and flare emissions [
5].
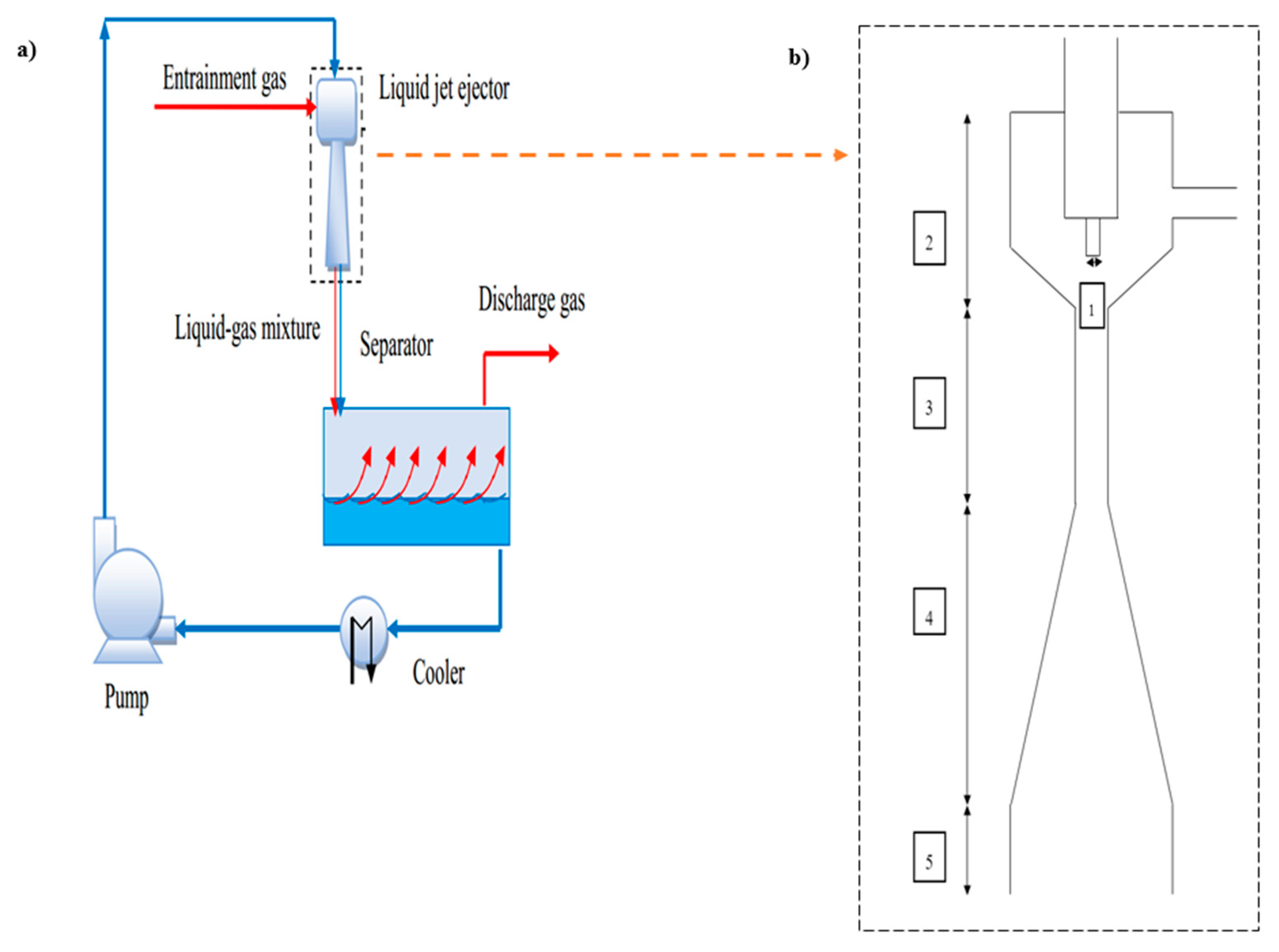
In the chemical-based processes that experts face with coaxial flow systems, ejectors are considered as one of the most critical elements in which a high-pressure current entrains a low-pressure current. This element is widely utilized in flare gas recovery units. To delve deeper into the role of the flare gas recovery unit, it should be highlighted that the recovery unit collects gases before entering to flare unit, and compressing and cooling them remarkably reduces the amount of discharged gas to the environment. The recovered gas extracted from the flare gas recovery unit is utilized as feed in other sections of the chemical plant. The common form of a flare gas recovery unit with a liquid-based ejector and a liquid–gas ejector’s geometry has been depicted in
Figure 1. The general ejector includes, as shown in this Figure, a nozzle (1), a suction chamber/converging part (2), where one phase is injected into the system at a high rate of speed via a nozzle, and a low-pressure zone is created in accordance with Bernoulli’s principle, a throat (3), where the gas and liquid phases mix and a dispersion of gas and liquid is formed, a pressure recovery part (4), and a contactor (5) for providing gas–liquid contact.
Because the ejector is so crucial in the flare gas recovery unit, a lot of research has been done on it in both theoretical and experimental ways. In this regard, computational fluid dynamics is applied to evaluate the performance of ejectors in different conditions and different fluids [
6,
7,
8]. For instance, Aidoun et al. [
9] provided a review paper about the most recent innovations, advances, and novel applications of single-phase ejectors. A wide range of aspects of mentioned ejector were scrutinized. Moreover, the potential of the single-phase ejector to employ as a single equipment or integrate with other elements in a combined system was assessed. Two-phase ejectors are another type of ejector in which two different materials flow inside the ejector. The simulation of these types of ejectors was precisely reviewed by Song et al. [
10]. Bauzvand et al. investigated the experimental and computational fluid dynamics of an ejector with a new geometry to improve its performance. The multi-nozzle and swirls effect features are combined in the proposed inlet geometry. With the applied changes, the ejector efficiency increased by 158% [
11]. El-Zahaby et al. [
12] tested the configuration of gas-gas ejectors based on numerical analysis. They considered different operation circumstances and geometries, and a CFD model was developed to assess the proposed system. The results demonstrated that the system’s performance is influenced by the current and character of the ejector. In another study [
13], an innovative liquid-liquid ejector was hydrodynamically evaluated by CFD. The results of validation demonstrated that the simulation’s findings had enough accuracy. Moreover, some critical variables, such as jet velocity, column to nozzle diameter ratio, and two phases flow ratio, were selected, and their impacts on the proposed system’s efficiency were assessed. Patel et al. [
14] conducted a numerical modeling of a multi-nozzle jet ejector using CFD. They evaluated three different sets of nozzles inside a jet ejector and compared the finding results. Additionally, they assessed the dynamic of break-up and bubble coalescence in the mentioned system by considering 3D numerical domains. Void fraction, velocity, and bubble size were three critical parameters tested in a dual-channel optical fiber probe in a liquid–gas annular ejector system by Aliyu et al. [
15]. Based on the results and comparing them with experimental data, the accuracy of measured parameters was verified. A single phase model based on CFD was developed by Sharma et al. [
16]. The authors simulated a gas–liquid nozzle to anticipate the gas induction rates. Additionally, the influences of variables such as the nozzle’s mixing length, the diameter of the nozzle, suction chamber geometry, etc., were scrutinized and reported. Operating circumstances are another critical parameter that was especially evaluated by Zheng et al. [
17]. To this end, a two-phase CFD-based model was developed for an ejector. In that investigation, Liquefied natural gas (LNG) and Boiling Off Gas (BOG) were chosen as primary and secondary fluids inside the simulated ejector. The simulation’s results revealed that the phase change had a remarkable impact on the system’s efficiency. The fluid type inside the liquid jet ejector was another parameter tested by Sharma et al. [
18]. Unlike other investigations in which the impacts of the turbulent phenomenon have been neglected, in that research, authors tried to evaluate the turbulence phenomena and its influences on the issues such as jet dynamic and rate of gas induction. They reported that the mentioned issue should be considered to reach a reliable and more correct CFD model. The mixing flow behavior and its impact on liquid–gas ejectors were modeled by Wang et al. [
8]. The model was developed based on the realizable k–ε turbulence mode by ANSYS-FLUENT software. By simulating the system, all critical variables were investigated for the ejector. A great number of scholars and experts have been widely working on both the computational evaluations and experimental-based investigations around ejectors and their characteristics [
19,
20,
21,
22].
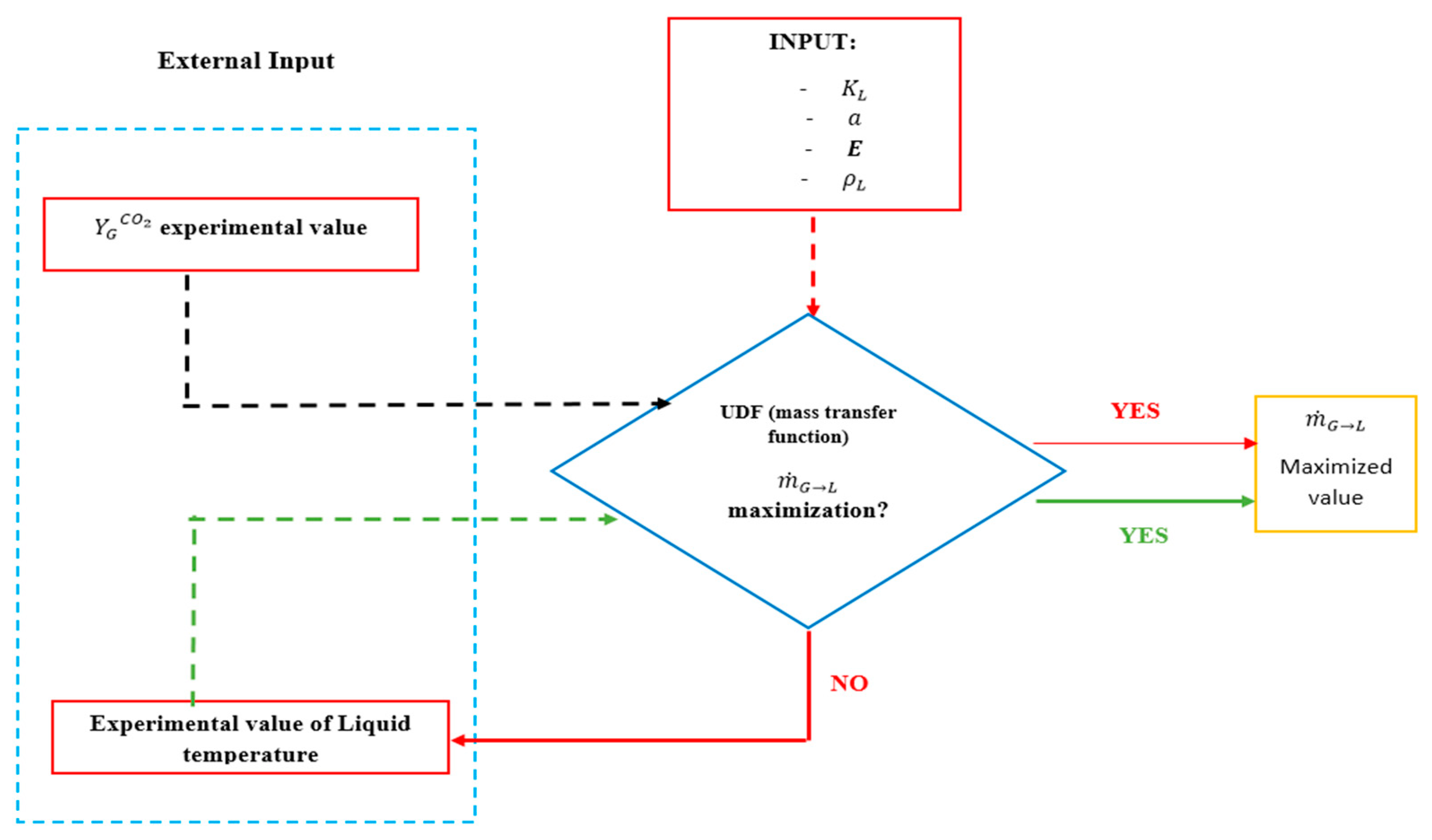
To the best of our knowledge, there has been no hydrodynamic study of carbon dioxide capture in an ejector in the literature prior to this study. The present work firstly evaluates the hydrodynamics of a 3D liquid–gas (water–air) ejector. In this study, the experimental values such as water flow rate and separator pressure are used from the experimental work by Mandal [
23]. The impacts of motive liquid flowrate and outlet pressure on the air entrainment rate and air hold-up were assessed using 3D CFD simulation. One of the important research gaps that has been neglected in other similar investigations is related to mixing shock and where it occurs. In the current study, the authors evaluated the 3D geometry of the simulated ejector with considering mixing shock. After evaluating the 3D geometry and finding its reliability and accuracy, hydrodynamic and mass transfer of a dual function ejector are assessed in a 2D liquid–gas (MEA solution–CO
2/air) ejector for simultaneously increasing gas pressure and capturing CO
2. This action is performed to assess the mass transfer through the liquid–gas ejector by using User-Defined Function (UDF) code. The mixture of carbon dioxide and air is selected as the gas phase, and the amine solution plays the liquid phase’s role in the 2D evaluation. The effects of ejector outlet pressure, amine concentration, and liquid-to-gas (L/G) ratio on the CO
2 removal are evaluated by using 2D CFD modeling. In all stages, grids and mesh independency, Eulerian–Eulerian approach as a multiphase model, and Standard k-
model as the turbulent behavior model of the flow, are implemented. The commercial CFD package ANSYS-FLUENT 17 is the software that is used to conduct this investigation.
2. Modeling Approach
Two common approaches exist for the numerical modeling of multiphase flows. One is Eulerian–Lagrangian method. The other is the Eulerian–Eulerian approach. In the first method, the dispersed phase is considered as an independent discrete phase. In order to solve the dispersed phase, a large number of particles, bubbles, or droplets must be calculated through the computed flow field. Furthermore, momentum, mass, and energy exchanges between the dispersed and fluid phases can be done effectively. On the contrary, the second method considers both phases (fluid and dispersed) as two continuous phases that continuously penetrate to each other. For each phase (not for each one of bubbles, droplets, or particles in the dispersed phase), governing equations are made up of a set of relationships that are uniform in structure for all phases [
24]. Since each particle, bubble, or droplet is tracked in a Lagrangian framework, the Eulerian–Lagrangian approach is computationally intensive. In addition, in this approach, owing to the convergence problem at higher hold-ups, the secondary phase should not exceed ten percent of the total volumetric flow rate. In the majority of cases, the volume fraction of the dispersed phase is more than ten percent; therefore, the more common model in CFD is based on the Eulerian–Eulerian approach. In this study, a three- and two-dimensional modeling with considering Eulerian–Eulerian approach is implemented, in which gas phase/liquid phase for 3D and 2D simulation are air/water and CO
2 + air/MEA solution, respectively. The liquid phase is considered as a continuous phase, but the gas phase is regarded as a dispersed fluid phase with a constant bubble diameter (1 mm). The turbulent behavior of the flow was modeled using a standard k-
model. Some assumptions are made in this simulation: (1) the flow conditions are isothermal (25 °C) and (2) the virtual mass force is neglected.
2.1. Continuity and Momentum Equations
The governing equations of the 3D ejector (water–air) include continuity and momentum equations. For the 2D (MEA solution–CO2/air) ejector, due to the presence of MEA and CO2, the species transport equation is also added.
The dimensional unsteady-state governing fluid flow equations of phase k are presented in Equations (2) and (3). Additionally, in the Eulerian–Eulerian approach, the term of volume fraction is shown. It is assumed that at a specified time, a volume fraction of phase k, (
), exists in a small place. If there are
n phases in total, Equation (1) is obtained:
This means that a large number of particles of diverse phases exist in a volume that is defined by the macroscopic length of the system, in which the sum of the volume fraction is equal to one. The continuity equations and momentum formulations are presented by the two following equations [
25,
26]:
where
is the volume fraction of phase k,
is the density of phase k,
is the velocity of phase k,
is the acceleration of gravity,
is the pressure,
is time,
shows the rate of mass transfer from the kth phase to the liquid phase that is zero for 3D simulation, and
demonstrates the total interfacial forces.
In the above formula,
denotes the shear stress in the kth phase and is calculated by the following equation [
25]:
where
shows the effective viscosity and presents a summation of molecular and turbulent viscosities.
2.1.1. Interfacial Forces
To effectively predict the gas distribution, it is necessary to take the interface forces, such as drag, lift, wall lubrication, turbulent dispersion, and virtual mass, into account. Compared to other interfacial forces, the virtual mass force had minimal impact on the results [
27]. Consequently, the virtual mass force is left out of the equation. Interfacial forces are denoted by the following expression:
The drag, lift, turbulent dispersion, and wall lubrication forces are denoted by , , , and .
Drag Force
The drag force is the result of hydrodynamic friction between the liquid and the dispersed phase, which means that particles move in liquid face resistance from this source. The drag force is defined as follows:
where the slip velocity is (
) and C
D is the coefficient of drag. C
D is largely determined by the Reynolds number (
Re) for spherical bubbles. The C
D of non-spherical bubbles is determined by the Eötvös number (Eo). A formula for calculating the drag coefficients of various bubble shapes is presented [
28]. When the bubbles’ Reynolds numbers are low, the bubbles retain their spherical shape, and C
D can be determined using
CD,sphere. However, as the bubbles’ Reynolds number is increased, the bubbles deform and take on the appearance of a cap bubble or an ellipse. The CD’s equivalent expression is as follows:
where
denotes the diameter of the bubble and
denotes the settling velocity of the bubble.
Lift Force
A lift force is experienced by the particle when the liquid is flowing at a shear rate or rotates at a rotational rate [
29]. The lift force is the primary cause of the void fraction’s uneven radial distribution in the dispersed phase, which is necessary to take into account when simulating the effect. The general expression is written as [
30]:
where
is the lift force coefficient. Tomiyama et al. [
31] carried out the experiment in an air–water system and found a correlation between the coefficient of lift and a modified Eo number when the change in bubble shape was taken into account. The coefficient of the lift force is defined as:
where
is defined as
.
where
is the modified
number,
is the coefficient of surface tension, and
is the maximum horizontal dimension of the bubble, which can be derived using following equation [
32]:
where
is the Eötvös number. The magnitude of the lift coefficient is proportional to
. When the
is small, the lift coefficient is greater than zero, and the bubbles retain their spherical form and migrate to the pipe wall. At high
, on the other hand, the lift coefficient is smaller than zero, causing the bubbles to deform and flow toward the pipe’s center.
Turbulent Dispersion Force
A consistent distribution of the dispersed phase occurs as a consequence of the force of turbulent dispersion, which takes into account the interaction between turbulent eddies and the particles they contact with.
An equation for the interphase drag term’s average time value may be generated by using the following formula [
33]:
where C
TD is a constant that can be changed by the user and it is set to 1 and σ
TD is the turbulent Prandtl number which is 0.9. The turbulent dynamic viscosity is denoted by µ
t.
Wall Lubrication Force
In order to ensure that the bubbles do not touch the wall, the wall lubrication force acts as a barrier [
34]. The wall lubricating force is expressed as:
where
represents the unit normal that points away from the wall and
denotes the coefficient of wall lubrication force. The following is an expression for C
WL [
34]:
where
is the distance to the wall.
2.2. Species Transport Equation
In this study, to observe the changes in the concentration of carbon dioxide in the gas phase, it is necessary to consider the species transport equation in the gas phase [
25]:
In this equation,
shows the diffusion coefficient,
is the volume fraction of gas that is defined based on C_VOF (cell, gas) function,
is the gas density that is defined based on C_R (cell, gas) function,
is the gas velocity,
is the CO
2 mass fractions in the gas phase, and
is the mass transfer of CO
2 from the gas to liquid phase that is determined by Equation (20) [
25,
35]. As shown schematically in
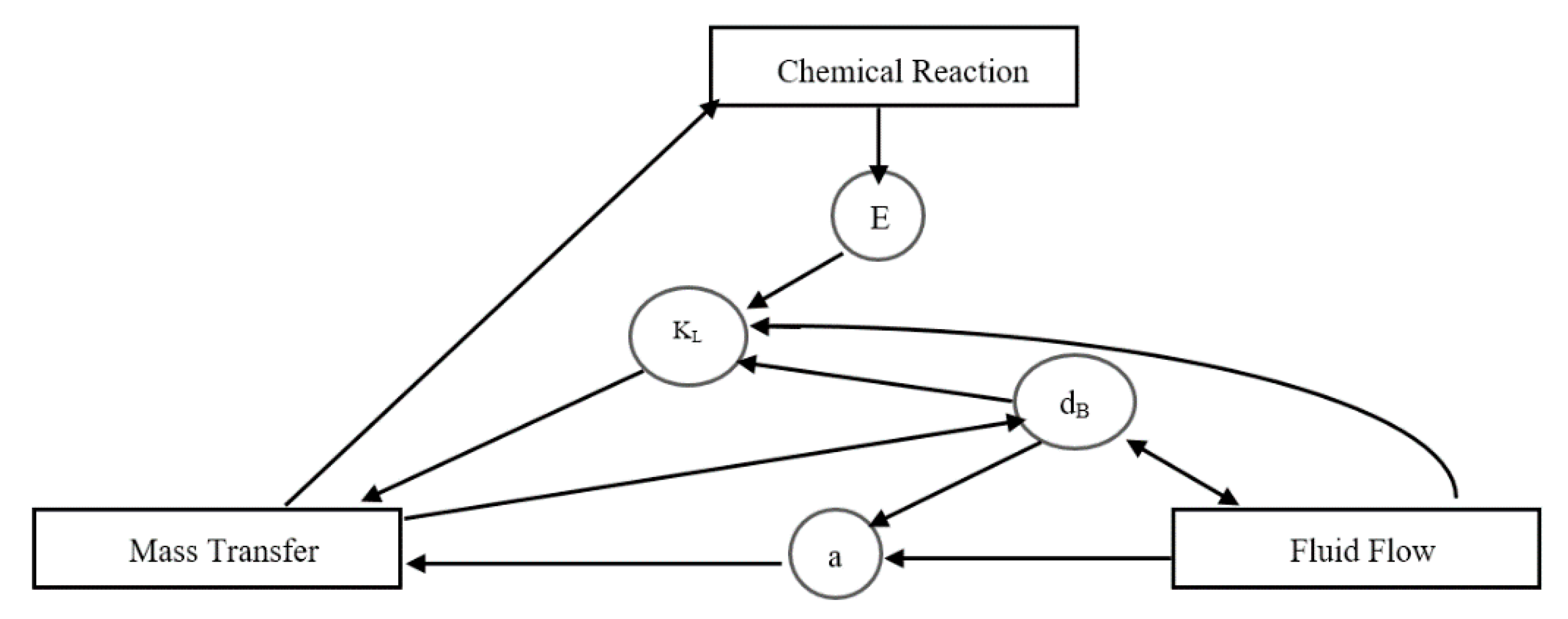
Figure 2, chemical reactions in a gas–liquid system include extremely complicated interconnections between the main processes. The pace of chemical reaction is regulated by the local concentration of the species and the mixing caused by the interphase mass transfer mechanism and scattered bubbles. The mass transfer coefficient, meanwhile, is a function of local hydrodynamics, which is itself impacted by bubble shrinkage as a result of physical or chemical absorption, as well as differences in physical characteristics as a result of inhomogeneous distributions of chemical species [
36].
where
shows the overall mass transfer coefficient of liquid phase,
is interfacial area per unit volume,
is bubble diameter that is equal to 1 mm, and
and
(=0.191) are CO
2 mass fractions in liquid and gas phases, respectively. Due to the point that there is a mass transfer resistance in the liquid phase, with respect to Henry’s law, the mass fraction of liquid phase that is in equilibrium with the mass fraction of the gas phase is calculated by [
25,
35]:
In this study, the value of
(=0.64) has been considered based on the data of an investigation carried out by Penttilä et al. [
37] and
and
are gas and liquid densities that are defined based on C_R (cell, gas) and C_R (cell, liq) functions, respectively.
Additionally, in Equation (6), the enhancement factor
is a constant related to chemical reactions and should be considered in calculations. In this study, this term is determined for modeled system via [
25,
35]:
where
= 1 denotes the stoichiometry coefficient for CO
2 based on the reaction between CO
2 and MEA as follows:
= 4.86 kmol/m
3 is the concentration of MEA; besides, the diffusivity constant for CO
2 in the MEA (
) is calculated by N
2O analogy, that is
m
2/s [
38], while the diffusivity constant for MEA in water (
) is considered based on data of Maceiras et al., which is equal to
m
2/s [
39].
Additionally,
is overall mass transfer coefficient in the liquid phase that is calculated for bubble movement based on the Sherwood relationship by H. Brauer [
40]:
where
is the bubble diameter,
is the diffusivity for CO
2,
is the Reynolds number, and
is the Schmidt number. Thus,
is determined by the following relation:
where
is the relative speed,
is the liquid density, and
is the liquid viscosity.
2.3. Turbulence Model
The turbulence model that is chosen has a significant impact on the outcomes of simulation, since it defines the liquid’s distribution of velocity. By solving two independent transport equations, two-equation turbulence models can determine both a turbulent length and time scale. Since the ejector contains turbulent flows with high Reynolds number, it is better to use standard
and realizable
models for the existing ejector. Because of the rotational effects, the
realizable model is expected to perform better, but the standard
model is chosen because it is simpler and requires less computational time than the
realizable model. The standard
model [
41] is a model based on model transport equations for the turbulence kinetic energy (k) and its dissipation rate (
). The exact equation and physical reasoning are used to derive the model transport equation for k and
, respectively. Transport equations for the standard
model, are given by:
where G
k denotes the kinetic energy generated by turbulence as a result of the average velocity gradients. The buoyancy-induced generation of turbulence kinetic energy is denoted by G
b. In compressible turbulence, Y
M is the effect of the fluctuating dilation. S
K and
are source terms.
= 1.42,
= 1.92,
= 0.09, and
= 0.012 are constants.
= 1 and
= 1.3 are the turbulent Prandtl numbers for k and
. S is the tensor of deformation and S
ij is the mainstream time strain rate. The initial values of turbulent kinetic energy (k) and turbulent dissipation rate (
) for air and water are considered 1 m
2/s
2 and 0.22 m
2/s
3, respectively.
2.4. Geometries, Grids, and Mesh Independency
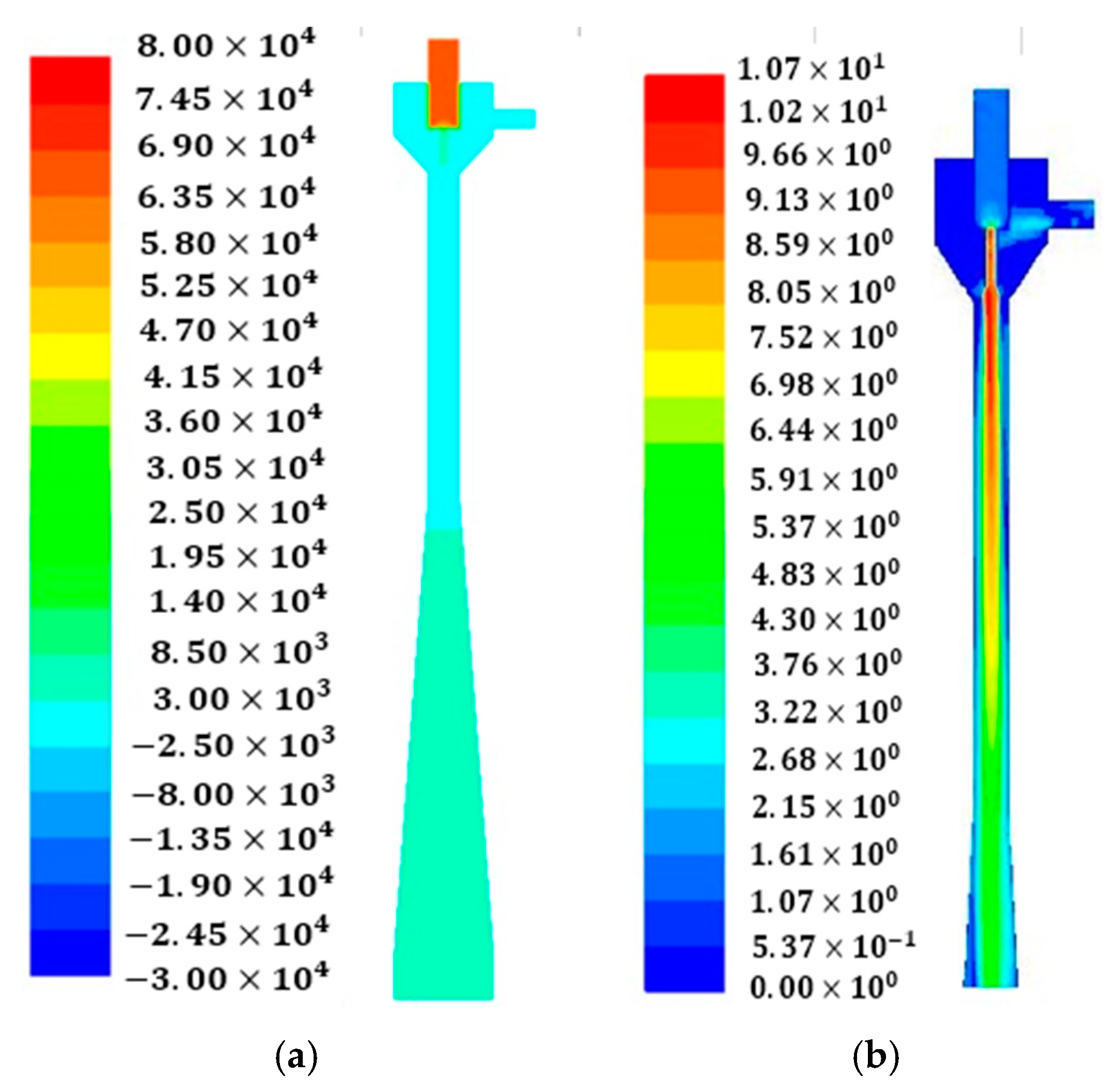
In this study, for the computational domain and grid generation, Fluent Meshing software is used. The dimensions of geometry are shown in
Table 1 and
Figure 3 [
42]. Additionally, 3D and 2D solution domain with uniform mesh is shown in
Figure 3. Due to the mixing shock, the concentration of grid density is centered on the exit of nozzle and also the throat. To acknowledge the stability of numerical results (mesh independency), the ejector with 3D design and d
nozzle = 12 mm is selected. The effect of three sets of grids (i.e., 107,793, 206,537, and 387,987 mesh elements) on the air entrainment rate, Q
g, at motive liquid flow rate (Q
L = 2.17 × 10
−4 m
3/s) is listed in
Table 2. The error percentage is calculated by obtaining air entrainment rate for each grids and comparing it with experimental data [
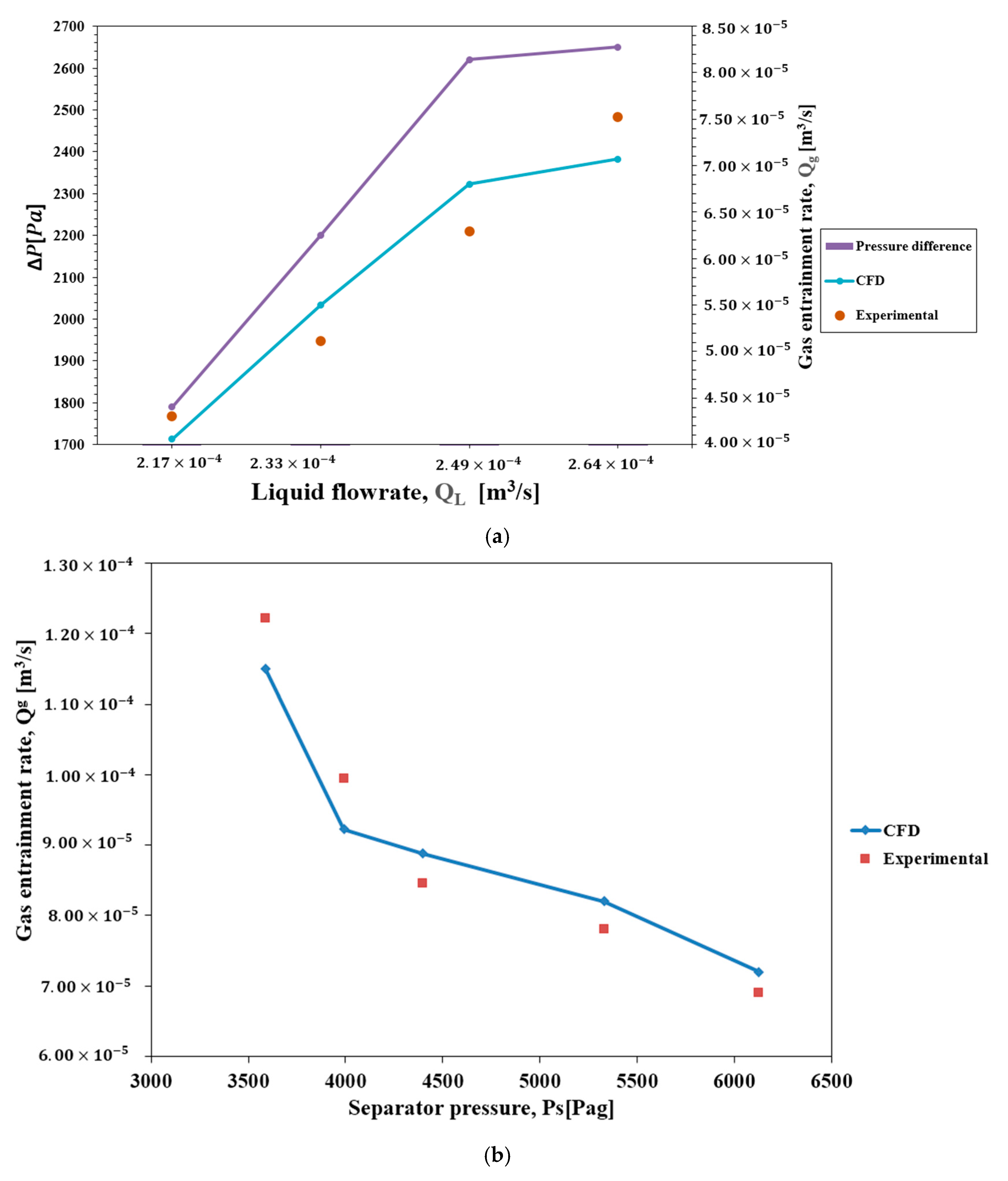
23]. It can be seen that the results achieved for grids with 206,537 and 387,987 cells confirm the stability of numerical results; however, the grid with 206,537 cells needs less computational time than the one with the 387,987 cells. Therefore, the grid with 206,537 cells was selected for this section.
The predicted outcomes could be significantly affected by the bubbles’ diameter. Based on the experimental work done by Mandal [
23], which is the basis of this study, an asymmetry toward larger bubble sizes was observed in the bubble size distributions, which differed from a normal distribution. Thus, according to this paper, we chose d
b = 1 mm as the model parameter for the simulation. It is noted that d
b = 1 mm is also used for the independency of grid.
To investigate the absorption of carbon dioxide by MEA inside the ejector, due to the existence of mass transfer and enhancing the convergence and analyzing time, a 2D-axisymmetric geometry has been considered with a quadrilateral and triangular-meshing scheme. For two-dimensional geometry, similar procedures to the three-dimensional geometry were applied, and the grid with 60,234 cells was selected. The results are shown in
Table 2. Additionally, a 2D solution domain with uniform mesh is shown in
Figure 3.
2.5. Fluid Properties and Boundary Conditions
In this work, for 3D simulation, air and water are, respectively, used as gas and liquid phases; the characteristics of those are listed in
Table 3. A velocity inlet boundary condition is applied to the water inlet zone (nozzle section). Since the other inlet boundary zone for air is open to the atmosphere, the inlet zero-gauge pressure is exerted to this boundary (suction section). According to the research done by Mandal [
23], at the ejector outlet, a 3250 Pa static gauge pressure (
) is used. A no-slip wall boundary condition is specified on the wall. The symmetry boundary condition is introduced for all of perpendicular faces.
For 2D simulation, the mixture of air and CO
2 is used as gas phase and MEA solution is used as liquid phase. The properties of these fluids are shown in
Table 3. The boundary conditions that are considered in the simulation process of 2D ejector are reported in
Table 4.
2.6. Algorithm
All numerical simulations in this study are carried out under non-steady-state conditions. FLUENT 17.0 commercial software is used to predict the distribution of the two phases. Volume fraction is discretized using the QUICK method. The First Order Upwind scheme was used for other equations. Additionally, the SIMPLE scheme was exerted for the pressure–velocity coupling. For all the simulations done in the present work, the convergence criteria for the solutions are considered when the achieved residuals are less than 1 × 10−3. One of the important parameters that affects the convergence criteria of simulation is the under-relaxation factor. The under-relaxation factor minimizes oscillations and contributes to the stability of the computation. In this study, under-relaxation factors for pressure, density, momentum, volume fraction, turbulent kinetic energy, and turbulent dissipation rates are considered 0.3, 1, 0.7, 0.2, 0.8, and 0.8, respectively.
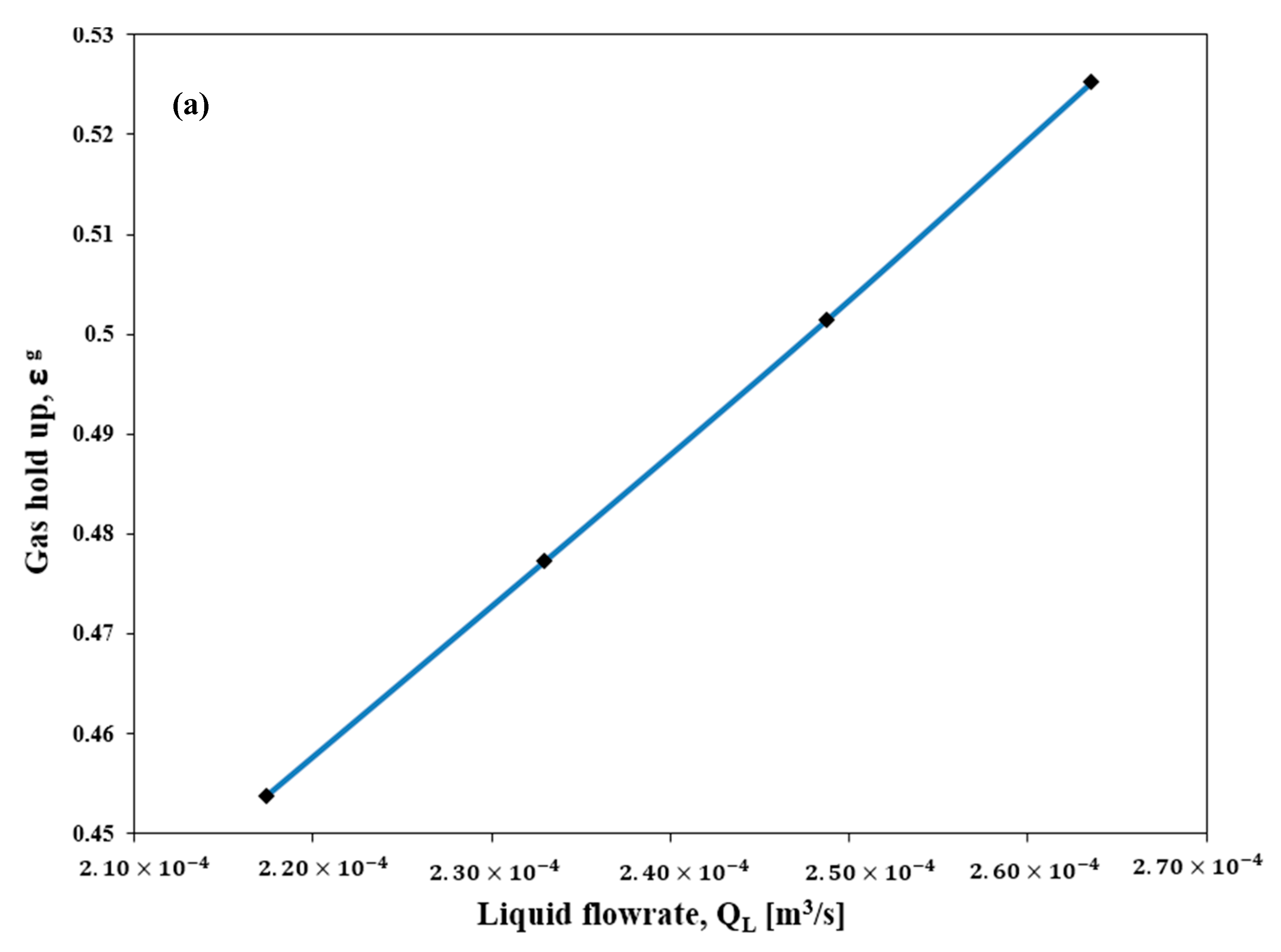
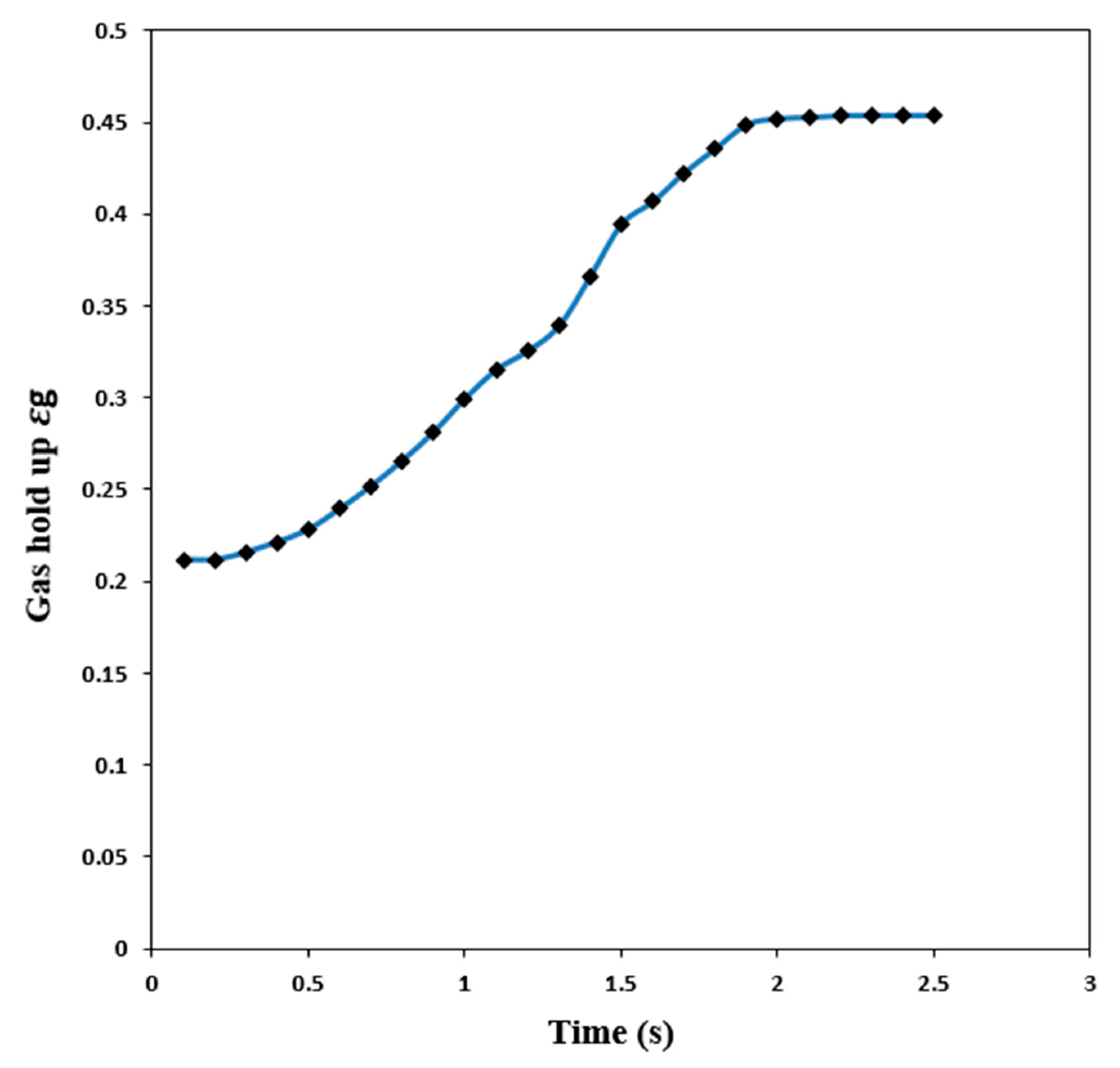
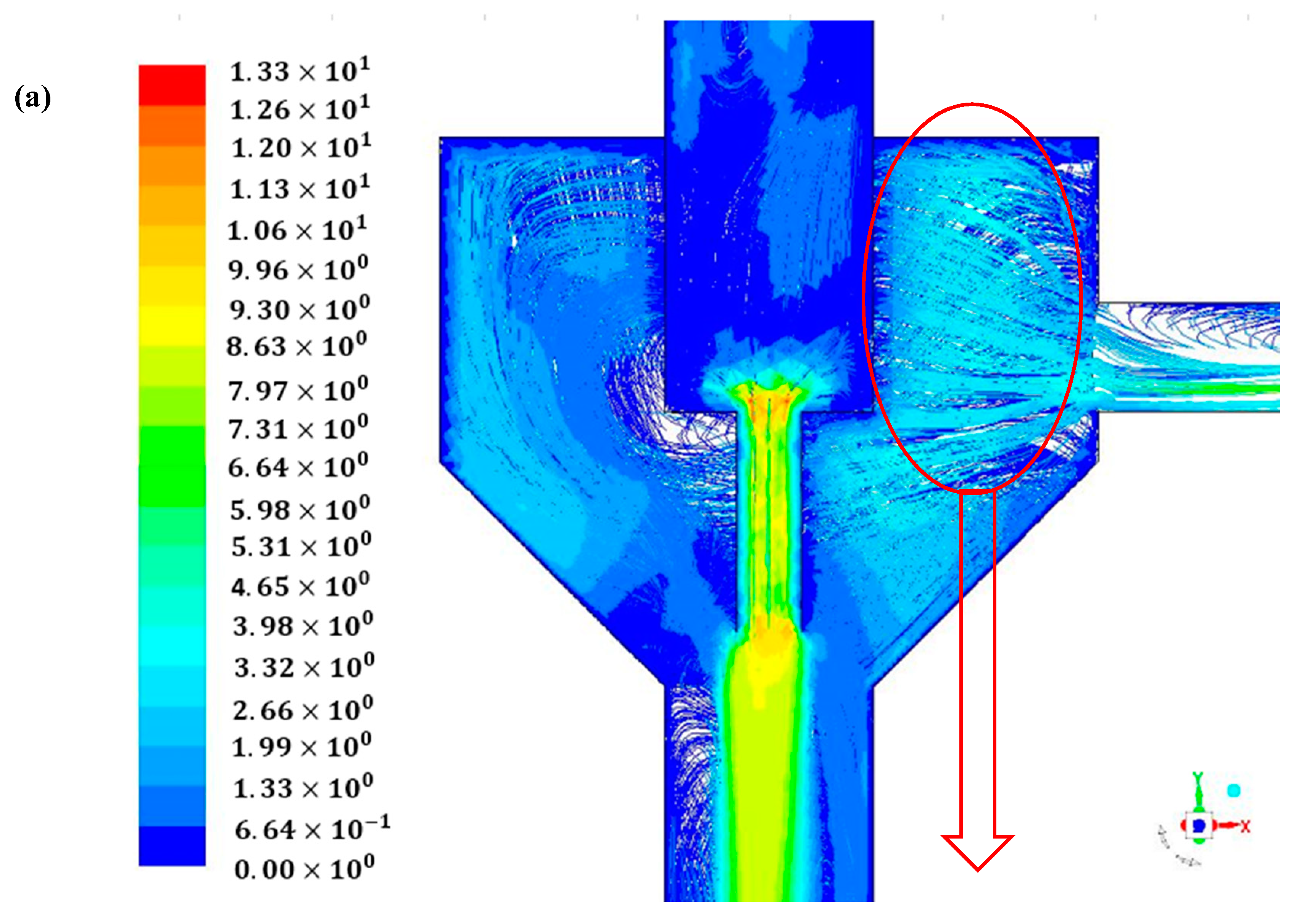
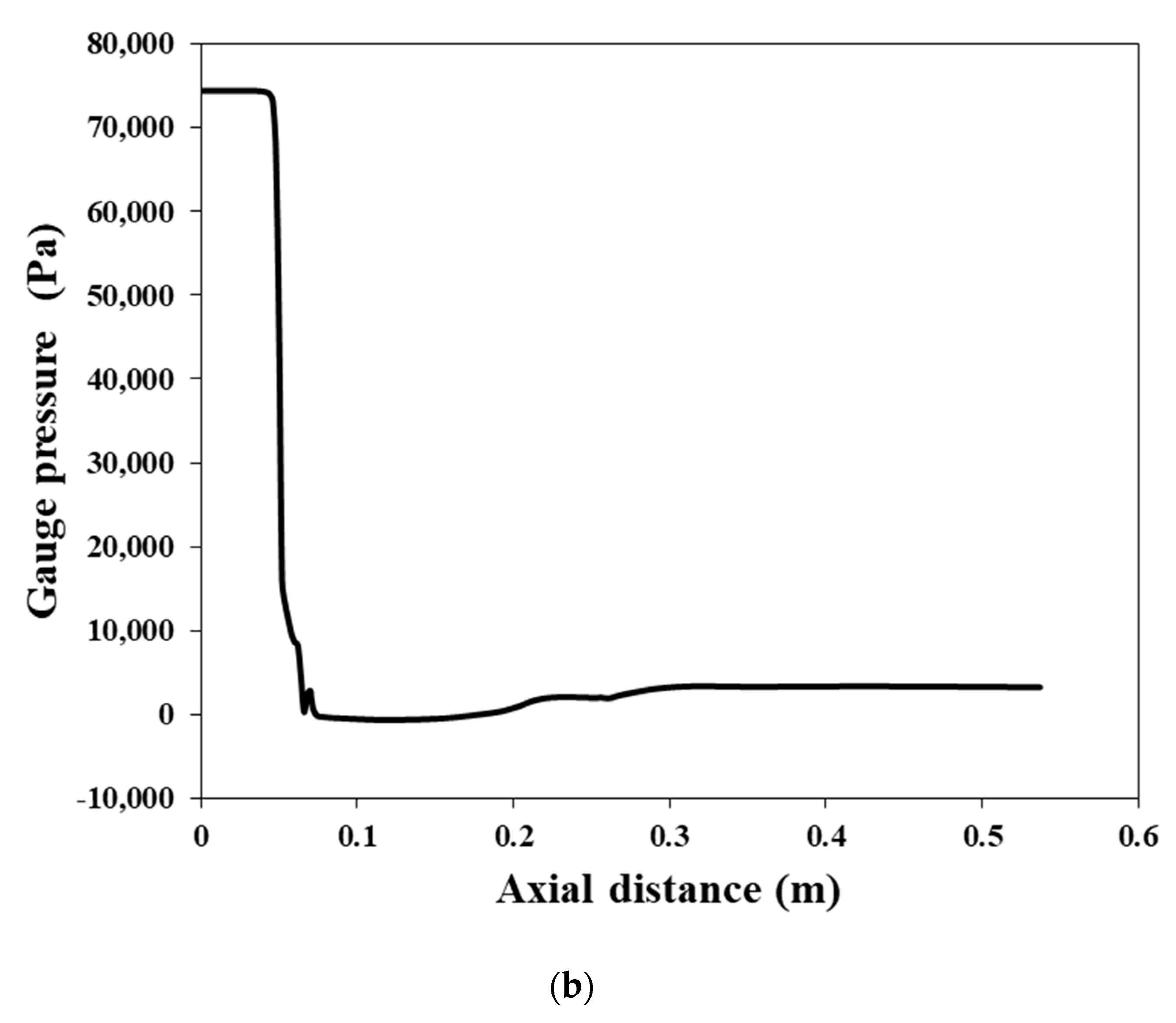
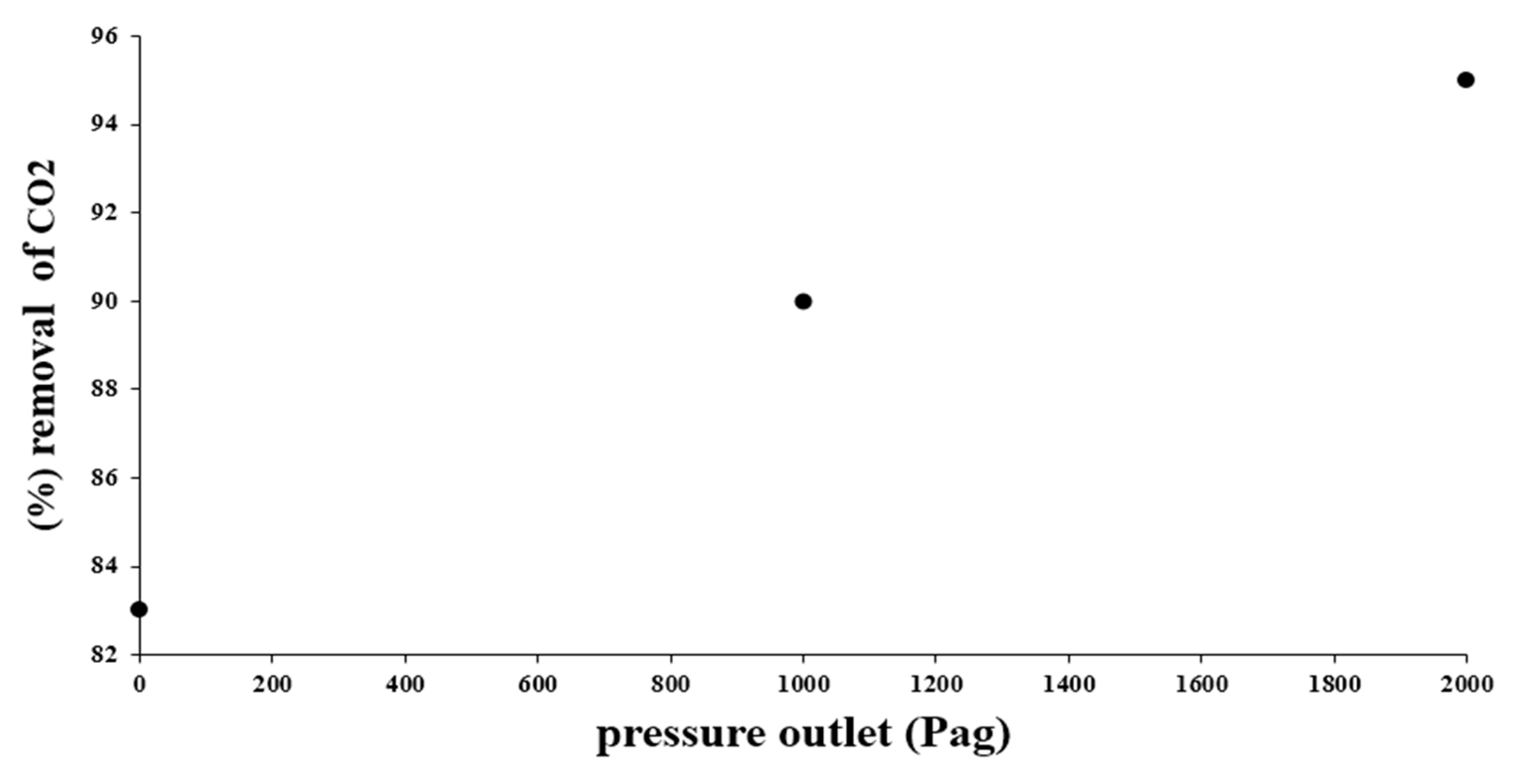
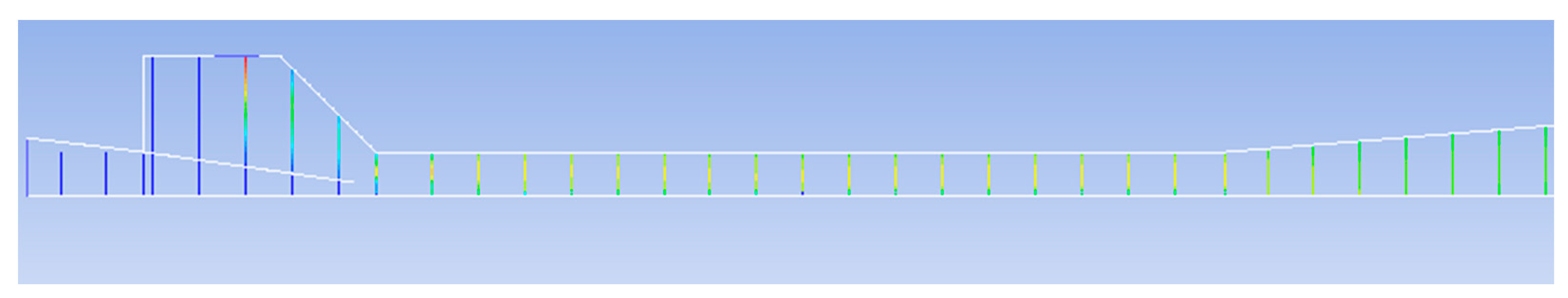
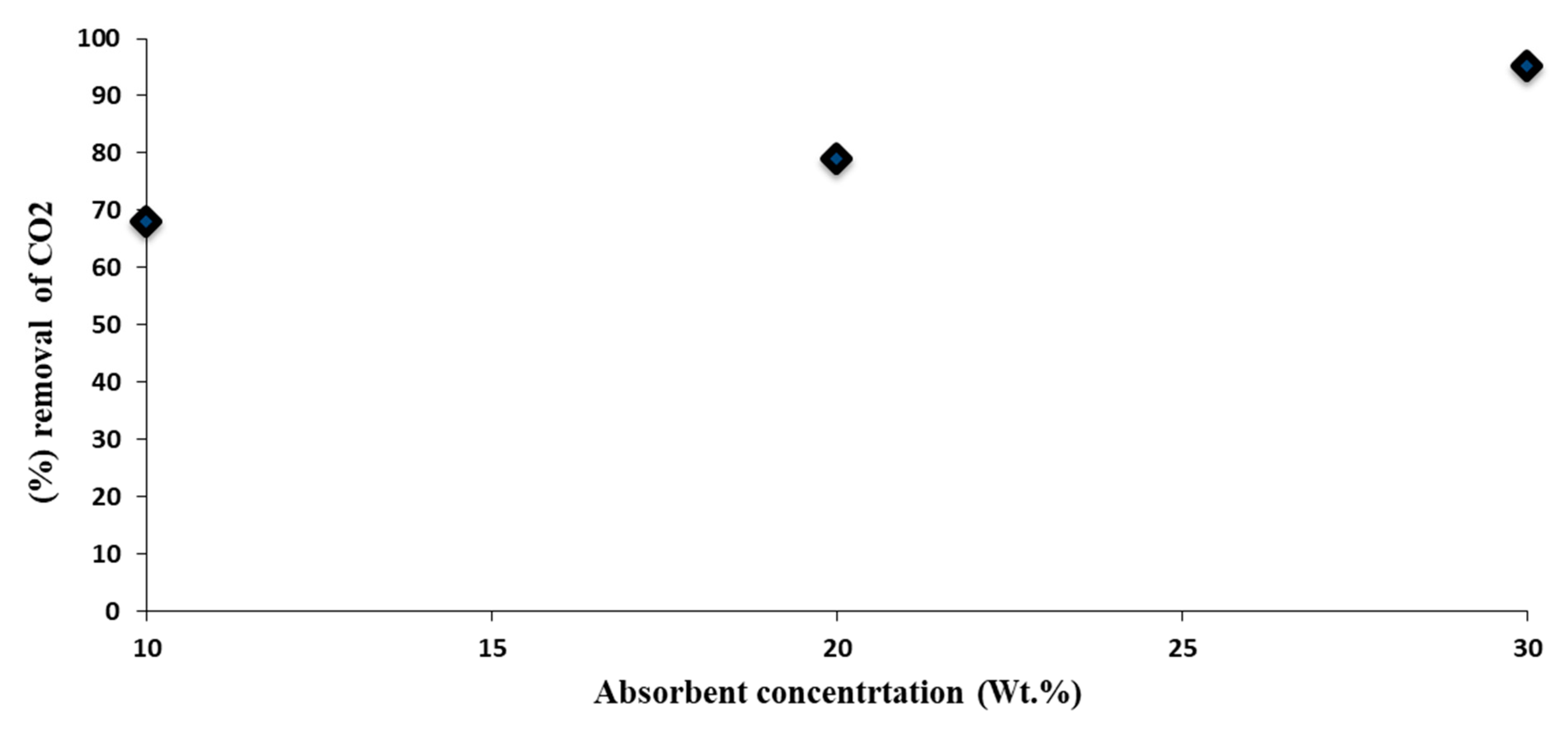
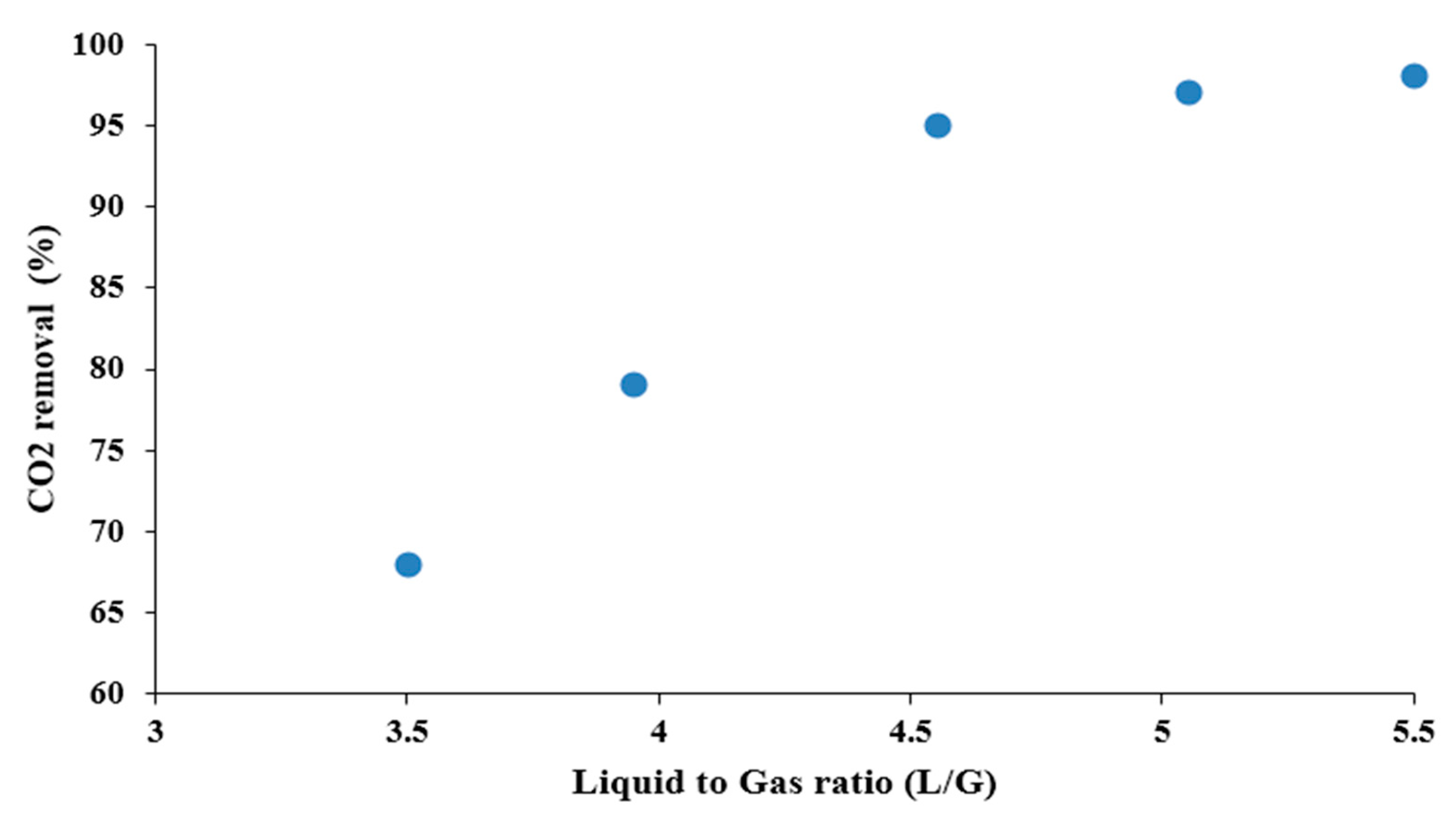
4. Conclusions
A CFD model is developed to illuminate the hydrodynamics and mass transfer characteristics of an ejector by employing the Eulerian–Eulerian multiphase fluid model and Standard k- model with 2D (air/CO2-MEA solution) and 3D (air–water) modeling. For 3D geometry, the model was validated initially by altering the rate of liquid fluid flow in the range of 2.17 × 10−4 to 2.64 × 10−4 m3/s and outlet pressure in the range of 3000 to 6500 Pa, then the effects of these two parameters on gas phase hold-up, recirculation, the location of mixing shock, and negative pressure were investigated. Experimental data and CFD results are in good agreement. Mixing shock and position of its occurrence were investigated in this study as important factors. This three-dimensional phenomenon could be explained satisfactorily based on the results obtained from the 3D simulation. For 2D geometry and by considering the User-Defined Functions code, the effect of different parameters on CO2 removal was considered. The following are the main findings: for the 3D ejector, it is found that with increasing the outlet pressure by about 70%, the rate of gas entrainment and gas hold up decrease by about 37% and 20%, respectively. The mentioned parameters showed increasing behavior of about 74% and 15%, respectively, when the mass flow rate of the liquid increased from 2.17 × 10−4 to 2.64 × 10−4 m3/s. It is observed that the location of mixing shock comes about at the end of the mixing tube. With an increasing mass flow rate of the liquid, the mixing shock is transferred from the ejector’s throat to the diffuser side. A greater negative pressure is observed when the mass flow rate of the liquid is increased. 2D ejector’s findings showed that %CO2 removal is increased with increasing outlet pressure, absorbent concentration, and liquid-to-gas ratio.