Bottle Aging Affected Aromatic and Phenolic Wine Composition More than Yeast Starter Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Winemaking and Aging in Bottles
2.2. Determination of Must and Wine Classical Parameters
2.3. Analysis of Wine Volatile Compounds by GC-MS
2.4. Determination of Wine Phenolic Compounds by HPLC-DAD
2.4.1. Sample Preparation for the Analysis of Non-Anthocyanin Phenolic Compounds
2.4.2. Analysis of Phenolic Compounds by HPLC-DAD
2.5. Statistical Analysis
3. Results and Discussion
3.1. Kinetics of Fermentation and Wine Enological Parameters
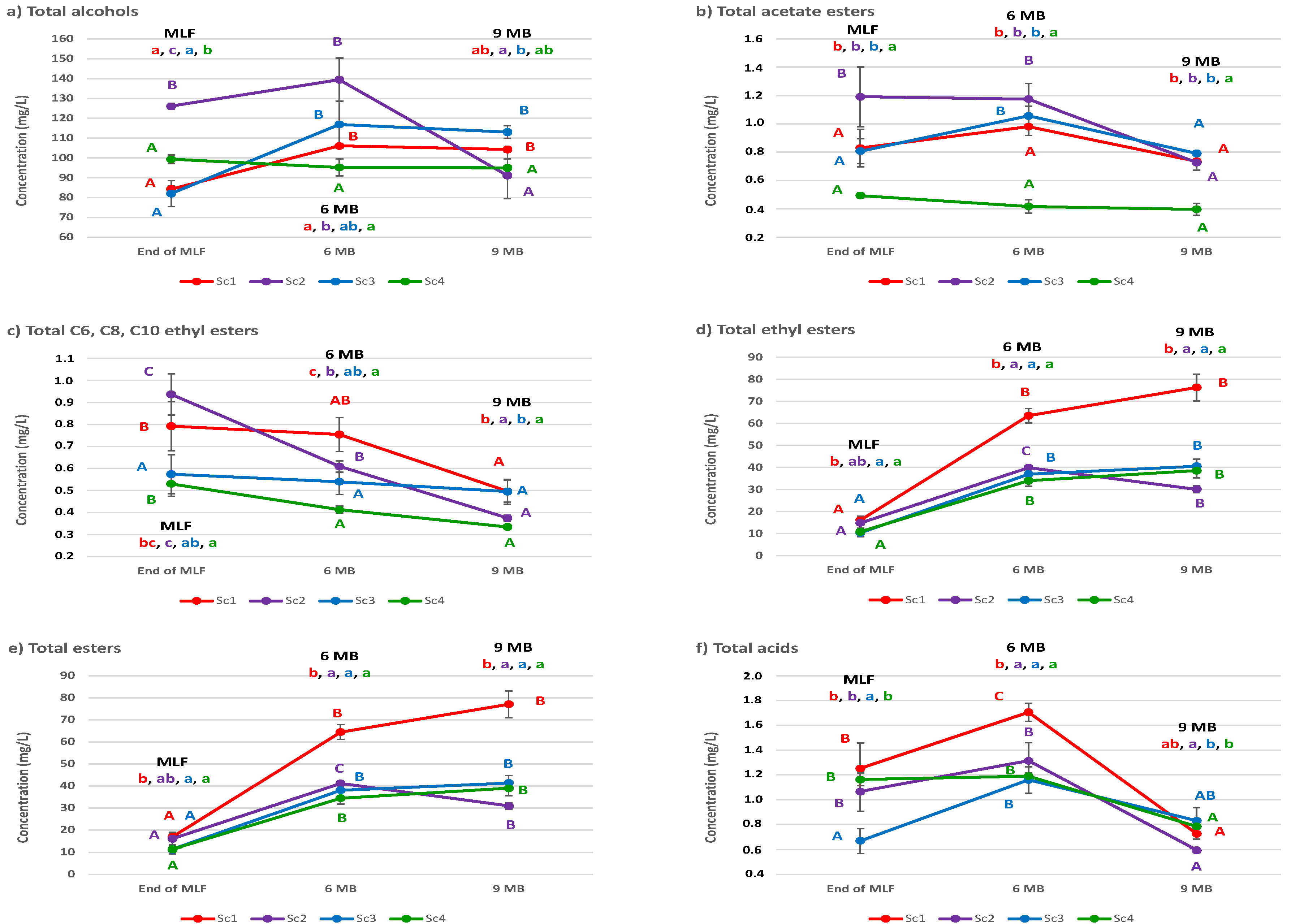
3.2. Wine Aromatic Composition
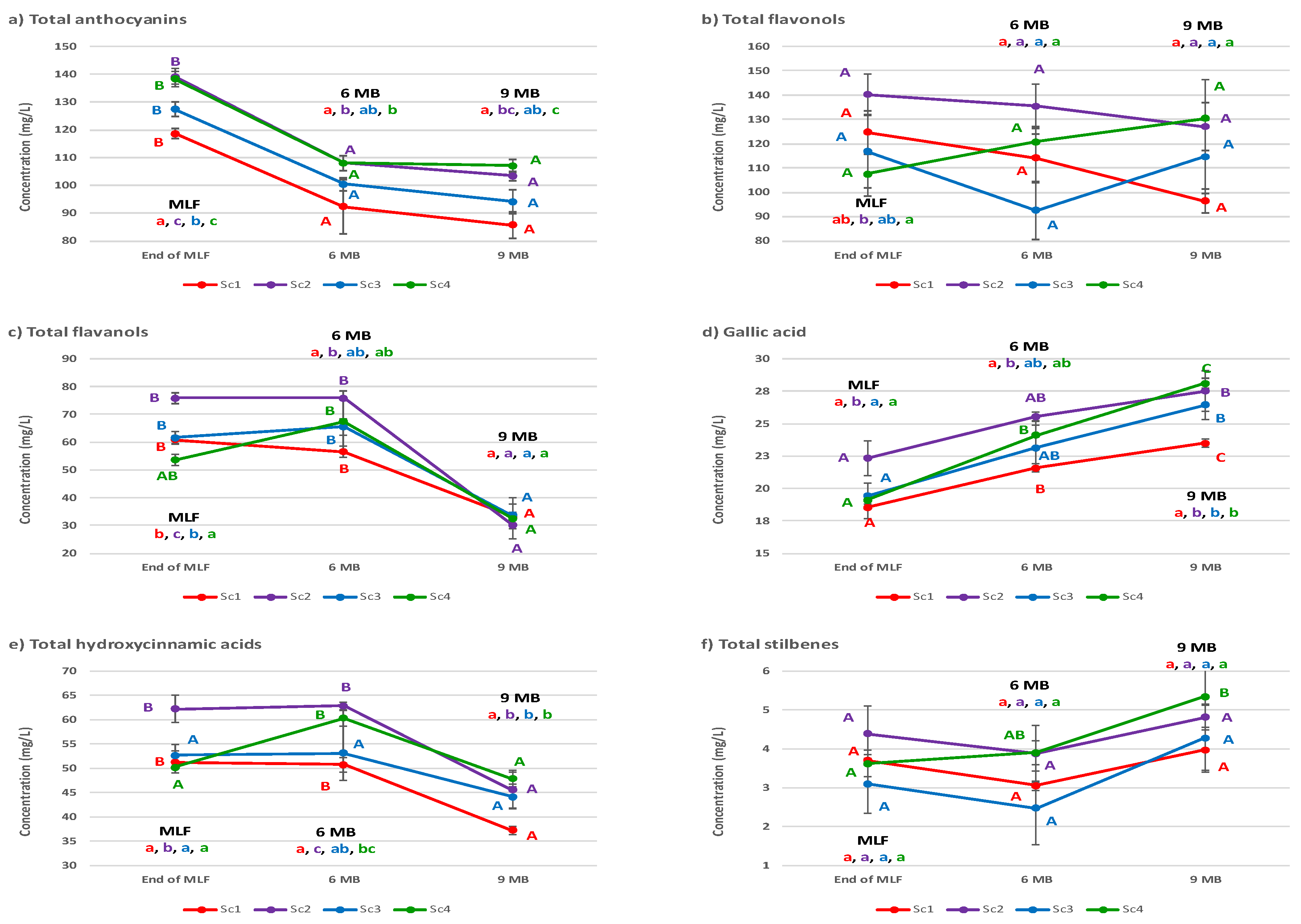
3.3. Wine Phenolic Composition
3.4. Discriminat and Factorial Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of yeast species and strains in wine flavor. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Ugliano, M.; Henschke, P.A. Yeasts and wine flavor. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 313–392. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary aroma: Influence of wine microorganisms in their aroma profile. Foods 2021, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Morales, P. Truth in wine yeast. Microb. Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Contribution of wild yeasts to the formation of volatile compounds in inoculated wine fermentations. Eur. Food Res. Technol. 2006, 222, 15–25. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Garde-Cerdán, T.; Rubio-Bretón, P.; Marín-San Román, S.; Baroja, E.; Sáenz de Urturi, I.; Pérez-Álvarez, E.P. Study of wine volatile composition of Tempranillo versus Tempranillo Blanco, a new white grape variety. Beverages 2021, 7, 72. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Intrigliolo, D.S.; Almajano, M.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Effects of water deficit irrigation on phenolic composition and antioxidant activity of Monastrell grapes under semiarid conditions. Antioxidants 2021, 10, 1301. [Google Scholar] [CrossRef]
- Osete-Alcaraz, A.; Gómez-Plaza, E.; Pérez-Porras, P.; Bautista-Ortín, A.B. Revisiting the use of pectinases in enology: A role beyond facilitating phenolic grape extraction. Food Chem. 2022, 372, 131282. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; García-Estévez, I. Wine color evolution and stability. In Red Wine Technology; Morata, A., Ed.; Elsevier: London, UK, 2018; pp. 195–205. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, W.; Meng, Y.; Zhang, Y.; Jin, G.; Fang, Z. Wine phenolic profile altered by yeast: Mechanisms and influences. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3579–3619. [Google Scholar] [CrossRef]
- Fernández-Espinar, M.T.; López, V.; Ramón, D.; Bartra, E.; Querol, A. Study of the authenticity of commercial wine yeast strains by molecular techniques. Int. J. Food Microbiol. 2001, 70, 1–10. [Google Scholar] [CrossRef]
- Borneman, A.; Forgan, A.H.; Kolouchova, R.; Fraser, J.A.; Schmidt, S.A. Whole genome comparison reveals high levels of inbreeding and strain redundancy across the spectrum of commercial wine strains of Saccharomyces cerevisiae. G3-Genes Genomes Genet. 2016, 6, 957–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Falcón, M.S.; Pérez-Lamela, C.; Martínez-Carballo, E.; Simal-Gándara, J. Determination of phenolic compounds in wines: Influence of bottle storage of young red wines on their evolution. Food Chem. 2007, 105, 248–259. [Google Scholar] [CrossRef]
- Echave, J.; Barral, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Bottle aging and storage of wines: A review. Molecules 2021, 26, 713. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Coello, M.S.; González-Viñas, M.A.; Garća-Romero, E.; Díaz-Maroto, M.C.; Cabezudo, M.D. Influence of storage temperature on the volatile compounds of young white wines. Food Control 2003, 14, 301–306. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of SO2 on the formation and evolution of volatile compounds in wines. Food Control 2007, 18, 1501–1506. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Marsellés-Fontanet, A.R.; Arias-Gil, M.; Ancín-Azpilicueta, C.; Martín-Belloso, O. Effect of storage conditions on the volatile composition of wines obtained from must stabilized by PEF during ageing without SO2. Innov. Food Sci. Emerg. Technol. 2008, 9, 469–476. [Google Scholar] [CrossRef]
- Giuffrida de Esteban, M.L.; Ubeda, C.; Heredia, F.J.; Catania, A.A.; Assof, M.V.; Fanzone, M.L.; Jofre, V.P. Impact of closure type and storage temperature on chemical and sensory composition of Malbec wines (Mendoza, Argentina) during aging in bottle. Food Res. Int. 2019, 125, 108553. [Google Scholar] [CrossRef]
- Pati, S.; Crupi, P.; Savastano, M.L.; Benucci, I.; Esti, M. Evolution of phenolic and volatile compounds during bottle storage of a white wine without added sulfite. J. Sci. Food Agric. 2020, 100, 775–784. [Google Scholar] [CrossRef]
- OIV. Compendium of Internationals Methods of Wine and Must Analysis; OIV: Paris, France, 2016. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology. The Chemistry of Wines, Stabilization and Treatments; John Wiley: Chichester, UK, 2000. [Google Scholar]
- Portu, J.; López-Alfaro, I.; Gómez-Alonso, S.; López, R.; Garde-Cerdán, T. Changes on grape phenolic composition induced by grapevine foliar applications of phenylalanine and urea. Food Chem. 2015, 180, 171–180. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Fernández-González, M.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. J. Agric. Food Chem. 2009, 57, 7883–7891. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.; Peinado, R.A.; Medina, M.; Moreno, J. Higher alcohols concentration and its relation with the biological aging evolution. Eur. Food Res. Technol. 2015, 222, 629–635. [Google Scholar] [CrossRef]
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Mauriello, G.; Capece, A.; D’Auria, M.; Garde-Cerdán, T.; Romano, P. SPME-GC method as a tool to differentiate VOC profiles in Saccharomyces cerevisiae wine yeasts. Food Microbiol. 2009, 26, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Bisson, L.F.; Karpel, J.E. Genetics of yeast impacting wine quality. Annu. Rev. Food Sci. Technol. 2010, 1, 139–162. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; Alegría, E.G.; Polo, M.C.; Tenorio, C.; Martín-Álvarez, P.J.; Calvo de la Banda, M.T.; Ruiz-Larrea, F.; Moreno-Arribas, M.V. Wine volatile and amino acid composition after malolactic fermentation: Effect of Oenococcus oeni and Lactobacillus plantarum starter cultures. J. Agric. Food Chem. 2005, 53, 8729–8735. [Google Scholar] [CrossRef]
- Gómez Gallego, M.A.; Gómez García-Carpintero, E.; Sánchez-Palomo, E.; González Viñas, M.A.; Hermosín-Gutiérrez, I. Evolution of the phenolic content, chromatic characteristics and sensory properties during bottle storage of red single-cultivar wines from Castilla La Mancha region. Food Res. Int. 2013, 51, 554–563. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Martínez-Vidaurre, J.M.; Garde-Cerdán, T. Anthocyanin composition of grapes from three different soil types in cv. Tempranillo A.O.C. Rioja vineyards. J. Sci. Food Agric. 2019, 99, 4833–4841. [Google Scholar] [CrossRef]
- Hermosín-Gutiérrez, I.; Sánchez-Palomo, E.; Vicario-Espinosa, A. Phenolic composition and magnitude of copigmentation in young and shortly aged red wines made from the cultivars, Cabernet Sauvignon, Cencibel, and Syrah. Food Chem. 2005, 92, 269–283. [Google Scholar] [CrossRef]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Evolution of polyphenols in red wines from Vitis vinifera L. During aging in bottle. I. Anthocyanins and pyranoanthocyanins. Eur. Food Res. Technol. 2005, 220, 607–614. [Google Scholar] [CrossRef]
- de Souza, J.F.; Nascimento, A.M.S.; Linhares, M.D.S.S.; Dutra, M.D.C.P.; Lima, M.S.; Pereira, G.E. Evolution of phenolic compound profiles and antioxidant activity of Syrah red and sparkling Moscatel wines stored in bottles of different colors. Beverages 2018, 4, 89. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Navarro, J.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Martínez-Gascueña, J.; Chacón-Vozmediano, J.L.; García-Romero, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S. Genotypic variation in phenolic composition of novel white grape genotypes (Vitis vinifera L.). J. Food Compos. Anal. 2021, 102, 103987. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Compos. Anal. 2007, 20, 618–626. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Uzkuç, N.M.Ç.; Bayhan, A.; Toklucu, A.K. Phenolics and color components of young Cabernet Sauvignon wines: Effect of spontaneous fermentation and bottle storage. Eur. Food Res. Technol. 2022. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.-N.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Lorenzo, C.; Carot, J.M.; Esteve, M.D.; Climent, M.D.; Salinas, M.R. Effects of composition, storage time, geographic origin and oak type on the accumulation of some volatile oak compounds and ethylphenols in wines. Food Chem. 2010, 122, 1076–1082. [Google Scholar] [CrossRef]
- Koh, Y.-C.; Ho, C.-T.; Pan, M.-H. Recent advances in health benefits of stilbenoids. J. Agric. Food Chem. 2021, 69, 10036–10057. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Hornedo-Ortega, R.; Garcia, F.; El Khawand, T.; Saucier, C.; Richard, T. Stilbenes in grape berries and wine and their potential role as anti-obesity agents: A review. Trends Food Sci. Technol. 2021, 112, 362–381. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Manzano, S.; González-Paramás, A.M. Wine, polyphenols, and Mediterranean diets. What else is there to say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef]
- Denat, M.; Perez, D.; Heras, J.M.; Querol, A.; Ferreira, V. The effects of Saccharomyces cerevisiae strains carrying alcoholic fermentation on the fermentative and varietal aroma profiles of young and aged Tempranillo wines. Food Chem. X 2021, 9, 100116. [Google Scholar] [CrossRef] [PubMed]
- Denat, M.; Ontanon, I.; Querol, A.; Ferreira, V. The diverse effects of yeast on the aroma of non-sulfite added white wines throughout aging. LWT-Food Sci. Technol. 2022, 158, 113111. [Google Scholar] [CrossRef]
- Gammacurta, M.; Marchand, S.; Albertin, W.; Moine, V.; de Revel, G. Impact of yeast strain on ester levels and fruity aroma persistence during aging of Bordeaux red wines. J. Agric. Food Chem. 2014, 62, 5378–5389. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [Green Version]
- Jagati´c Korenika, A.-M.; Tomaz, I.; Preiner, D.; Plichta, V.; Jeromel, A. Impact of commercial yeasts on phenolic profile of Plavac Mali wines from Croatia. Fermentation 2021, 7, 92. [Google Scholar] [CrossRef]
- Carew, A.L.; Smith, P.; Close, D.C.; Curtin, C.; Dambergs, R.G. Yeast effects on Pinot noir wine phenolics, color, and tannin composition. J. Agric. Food Chem. 2013, 61, 9892–9898. [Google Scholar] [CrossRef]
- Caridi, A.; De Bruno, A.; De Salvo, E.; Piscopo, A.; Poiana, M.; Sidari, R. Selected yeasts to enhance phenolic content and quality in red wine from low pigmented grapes. Eur. Food Res. Technol. 2017, 243, 367–378. [Google Scholar] [CrossRef]



| Sc1 | Sc2 | Sc3 | Sc4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| End of MLF | 6 MB | 9 MB | End of MLF | 6 MB | 9 MB | End of MLF | 6 MB | 9 MB | End of MLF | 6 MB | 9 MB | |
| Alcohol degree (% v/v) | 13.8 ± 0.1 b | - | - | 13.4 ± 0.0 ab | - | - | 13.1 ± 0.3 a | - | - | 13.6 ± 0.2 ab | - | - |
| pH | 4.04 ± 0.06 a | - | - | 4.14 ± 0.02 a | - | - | 4.13 ± 0.08 a | - | - | 4.15 ± 0.00 a | - | - |
| Total acidity (g/L) * | 5.10 ± 0.11 b | - | - | 3.83 ± 0.21 a | - | - | 3.77 ± 0.24 a | - | - | 4.05 ± 0.11 a | - | - |
| Malic acid (g/L) | n.d. | - | - | n.d. | - | - | n.d. | - | - | n.d. | - | - |
| Lactic acid (g/L) | 2.62 ± 0.10 c | - | - | 2.26 ± 0.11 b | - | - | 2.15 ± 0.05 b | - | - | 1.90 ± 0.03 a | - | - |
| Volatile acidity (g/L) ** | 0.50 ± 0.02 A, b | 0.46 ± 0.00 A, b | 0.49 ± 0.04 A, b | 0.33 ± 0.04 A, a | 0.35 ± 0.01 A, a | 0.34 ± 0.01 A, a | 0.48 ± 0.07 A, b | 0.48 ± 0.07 A, b | 0.49 ± 0.10 A, b | 0.27 ± 0.01 A, a | 0.28 ± 0.01 A, a | 0.30 ± 0.01 A, a |
| YAN (mg N/L) | 8.50 ± 1.98 a | - | - | 21.5 ± 11.8 a | - | - | 9.50 ± 0.8 a | - | - | 22.5 ± 12.6 a | - | - |
| OD 420 nm | 0.24 ± 0.01 A, b | 0.28 ± 0.02 AB, a | 0.30 ± 0.02 B, a | 0.24 ± 0.01 A, ab | 0.29 ± 0.01 B, a | 0.30 ± 0.00 B, a | 0.22 ± 0.00 A, a | 0.27 ± 0.00 B, a | 0.29 ± 0.00 C, a | 0.23 ± 0.01 A, ab | 0.27 ± 0.01 B, a | 0.29 ± 0.01 B, a |
| OD 520 nm | 0.30 ± 0.02 A, b | 0.36 ± 0.04 A, a | 0.37 ± 0.05 A, a | 0.29 ± 0.02 A, ab | 0.35 ± 0.01 B, a | 0.35 ± 0.01 B, a | 0.25 ± 0.01 A, a | 0.34 ± 0.01 B, a | 0.35 ± 0.03 B, a | 0.29 ± 0.01 A, ab | 0.34 ± 0.00 B, a | 0.35 ± 0.01 B, a |
| OD 620 nm | 0.07 ± 0.00 A, b | 0.11 ± 0.01 B, a | 0.12 ± 0.01 B, a | 0.06 ± 0.00 A, ab | 0.11 ± 0.00 B, a | 0.12 ± 0.00 C, a | 0.06 ± 0.00 A, a | 0.11 ± 0.00 B, a | 0.12 ± 0.00 C, a | 0.06 ± 0.00 A, ab | 0.11 ± 0.00 B, a | 0.12 ± 0.00 C, a |
| Color intensity (CI) | 6.11 ± 0.27 A, b | 7.51 ± 0.74 A, a | 8.00 ± 0.74 A, a | 5.92 ± 0.35 A, ab | 7.47 ± 0.22 B, a | 7.69 ± 0.16 B, a | 5.25 ± 0.07 A, a | 7.19 ± 0.11 B, a | 7.51 ± 0.28 B, a | 5.76 ± 0.25 A, ab | 7.22 ± 0.13 B, a | 7.64 ± 0.29 B, a |
| TPI | 43.7 ± 0.7 A, a | 43.4 ± 0.4 A, a | 42.0 ± 0.5 A, a | 49.2 ± 1.4 A, b | 48.9 ± 1.1 A, b | 47.3 ± 1.0 A, b | 44.7 ± 0.1 A, a | 44.7 ± 1.1 A, a | 42.8 ± 0.6 A, a | 50.0 ± 1.2 A, b | 49.2 ± 1.7 A, b | 48.0 ± 2.5 A, b |
| Total polyphenols (mg/L) | 1719 ± 64 B, a | 1625 ± 15 AB, a | 1537 ± 11 A, a | 2086 ± 226 A, a | 1927 ± 59 A, b | 1767 ± 47 A, b | 1778 ± 54 B, a | 1692 ± 14 B, a | 1542 ± 28 A, a | 2046 ± 144 A, a | 1928 ± 66 A, b | 1769 ± 77 A, b |
| Yeast Strain (%) | Aging Time (%) | YS × AT (%) | Residual (%) | |
|---|---|---|---|---|
| Total alcohols | 26.9 *** | 17.8 *** | 48.0 *** | 7.24 |
| Total acetate esters | 68.6 *** | 14.7 *** | 11.0 * | 5.71 |
| Total C6, C8, C10 ethyl esters | 31.3 *** | 42.8 *** | 19.4 ** | 6.48 |
| Total ethyl esters | 27.0 *** | 59.3 *** | 12.6 *** | 1.12 |
| Total esters | 27.1 *** | 59.0 *** | 12.7 *** | 1.16 |
| Total acids | 15.0 *** | 60.5 *** | 19.2 ** | 5.38 |
| Total anthocyanins | 20.8 *** | 76.1 *** | 0.46 ns | 2.66 |
| Total flavonols | 34.0 * | 2.72 ns | 28.6 ns | 34.7 |
| Total flavanols | 5.87 ** | 83.2 *** | 7.63 * | 3.33 |
| Gallic acid | 19.8 *** | 70.8 *** | 4.48 ns | 4.92 |
| Total hydroxycinnamic acids | 26.2 *** | 56.4 *** | 11.5 * | 5.88 |
| Total stilbenes | 28.1 * | 38.0 ** | 0.63 ns | 24.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garde-Cerdán, T.; Sáenz de Urturi, I.; Murillo-Peña, R.; Iribarren, M.; Marín-San Román, S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Bottle Aging Affected Aromatic and Phenolic Wine Composition More than Yeast Starter Strains. Appl. Sci. 2022, 12, 4478. https://doi.org/10.3390/app12094478
Garde-Cerdán T, Sáenz de Urturi I, Murillo-Peña R, Iribarren M, Marín-San Román S, Rubio-Bretón P, Pérez-Álvarez EP. Bottle Aging Affected Aromatic and Phenolic Wine Composition More than Yeast Starter Strains. Applied Sciences. 2022; 12(9):4478. https://doi.org/10.3390/app12094478
Chicago/Turabian StyleGarde-Cerdán, Teresa, Itziar Sáenz de Urturi, Rebeca Murillo-Peña, Miquel Iribarren, Sandra Marín-San Román, Pilar Rubio-Bretón, and Eva P. Pérez-Álvarez. 2022. "Bottle Aging Affected Aromatic and Phenolic Wine Composition More than Yeast Starter Strains" Applied Sciences 12, no. 9: 4478. https://doi.org/10.3390/app12094478
APA StyleGarde-Cerdán, T., Sáenz de Urturi, I., Murillo-Peña, R., Iribarren, M., Marín-San Román, S., Rubio-Bretón, P., & Pérez-Álvarez, E. P. (2022). Bottle Aging Affected Aromatic and Phenolic Wine Composition More than Yeast Starter Strains. Applied Sciences, 12(9), 4478. https://doi.org/10.3390/app12094478






