How Does the Lumbopelvic Complex Cope with the Obstetrical Load during Standing? Ergonomic Aspects of Body Posture in Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Musculoskeletal Model of a Pregnant Woman and Simulation
- VARIANT I—taking into account a change in the body mass in the following trimesters of pregnancy;
- VARIANT II—taking into account a change in the body mass and alignment of individual body segments as a consequence of changing the angle of pelvic inclination in the sagittal plane in the following trimesters of pregnancy.
2.2. Data Analysis
- Lordosis in the lumbar spine—defined as the angle of the bending of the lumbar lordosis between the line located parallel to the upper vertebral body L1, and the line of extension of the lower edge of vertebral body L5;
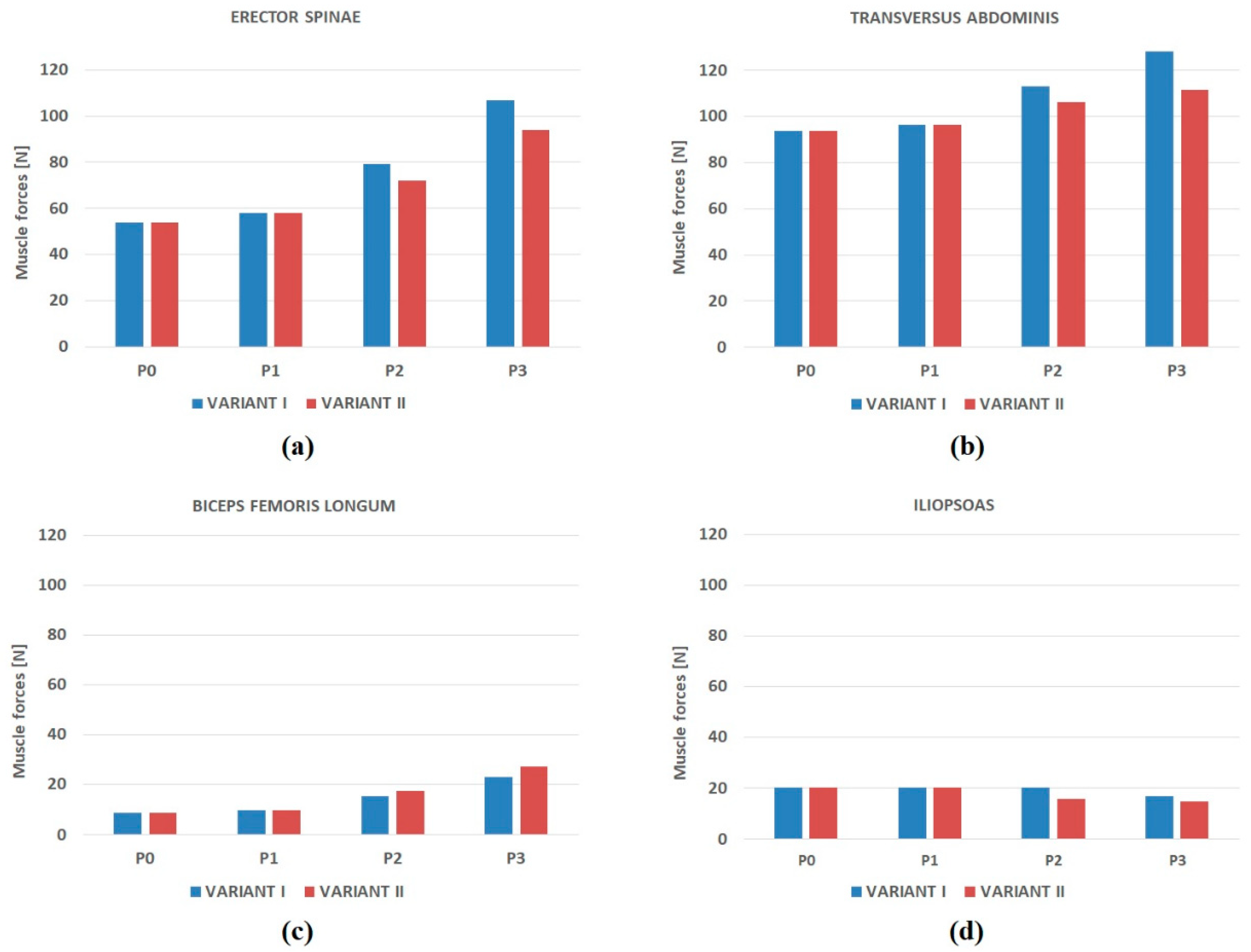
- Muscular forces generated in the locomotor system during the adoption of a standing position by the female (i.e., erector spinae, transverse abdominal muscle, as well as the group of the flexors and extensors of the hip joint);
- Muscular fatigue expressed by the value of the optimisation task objective function; the higher the function value, the higher the muscular fatigue [51];
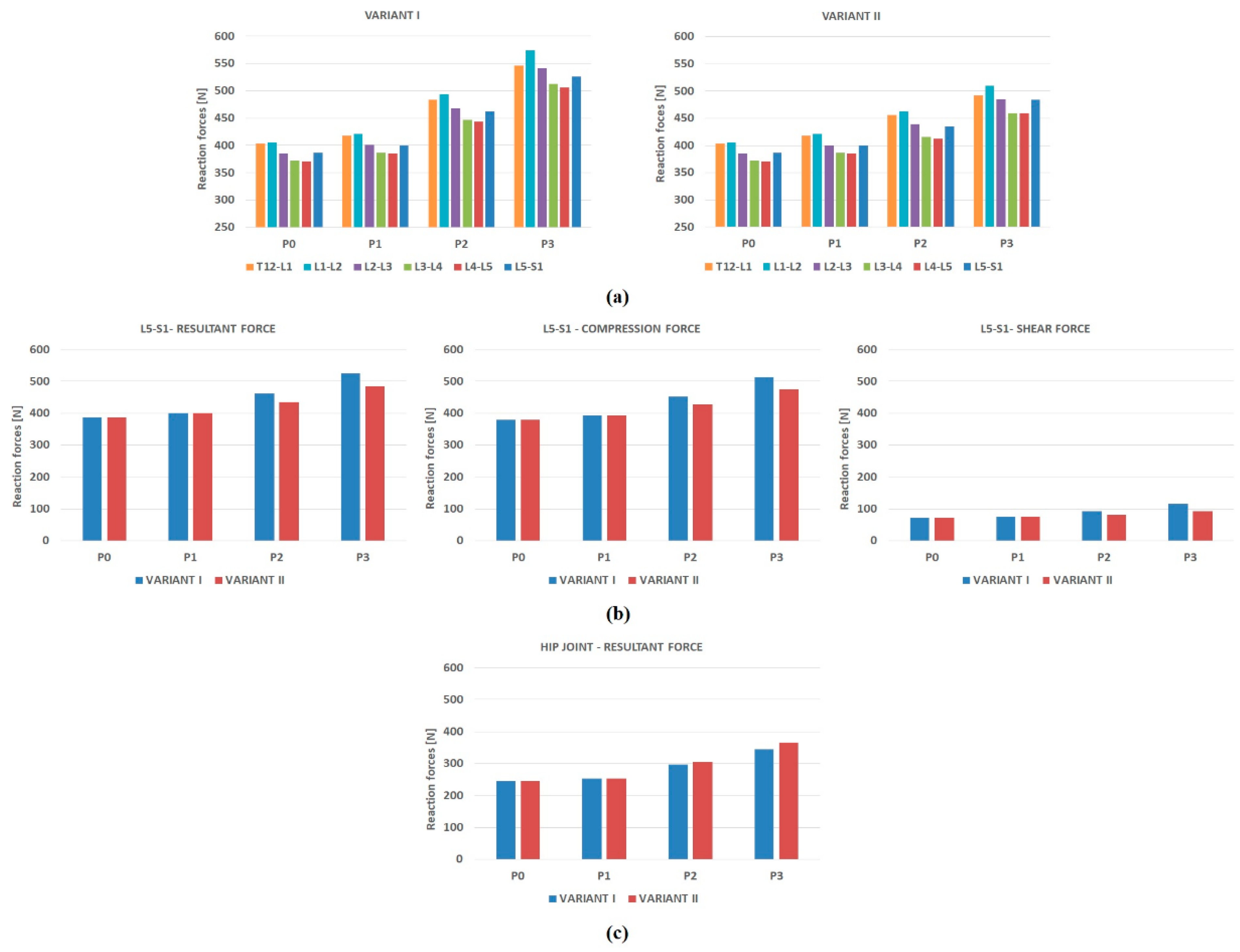
- Resultant reaction forces generated in the intervertebral joints of the lumbar spine, resultant force, compression and shear force in segment L5-S1 and the resultant force in the hip joint.
3. Results
3.1. Muscle Forces
3.2. Muscle Fatigue
3.3. Joint Reaction Forces
4. Discussion
5. Conclusions
Limitation of the Study and Directions for Further Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Conti, M.; Calderon, I.; Rudge, M. Musculoskeletal Discomforts during Pregnancy—An Obstetric and Physiotherapy View. Femina 2003, 31, 531–535. [Google Scholar]
- Calguneri, M.; Bird, H.A.; Wright, V. Changes in Joint Laxity Occurring during Pregnancy. Ann. Rheum. Dis. 1982, 41, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Aldabe, D.; Ribeiro, D.C.; Milosavljevic, S.; Bussey, M.D. Pregnancy-Related Pelvic Girdle Pain and Its Relationship with Relaxin Levels during Pregnancy: A Systematic Review. Eur. Spine J. 2012, 21, 1769–1776. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; João, S.M.A.; Sacco, I.C.N. Static and Dynamic Biomechanical Adaptations of the Lower Limbs and Gait Pattern Changes during Pregnancy. Womens Health (Lond. Engl.) 2013, 9, 99–108. [Google Scholar] [CrossRef]
- Been, E.; Kalichman, L. Lumbar Lordosis. Spine J. 2014, 14, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Pool-Goudzwaard, A.L.; Slieker ten Hove, M.C.P.H.; Vierhout, M.E.; Mulder, P.H.; Pool, J.J.M.; Snijders, C.J.; Stoeckart, R. Relation between Low Back and Pelvic Pain, Pelvic Floor Activity and Pelvic Floor Disorders. In Biomechanics of the Sacroiliac Joints and the Pelvic Floor; Springer: Berlin/Heidelberg, Germany, 2003; pp. 89–104. [Google Scholar]
- Gilleard, W.L. Trunk Motion and Gait Characteristics of Pregnant Women When Walking: Report of a Longitudinal Study with a Control Group. BMC Pregnancy Childbirth 2013, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.A.; Chu, S.R. Musculoskeletal Anatomic, Gait, and Balance Changes in Pregnancy and Risk for Falls. In Musculoskeletal Health in Pregnancy and Postpartum; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–18. [Google Scholar]
- Sabino, J.; Grauer, J.N. Pregnancy and Low Back Pain. Curr. Rev. Musculoskelet. Med. 2008, 1, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Mogren, I.M.; Pohjanen, A.I. Low Back Pain and Pelvic Pain during Pregnancy: Prevalence and Risk Factors. Spine (Phila. PA. 1976) 2005, 30, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Mogren, I.M. Previous Physical Activity Decreases the Risk of Low Back Pain and Pelvic Pain during Pregnancy. Scand. J. Public Health 2005, 33, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Mens, J.M.A.; Vleeming, A.; Stoeckart, R.; Stam, H.J.; Snijders, C.J. Understanding Peripartum Pelvic Pain: Implications of a Patient Survey. Spine (Phila. PA. 1976) 1996, 21, 1363–1370. [Google Scholar] [CrossRef]
- Gilleard, W.L. The Structure and Function of the Abdominal Muscles during Pregnancy and the Immediate Post-Birth Period. Master’s Thesis, Department of Human Movement Science, University of Wollongong, Wollongong, Australia, 1992. Available online: https://ro.uow.edu.au/theses/2843 (accessed on 3 January 2022).
- Gilleard, W.L.; Brown, J.M.M. Structure and Function of the Abdominal Muscles in Primigravid Subjects during Pregnancy and the Immediate Postbirth Period. Phys. Ther. 1996, 76, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Golomer, E.; Ducher, D.; Arfi, G.S.; Sud, R. Simple Locomotion and during Load Carrying in Pregnant Women. J. Gynecol. Obstet. Biol. Reprod. 1991, 20, 406–412. [Google Scholar]
- Hainline, B. Low-Back Pain in Pregnancy. Adv. Neurol. 1994, 64, 65–76. [Google Scholar] [PubMed]
- Dumas, G.A.; Reid, J.G.; Wolfe, L.A.; Griffin, M.P.; McGrath, M.J. Exercise, Posture, and Back Pain during Pregnancy. Part 1. Exercise and Posture. Clin. Biomech. 1995, 10, 98–103. [Google Scholar] [CrossRef]
- Whitcome, K.K. Obstetric Load and the Evolution of Human Lumbopelvic Sexual Dimorphism. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2006. [Google Scholar]
- Martinez-Marti, F.; Martinez-Garcia, M.S.; Carvajal, M.A.; Palma, A.J.; Anguiano, M.; Lallena, A.M. Fractal Behavior of the Trajectories of the Foot Centers of Pressure during Pregnancy. Biomed. Phys. Eng. Express 2019, 5, 025007. [Google Scholar] [CrossRef]
- Ramachandra, P.; Kumar, P.; Kamath, A.; Maiya, A.G. Do Structural Changes of the Foot Influence Plantar Pressure Patterns During Various Stages of Pregnancy and Postpartum? Foot Ankle Spec. 2017, 10, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.E.; Conner-Kerr, T. An Analysis of Posture and Back Pain in the First and Third Trimesters of Pregnancy. J. Orthop. Sports Phys. Ther. 1998, 28, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; Whittle, M.W. The Effects of Pelvic Movement on Lumbar Lordosis in the Standing Position. J. Orthop. Sports Phys. Ther. 1996, 24, 130–135. [Google Scholar] [CrossRef]
- Buck, S. The Evolutionary History of the Modern Birth Mechanism: Looking at Skeletal and Cultural Adaptations. Totem Univ. West. Ont. J. Anthropol. 2011, 19, 81–92. [Google Scholar]
- Östgaard, H.C.; Andersson, G.B.; Schultz, A.B.; Miller, J.A. Influence of Some Biomechanical Factors on Low-Back Pain in Pregnancy. Spine (Phila. PA. 1976) 1993, 18, 61–65. [Google Scholar] [CrossRef]
- Catena, R.D.; Wolcott, W.C. Self-Selection of Gestational Lumbopelvic Posture and Bipedal Evolution. Gait Posture 2021, 89, 7–13. [Google Scholar] [CrossRef]
- Butler, E.E.; Colón, I.; Druzin, M.L.; Rose, J. Postural Equilibrium during Pregnancy: Decreased Stability with an Increased Reliance on Visual Cues. Am. J. Obstet. Gynecol. 2006, 195, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Opala-Berdzik, A.; Błaszczyk, J.W.; Bacik, B.; Cieślińska-Świder, J.; Świder, D.; Sobota, G.; Markiewicz, A. Static Postural Stability in Women during and after Pregnancy: A Prospective Longitudinal Study. PLoS ONE 2015, 10, e0124207. [Google Scholar] [CrossRef] [PubMed]
- Biviá-Roig, G.; Lisón, J.F.; Sánchez-Zuriaga, D. Changes in Trunk Posture and Muscle Responses in Standing during Pregnancy and Postpartum. PLoS ONE 2018, 13, e0194853. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.A.C.; Santos-Rocha, R.; Vieira, F.; Aguiar, L.; Veloso, A.P. Three-Dimensional Kinetic Adaptation of Gait throughout Pregnancy and Postpartum. Scientifica 2015, 2015, 580374. [Google Scholar] [CrossRef] [PubMed]
- Forczek, W.; Ivanenko, Y.; Curyło, M.; Frączek, B.; Masłoń, A.; Salamaga, M.; Suder, A. Progressive Changes in Walking Kinematics throughout Pregnancy—A Follow up Study. Gait Posture 2019, 68, 518–524. [Google Scholar] [CrossRef]
- Forczek, W.; Ivanenko, Y.; Salamaga, M.; Sylos-Labini, F.; Frączek, B.; Masłoń, A.; Curyło, M.; Suder, A. Pelvic Movements during Walking throughout Gestation—The Relationship between Morphology and Kinematic Parameters. Clin. Biomech. 2020, 71, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liang, M.; Lian, W. A Comparison of Foot Kinematics between Pregnant and Non-Pregnant Women Using the Oxford Foot Model during Walking. Int. J. Biomed. Eng. Technol. 2020, 34, 20–30. [Google Scholar] [CrossRef]
- Mei, Q.; Gu, Y.; Fernandez, J. Alterations of Pregnant Gait during Pregnancy and Post-Partum. Sci. Rep. 2018, 8, 2217. [Google Scholar] [CrossRef] [PubMed]
- Bertuit, J.; Leyh, C.; Rooze, M.; Feipel, V. Pregnancy-Related Changes in Centre of Pressure during Gait. Acta Bioeng. Biomech. 2017, 19, 95–102. [Google Scholar] [CrossRef]
- Nowakowska-Lipiec, K.; Michnik, R.; Linek, P.; Myśliwiec, A.; Jochymczyk-Woźniak, K.; Gzik, M. A Numerical Study to Determine the Effect of Strengthening and Weakening of the Transversus Abdominis Muscle on Lumbar Spine Loads. Comput. Methods Biomech. Biomed. Engin. 2020, 23, 1287–1296. [Google Scholar] [CrossRef]
- Michnik, R.; Zadoń, H.; Nowakowska-Lipiec, K.; Jochmyczyk-Woźniak, K.; Myśliwiec, A.; Mitas, A.W. The Effect of the Pelvis Position in the Sagittal Plane on Loads in the Human Musculoskeletal System. Acta Bioeng. Biomech. 2020, 22, 33–42. [Google Scholar] [CrossRef]
- Jurkojć, J.; Michnik, R.; Pauk, J. Identification of Muscle Forces Acting in Lower Limbs with the Use of Planar and Spatial Mathematical Model. J. Vibroeng. 2009, 11, 566–570. [Google Scholar]
- Zadoń, H.; Nowakowska-Lipiec, K.; Michnik, R. A Sitting or Standing Position—Which One Exerts More Loads on the Musculoskeletal System of the Lumbar Spine? Comparative Tests Based on the Methods of Mathematical Modelling. Acta Bioeng. Biomech. 2021, 23, 113–120. [Google Scholar] [CrossRef]
- Nakashima, M.; Mori, Y. Effect of Mechanical Unbalance Induced by Pregnancy on the Muscle Load of the Erector Spinae during a Sit-to-Stand Motion. J. Biomech. Sci. Eng. 2014, 9, 1–11. [Google Scholar] [CrossRef][Green Version]
- Haddox, A.G.; Hausselle, J.; Azoug, A. Changes in Segmental Mass and Inertia during Pregnancy: A Musculoskeletal Model of the Pregnant Woman. Gait Posture 2020, 76, 389–395. [Google Scholar] [CrossRef]
- Morino, S.; Takahashi, M. Musculoskeletal Model of a Pregnant Woman Considering Stretched Rectus Abdominis and Co-Contraction Muscle Activation. In Proceedings of the IEEE International Conference on Multisensor Fusion and Integration for Intelligent Systems, Daegu, Korea, 16–18 November 2017; pp. 452–457. [Google Scholar]
- Catena, R.D.; Connolly, C.P.; McGeorge, K.M.; Campbell, N. A Comparison of Methods to Determine Center of Mass during Pregnancy. J. Biomech. 2018, 71, 217–224. [Google Scholar] [CrossRef]
- De Zee, M.; Hansen, L.; Wong, C.; Rasmussen, J.; Simonsen, E.B. A Generic Detailed Rigid-Body Lumbar Spine Model. J. Biomech. 2007, 40, 1219–1227. [Google Scholar] [CrossRef]
- Hansen, L.; De Zee, M.; Rasmussen, J.; Andersen, T.B.; Wong, C.; Simonsen, E.B. Anatomy and Biomechanics of the Back Muscles in the Lumbar Spine with Reference to Biomechanical Modeling. Spine (Phila. PA. 1976) 2006, 31, 1888–1899. [Google Scholar] [CrossRef]
- Rajaee, M.A.; Arjmand, N.; Shirazi-Adl, A.; Plamondon, A.; Schmidt, H. Comparative Evaluation of Six Quantitative Lifting Tools to Estimate Spine Loads during Static Activities. Appl. Ergon. 2015, 48, 22–32. [Google Scholar] [CrossRef]
- Koblauch, H. Low Back Load in Airport Baggage Handlers. Ph.D. Thesis, Univeristy of Copenhagen, Copenhagen, Denmark, 2015. [Google Scholar]
- Liu, T.; Khalaf, K.; Adeeb, S.; El-Rich, M. Numerical Investigation of Intra-Abdominal Pressure Effects on Spinal Loads and Load-Sharing in Forward Flexion. Front. Bioeng. Biotechnol. 2019, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Gilleard, W.L.; Crosbie, J.; Smith, R. Static Trunk Posture in Sitting and Standing during Pregnancy and Early Postpartum. Arch. Phys. Med. Rehabil. 2002, 83, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Krkeljas, Z. Changes in Gait and Posture as Factors of Dynamic Stability during Walking in Pregnancy. Hum. Mov. Sci. 2018, 58, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Otayek, J.; Bizdikian, A.J.; Yared, F.; Saad, E.; Bakouny, Z.; Massaad, A.; Ghanimeh, J.; Labaki, C.; Skalli, W.; Ghanem, I.; et al. Influence of Spino-Pelvic and Postural Alignment Parameters on Gait Kinematics. Gait Posture 2020, 76, 318–326. [Google Scholar] [CrossRef]
- Prilutsky, B.I.; Zatsiorsky, V.M. Optimization-Based Models of Muscle Coordination. Exerc. Sport Sci. Rev. 2002, 30, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Borg-Stein, J.; Dugan, S.A.; Gruber, J. Musculoskeletal Aspects of Pregnancy. Am. J. Phys. Med. Rehabil. 2005, 84, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Betsch, M.; Wehrle, R.; Dor, L.; Rapp, W.; Jungbluth, P.; Hakimi, M.; Wild, M. Spinal Posture and Pelvic Position during Pregnancy: A Prospective Rasterstereographic Pilot Study. Eur. Spine J. 2015, 24, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Farfan, H.F. Form and Function of the Musculoskeletal System as Revealed by Mathematical Analysis of the Lumbar Spine: An Essay. Spine (Phila. PA. 1976) 1995, 20, 1462–1474. [Google Scholar] [CrossRef]
- Bailey, J.F.; Sparrey, C.J.; Been, E.; Kramer, P.A. Morphological and Postural Sexual Dimorphism of the Lumbar Spine Facilitates Greater Lordosis in Females. J. Anat. 2016, 229, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.S.; Elias, L.A.; Gomide, A.B.; Vieira, M.F.; Do Amaral, W.N. A Longitudinal Assessment of Myoelectric Activity, Postural Sway, and Low-Back Pain during Pregnancy. Acta Bioeng. Biomech. 2017, 19, 77–83. [Google Scholar] [CrossRef]
- Bogduk, N.; Macintosh, J.E.; Pearcy, M.J. A Universal Model of the Lumbar Back Muscles in the Upright Position. Spine (Phila. PA. 1976) 1992, 17, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Richardson, C.A. Inefficient Muscular Stabilization of the Lumbar Spine Associated with Low Back Pain: A Motor Control Evaluation of Transversus Abdominis. Spine (Phila. PA. 1976) 1996, 21, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Roussouly, P.; Pinheiro-Franco, J.L. Biomechanical Analysis of the Spino-Pelvic Organization and Adaptation in Pathology. Eur. Spine J. 2011, 20 (Suppl. S5), 609–618. [Google Scholar] [CrossRef] [PubMed]
- Been, E.; Barash, A.; Marom, A.; Kramer, P.A. Vertebral Bodies or Discs: Which Contributes More to Human-like Lumbar Lordosis? Clin. Orthop. Relat. Res. 2010, 468, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Farfan, H.F. Biomechanics of the Lumbar Spine. In Managing Low Back Pain; Kirkaldy-Willis, W.H., Ed.; Churchill Livingstone: New York, NY, USA, 1983; pp. 9–21. [Google Scholar]
- Tardieu, C.; Haeusler, M. The Acquisition of Human Verticality with an Emphasis on Sagittal Balance. In Sagittal Balance of the Spine; Roussouly, P., Pinheiro-Franco, J., Labelle, H., Gehrechen, M., Eds.; Thieme Medical Publishers: Leipzig, Germany, 2019; pp. 13–22. [Google Scholar]
- Piper, T.J.; Jacobs, E.; Haiduke, M.; Waller, M.; McMillan, C. Core Training Exercise Selection during Pregnancy. Strength Cond. J. 2012, 34, 55–62. [Google Scholar] [CrossRef]
- Pujol, T.J.; Barnes, J.T.; Elder, C.L.; LaFontaine, T. Resistance Training During Pregnancy. Strength Cond. J. 2007, 29, 44–46. [Google Scholar] [CrossRef]
- Whitcome, K.K.; Shapiro, L.J.; Lieberman, D.E. Fetal Load and the Evolution of Lumbar Lordosis in Bipedal Hominins. Nature 2007, 450, 1075–1078. [Google Scholar] [CrossRef]
- Moore, K.; Dumas, G.A.; Reid, J.G. Postural Changes Associated with Pregnancy and Their Relationship with Low-Back Pain. Clin. Biomech. 1990, 5, 169–174. [Google Scholar] [CrossRef]
- Sulima, G.; Bryekhov, O.; Kosobokova, E.; Poliakov, M.; Kalinin, M.; Kovalenko, O.; Volkov, V. Biomechanical Aspects Of Lumbar Hyperlordosis And Low Back Pain During Pregnancy. Internet J. Minim. Invasive Spinal Technol. 2010, 4. [Google Scholar]

| Mean ± SD | ||||
|---|---|---|---|---|
| P0 | P1 | P2 | P3 | |
| Pregnancy week | 12.00 ± 0.78 | 24.86 ± 1.03 | 35.46 ± 0.66 | |
| Body height (m) | 1.67 ± 0.04 | 1.67 ± 0.04 | 1.67 ± 0.04 | 1.67 ± 0.04 |
| Body mass (kg) | 59.30 ± 7.72 | 60.42 ± 6.73 | 66.39 ± 7.96 | 70.99 ± 9.14 |
| BMI (kg/m2) | 21.31 ± 2.24 | 21.68 ± 2.01 | 23.72 ± 2.32 | 25.57 ± 2.88 |
| Pelvic tilt (deg) | 9.90 ± 2.86 | 9.85 ± 3.54 | 12.03 ± 3.69 | 14.52 ± 3.77 |
| Pelvic width (cm) | 24.84 ± 1.27 | 24.96 ± 1.31 | 26.61 ± 1.95 | 27.69 ± 2.23 |
| Pelvic width in relation to the width in P0 (%) | 100% | 100.5% | 107.1% | 111.5% |
| Lumbar Lordosis Angle (Deg) | ||||
|---|---|---|---|---|
| P0 | P1 | P2 | P3 | |
| Variant I | 42.03 | 41.91 | 40.79 | 40.12 |
| Variant II | 42.03 | 41.87 | 43.49 | 46.20 |
| Muscle Fatigue Function * | ||||
|---|---|---|---|---|
| P0 | P1 | P2 | P3 | |
| Variant I | 0.09 | 0.10 | 0.17 | 0.30 |
| Variant II | 0.09 | 0.10 | 0.14 | 0.23 |
| Difference in the value of the muscle fatigue function between Variant I and II | 0 | 0 | 17.6% | 23.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michnik, R.; Zadoń, H.; Nowakowska-Lipiec, K.; Forczek-Karkosz, W. How Does the Lumbopelvic Complex Cope with the Obstetrical Load during Standing? Ergonomic Aspects of Body Posture in Pregnant Women. Appl. Sci. 2022, 12, 4330. https://doi.org/10.3390/app12094330
Michnik R, Zadoń H, Nowakowska-Lipiec K, Forczek-Karkosz W. How Does the Lumbopelvic Complex Cope with the Obstetrical Load during Standing? Ergonomic Aspects of Body Posture in Pregnant Women. Applied Sciences. 2022; 12(9):4330. https://doi.org/10.3390/app12094330
Chicago/Turabian StyleMichnik, Robert, Hanna Zadoń, Katarzyna Nowakowska-Lipiec, and Wanda Forczek-Karkosz. 2022. "How Does the Lumbopelvic Complex Cope with the Obstetrical Load during Standing? Ergonomic Aspects of Body Posture in Pregnant Women" Applied Sciences 12, no. 9: 4330. https://doi.org/10.3390/app12094330
APA StyleMichnik, R., Zadoń, H., Nowakowska-Lipiec, K., & Forczek-Karkosz, W. (2022). How Does the Lumbopelvic Complex Cope with the Obstetrical Load during Standing? Ergonomic Aspects of Body Posture in Pregnant Women. Applied Sciences, 12(9), 4330. https://doi.org/10.3390/app12094330








