Assessment of Fish Health: Seasonal Variations in Blood Parameters of the Widely Spread Mediterranean Scorpaenid Species, Scorpaena porcus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Sampling and Blood Collection
2.2. Haematological and Biochemical Analyses
2.3. Statistical Analyses
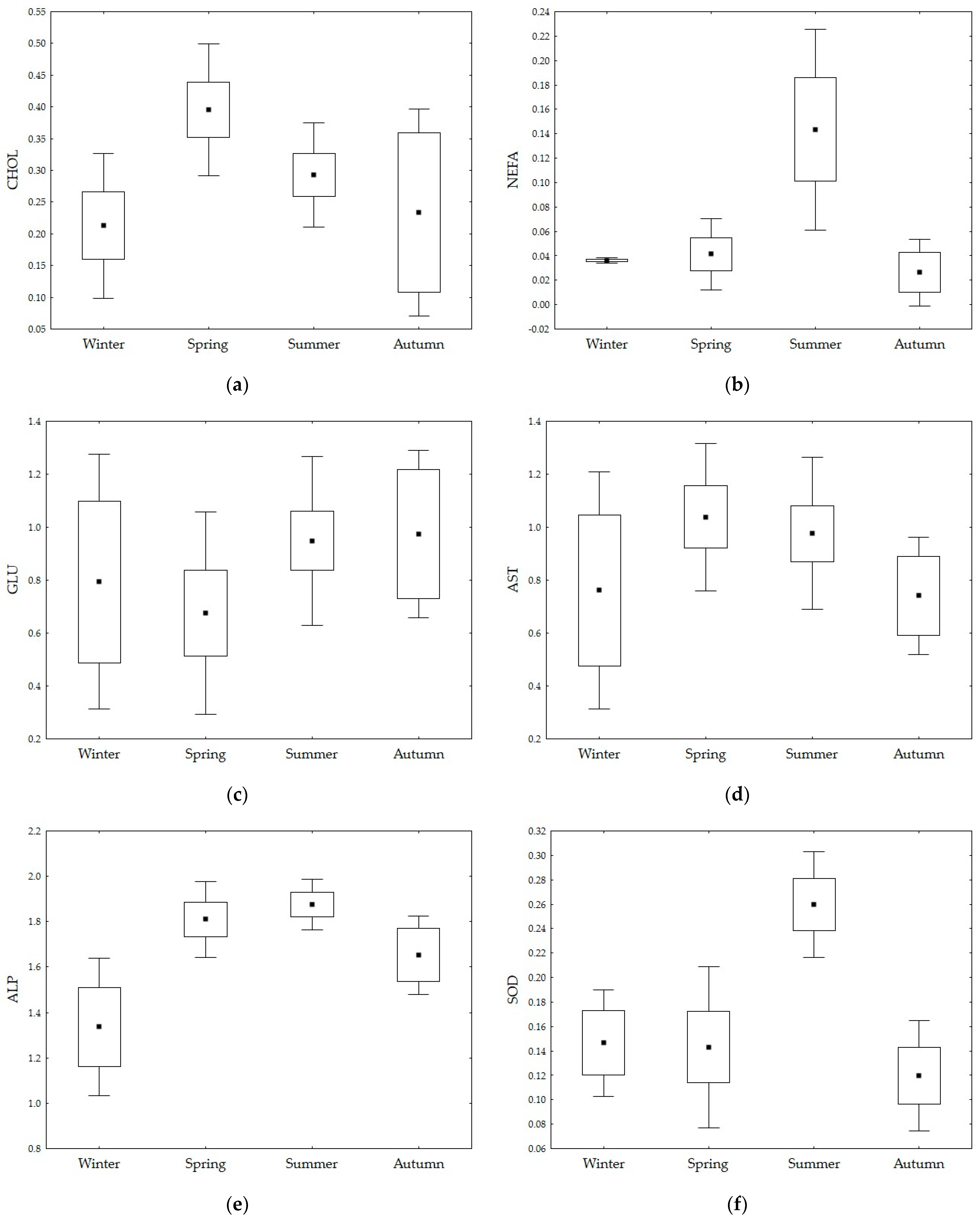
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Romão, S.; Donatti, L.; Fretia, M.O.; Teixeira, J.; Kusma, J. Blood parameter Analysis and Morphological Alterations as Biomarkjers on the health of Hoplias malabricus and Geophagus brasiliensis. Braz. Arch. Biol. Technol. 2006, 49, 441–448. [Google Scholar] [CrossRef]
- Ferri, J.; Stagličić, N.; Matić-Skoko, S. The black scorpionfish, Scorpaena porcus (Scorpaenidae): Could it serve as reliable indicator of Mediterranean coastal communities’ health? Ecol. Indic. 2012, 18, 25–30. [Google Scholar] [CrossRef]
- Tenji, D.; Micic, B.; Sipos, S.; Miljanovic, B.; Teodorovic, I.; Kaisarevic, S. Fish biomarkers from a different perspective: Evidence of adaptive strategy of Abramis brama (L.) to chemical stress. Environ. Sci. Eur. 2020, 32, 47. [Google Scholar] [CrossRef]
- Adams, S.M. Assessing cause and effect of multiple stressors on marine systems. Mar. Pollut. Bull. 2005, 51, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Lowe, D.; Bolognesi, C.; Fabbri, E.; Koehler, A. The use of biomarkers in biomonitoring: A 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 281–300. [Google Scholar] [CrossRef]
- Roche, H.; Bogé, G. Fish blood parameters as a potential tool for identification of stress caused by environmental factors and chemical intoxication. Mar. Environ. Res. 1996, 41, 27–43. [Google Scholar] [CrossRef]
- Lusková, V. Factors Affecting Hematological Indices in Free-living Fish Populations. Acta Vet. Brno 1998, 67, 249. [Google Scholar] [CrossRef] [Green Version]
- Ivanc, A.; Haskovic, E.; Jeremic Dekic, S.R. Haematological evaluation of welfare and health of fish. Praxis Vet. 2005, 53, 191–202. [Google Scholar]
- Vázquez, G.; Guerrero, G.A. Characterization of blood cells and hematological parameters in Cichlasoma dimerus (Teleostei, Perciformes). Tissue Cell 2007, 39, 151–160. [Google Scholar] [CrossRef]
- Seriani, R.; Abessa, D.M.S.; Kirschbaum, A.A.; Pereira, C.D.S.; Romano, P.; Ranzani-Paiva, M.J.T. Relationship between water toxicity and hematological changes in Oreochromis niloticus. Braz. J. Aquat. Sci. Technol. 2011, 15, 47–53. [Google Scholar]
- Ferri, J.; Topić Popović, N.; Čož-Rakovac, R.; Beer-Ljubić, B.; Strunjak-Perović, I.; Škeljo, F.; Jadan, M.; Petrić, M.; Barišić, J.; Šimpraga, M.; et al. The effect of artificial feed on blood biochemistry profile and liver histology of wild saddled bream, Oblada melanura (Sparidae). Mar. Environ. Res. 2011, 71, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.C.; Patra, A.K.; Sarkar, B.; Pal, A. Seasonal changes in hematological parameters of Catla catla (Hamilton 1822). Comp. Clin. Path A 2012, 21, 1473–1487. [Google Scholar] [CrossRef]
- Wade, N.M.; Clark, T.D.; Maynard, B.T.; Atherton, S.; Wilkinson, R.J.; Smullen, R.P.; Taylor, R.S. Effects of an unprecedented summer heatwave on the growth performance, flesh colour and plasma biochemistry of marine cage-farmed Atlantic salmon (Salmo salar). J. Therm. Biol. 2019, 80, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, P.; Walker, S.; Johnston, H.; Johnston, C.; Symonds, J. Comparative assessment of blood biochemistry and haematology normal ranges between Chinook salmon (Oncorhynchus tshawytscha) from seawater and freshwater farms. Aquaculture 2021, 537, 736464. [Google Scholar] [CrossRef]
- Jan, K.; Ahmed, I. The influence of sex and season on some hematological and biochemical parameters of snow trout Schizothorax labiatus in the Indian Himalayan Region. Fish. Sci. 2021, 87, 39–54. [Google Scholar] [CrossRef]
- Çelik, E.S. Blood Chemistry (Electrolytes, Lipoproteins and Enzymes) Values of Black Scorpion Fish (Scorpaena porcus Linneaus, 1758) in the Dardanelles, Turkey. J. Biol. Sci. 2004, 4, 716–719. [Google Scholar]
- Çelik, E.S. Çanakkale Boğazı’ndan Avlanan İskorpit Balığı (Scorpaena porcus Linnaeus, 1758)’nın Kan Glukoz Düzeyindeki Aylık Değişmeler. Su. Ürünleri. Dergisi. 2005, 22, 115–118. [Google Scholar]
- Rudneva, I.; Skuratovskaya, E. Gender peculiarities of blood antioxidant enzyme activity of some Black Sea coastal fish species. J. Ichthyol. 2009, 49, 119–122. [Google Scholar] [CrossRef]
- Rudneva, I.; Skuratovskaya, E.; Kuzminova, N.; Kovyrshina, T. Age composition and antioxidant enzyme activities in blood of Black Sea teleosts. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 229–239. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.; Kaim-Malka, R.A.; Ledoyer, M.; Jacob-Abraham, S.S. Food partitioning among Scorpaenid fishes in Mediterranean seagrass beds. J. Fish Biol. 1989, 34, 715–734. [Google Scholar] [CrossRef]
- Rudneva, I.I.; Shevchenko, N.F.; Zalevskaya, I.N.; Zherko, N.V. Biomonitoring of the coastal waters of the Black Sea. Water Resour. 2005, 32, 215–222. [Google Scholar] [CrossRef]
- Topic Popovic, N.; Strunjak-Perovic, I.; Coz-Rakovac, R.; Barisic, J.; Jadan, M.; Persin Berakovic, A.; Sauerborn Klobucar, R. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J. Appl. Ichthyol. 2012, 28, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, O.I.; Mahmoud, U.M.; Fazio, F.; Sayed, A. SDS-PAGE technique as biomarker for fish toxicological studies. Toxicol. Rep. 2018, 5, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.A.; Ahmed, I. Impact of environmental changes on plasma biochemistry and hematological parameters of Himalayan snow trout, Schizothorax plagiostomus. Comp. Clin. Path 2019, 28, 793–804. [Google Scholar] [CrossRef]
- Ferri, J. Fishery–Biological and Ecological Characteristics of the Black Scorpionfish, Scorpaena porcus (Linnaeus, 1758) in the Eastern Adriatic Sea. Ph.D. Thesis, University of Split, Split, Croatia, 21 April 2011. [Google Scholar]
- Kavadias, S.; Castritsi-Catharios, J.; Dessypris, A.; Miliou, H. Seasonal variation in steroid hormones and blood parameters in cage-farmed European sea bass (Dicentrarchus labrax L.). J. Appl. Ichthyol. 2004, 20, 58–63. [Google Scholar] [CrossRef]
- Valls, E.; Navarro, J.; Barria, C.; Coll, M.; Fernandez-Borrais, J.; Rotllant, G. Seasonal, ontogenetic and sexual changes in lipid metabolism of the small-spotted catshark (Scyliorhinus canicula) in deep-sea free-living conditions. J. Exp. Mar. Biol. Ecol. 2016, 483, 59–63. [Google Scholar] [CrossRef]
- Ihuț, A.; Răducu, C.; Cocan, D.; Munteanu, C.; Luca, I.D.; Uiuiu, P.; Lațiu, C.; Rus, V.; Mireşan, V. Seasonal variation of blood biomarkers in huchen, Hucho hucho (Actinopterygii: Salmoniformes: Salmonidae) reared in captivity. Acta Ichthyol. Piscat. 2020, 50, 381–390. [Google Scholar] [CrossRef]
- Coz-Rakovac, R.; Smuc, T.; Topic Popovic, N.; Strunjak-Perovic, I.; Hacmanjek, M.; Jadan, M. Novel methods for assessing fish blood biochemical data. J. Appl. Ichthyol. 2008, 24, 77–80. [Google Scholar] [CrossRef]
- Coz-Rakovac, R.; Strunjak-Perovic, I.; Topic Popovic, N.; Hacmanjek, M.; Smuc, T.; Jadan, M.; Lipej, Z.; Homen, Z. Cage culture effects on mullets (Mugilidae) liver histology and blood chemistry profile. J. Fish Biol. 2008, 72, 2557–2569. [Google Scholar] [CrossRef]
- Coz-Rakovac, R.; Strunjak-Perovic, I.; Hacmanjek, M.; Popovic, N.T.; Lipej, Z.; Sostaric, B. Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the North Adriatic Sea. Vet. Res. Commun. 2005, 29, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Hoar, W.S.; Randall, D.J.; Farrell, A.P. The Cardiovascular System Part A, Fish Physiology Series Vol. XII; Academic Press: San Diego, CA, USA, 1992; p. 340. [Google Scholar]
- Farell, A.P.; Jones, D.R. The cardiovascular system. In Fish Physiology, Part B; Hoar, W.S., Randall, D.J., Farell, A.P., Eds.; Academic Press: San Diego, CA, USA, 1992; pp. 1–73. [Google Scholar]
- Wagner, T.; Congleton, J.L. Blood chemistry correlates of nutritional condition, tissue damage, and stress in migrating juvenile chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. 2004, 61, 1066–1074. [Google Scholar] [CrossRef]
- Cataldi, E.; Di Marco, P.; Mandich, A.; Cataudella, S. Serum parameters of Adriatic sturgeon Acipenser naccarii (Pisces: Acipenseriformes): Effects of temperature and stress. Comp. Biochem. Physiol. A 1998, 121, 351–354. [Google Scholar] [CrossRef]
- Percin, F.; Konyalioglu, S. Serum biochemical profiles of captive and wild northern bluefin tuna (Thunnus thynnus L. 1758) in the Eastern Mediterranean. Aquac. Res. 2008, 39, 945–953. [Google Scholar] [CrossRef]
- Manna, S.K.; Das, N.; Bera, A.K.; Baitha, R.; Maity, S.; Debnath, D.; Panikkar, P.; Nag, S.K.; Sarkar, S.D.; Das, B.K.; et al. Reference haematology and blood biochemistry profiles of striped catfish (Pangasianodon hypophthalmus) in summer and winter seasons. Aqua. Rep. 2021, 21, 100836. [Google Scholar] [CrossRef]
- Luskova, V. Annual cycles and normal values of hematological parameters in fishes. Acta Sci. Nat. Brno 2007, 31, 70–78. [Google Scholar]
| Number | TL Range (cm) | W Range (g) | Mean TL ± SD | Mean W ± SD | Mean CI ± SD | |
|---|---|---|---|---|---|---|
| Winter | 21 | 14.9–25.9 | 59–446 | 18.0 ± 2.83 | 125.5 ± 89.14 | 1.91 ± 0.23 |
| Spring | 24 | 14.1–26.0 | 49–356 | 18.5 ± 3.78 | 131.2 ± 87.05 | 1.83 ± 0.19 |
| Summer | 34 | 14.4–23.1 | 55–246 | 17.3 ± 2.05 | 100.4 ± 40.22 | 1.85 ± 0.17 |
| Autumn | 17 | 14.0–20.8 | 50–189 | 17.1 ± 1.87 | 99.3 ± 38.93 | 1.87 ± 0.16 |
| Mean ± SD | ||||
|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | |
| Protein profile parameters | ||||
| TP (g/L) | 29.88 ± 6.01 | 35.13 ± 7.58 | 35.25 ± 11.15 | 32.59 ± 9.93 |
| UREA (mmol/L) | 0.47 ± 0.39 | 0.84 ± 0.47 | 0.36 ± 0.33 | 0.49 ± 0.53 |
| CREA (mmol/L) | 44.71 ± 6.64 | 50.08 ± 25.83 | 47.37 ± 9.03 | 42.17 ± 13.52 |
| Lipid profile parameters | ||||
| CHOL (mmol/L) | 0.68 ± 0.39 | 1.55 ± 0.52 | 0.99 ± 0.33 | 0.83 ± 0.75 |
| TRIG (mmol/L) | 0.22 ± 0.15 | 0.69 ± 0.44 | 0.35 ± 0.18 | 0.50 ± 0.38 |
| NEFA (mmol/L) | 0.09 ± 0.01 | 0.10 ± 0.07 | 0.37 ± 0.30 | 0.06 ± 0.07 |
| Carbohydrate profile parameter | ||||
| GLU (mmol/L) | 8.07 ± 5.61 | 5.94 ± 6.76 | 9.86 ± 5.87 | 10.44 ± 6.93 |
| Enzyme profile parameters | ||||
| AST (U/L) | 9.07 ± 14.88 | 12.50 ± 10.14 | 10.47 ± 8.84 | 5.13 ± 2.85 |
| ALP (U/L) | 24.87 ± 13.30 | 68.07 ± 25.65 | 76.44 ± 20.12 | 47.42 ± 20.31 |
| GGT (U/L) | 2.64 ± 2.21 | 4.61 ± 10.66 | 3.32 ± 3.07 | 3.06 ± 1.47 |
| LDH (U/L) | 586.68 ± 561.25 | 881.68 ± 794.10 | 555.38 ± 586.37 | 422.80 ± 680.85 |
| CK (U/L) | 9.84 ± 6.91 | 16.94 ± 9.62 | 17.14 ± 18.34 | 11.38 ± 4.55 |
| Antioxidative profile parameters | ||||
| GPx (U/L) | 5474.33 ± 1960.31 | 4535.66 ± 1487.62 | 4446.46 ± 1342.74 | 4984.64 ± 2062.51 |
| SOD (U/mL) | 0.41 ± 0.14 | 0.41 ± 0.23 | 0.83 ± 0.19 | 0.32 ± 0.14 |
| SS | df | MS | F | p | |
|---|---|---|---|---|---|
| TP (g/L) | 0.065 | 3 | 0.022 | 1.630 | 0.188 |
| CREA (mmol/L) | 0.028 | 3 | 0.009 | 0.448 | 0.719 |
| CHOL (mmol/L) | 0.411 | 3 | 0.137 | 11.615 | 0.000 |
| NEFA (mmol/L) | 0.134 | 3 | 0.045 | 15.679 | 0.000 |
| GLU (mmol/L) | 1.235 | 3 | 0.412 | 3.068 | 0.033 |
| AST (U/L) | 1.086 | 3 | 0.362 | 3.842 | 0.013 |
| ALP (U/L) | 2.682 | 3 | 0.894 | 23.776 | 0.000 |
| GGT (U/L) | 0.085 | 3 | 0.028 | 0.385 | 0.764 |
| LDH (U/L) | 2.024 | 3 | 0.675 | 1.994 | 0.121 |
| CK (U/L) | 0.496 | 3 | 0.165 | 1.576 | 0.205 |
| GPx (U/L) | 0.052 | 3 | 0.017 | 0.640 | 0.591 |
| SOD (U/mL) | 0.211 | 3 | 0.070 | 26.007 | 0.000 |
| TL | TP | UREA | CREA | CHOL | TRIG | NEFA | GLU | AST | ALP | GGT | LDH | CK | GPx | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Winter | ||||||||||||||
| TP | 0.37 | |||||||||||||
| UREA | 0.02 | −0.14 | ||||||||||||
| CREA | 0.23 | 0.57 | 0.02 | |||||||||||
| CHOL | 0.46 * | 0.26 | 0.47 | −0.05 | ||||||||||
| TRIG | 0.32 | 0.08 | −0.14 | −0.17 | 0.46 | |||||||||
| NEFA | 0.17 | 0.52 | 0.05 | 0.79 | 0.44 | 0.52 | ||||||||
| GLU | −0.04 | 0.04 | 0.11 | 0.76 * | 0.23 | −0.01 | 0.19 | |||||||
| AST | 0.53 | 0.45 | −0.26 | −0.08 | 0.05 | 0.03 | 0.14 | −0.54 | ||||||
| ALP | 0.22 | −0.30 | 0.25 | 0.14 | 0.08 | −0.05 | 0.49 | 0.12 | 0.01 | |||||
| GGT | 0.66 * | 0.14 | −0.24 | 0.26 | 0.09 | 0.22 | 0.71 | −0.39 | 0.28 | 0.42 | ||||
| LDH | −0.57 * | 0.26 | 0.21 | 0.37 | −0.17 | −0.14 | 0.13 | 0.43 | −0.30 | −0.45 | −0.53 | |||
| CK | −0.45 | −0.20 | −0.25 | 0.13 | 0.04 | −0.13 | −0.78 | 0.36 | −0.33 | −0.21 | −0.28 | 0.16 | ||
| GPx | 0.16 | 0.36 | 0.32 | −0.07 | 0.18 | −0.20 | 0.38 | 0.18 | −0.05 | −0.27 | −0.10 | 0.11 | −0.57 | |
| SOD | −0.82 * | −0.31 | −0.13 | −0.12 | −0.67 * | −0.50 | −0.33 | 0.28 | −0.45 | −0.18 | −0.70 * | 0.45 | 0.22 | 0.00 |
| Summer | ||||||||||||||
| TP | 0.61 * | |||||||||||||
| UREA | −0.53 * | −0.23 | ||||||||||||
| CREA | −0.30 | 0.03 | 0.36 | |||||||||||
| CHOL | 0.28 | 0.53 * | 0.16 | 0.47 * | ||||||||||
| TRIG | 0.02 | 0.44 * | 0.45 * | 0.49 * | 0.87 * | |||||||||
| NEFA | −0.32 | −0.02 | 0.52 * | 0.44 | 0.62 * | 0.77 * | ||||||||
| GLU | 0.02 | 0.01 | 0.41 * | 0.55 * | 0.65 * | 0.62 * | 0.52 * | |||||||
| AST | 0.24 | 0.21 | −0.00 | −0.08 | −0.01 | −0.00 | −0.44 | −0.12 | ||||||
| ALP | −0.60 * | −0.24 | 0.24 | 0.38 | 0.06 | 0.19 | 0.42 | 0.05 | −0.18 | |||||
| GGT | 0.11 | −0.02 | −0.35 | −0.17 | −0.09 | −0.08 | −0.19 | −0.10 | 0.14 | −0.36 | ||||
| LDH | −0.08 | −0.01 | 0.35 | 0.43 * | 0.28 | 0.42 | 0.07 | 0.35 | 0.34 | −0.11 | 0.02 | |||
| CK | −0.29 | −0.10 | 0.38 | 0.27 | −0.21 | 0.20 | 0.29 | −0.02 | −0.29 | 0.08 | −0.30 | 0.04 | ||
| GPx | 0.61 * | 0.59 * | −0.24 | −0.16 | 0.40 | 0.41 | −0.11 | 0.08 | 0.22 | −0.21 | 0.32 | 0.03 | 0.00 | |
| SOD | −0.20 | −0.51 * | −0.25 | −0.30 | −0.84 * | −0.85 * | −0.50 * | −0.48 * | 0.13 | 0.07 | −0.12 | −0.42 | 0.00 | −0.50 * |
| Spring | ||||||||||||||
| TP | 0.22 | |||||||||||||
| UREA | −0.14 | −0.36 * | ||||||||||||
| CREA | 0.16 | 0.52 * | −0.07 | |||||||||||
| CHOL | 0.13 | 0.38 | 0.27 | 0.42 * | ||||||||||
| TRIG | 0.39 | 0.53 * | 0.07 | 0.26 | 0.37 | |||||||||
| NEFA | 0.16 | 0.41 | 0.07 | 0.04 | 0.44 | 0.88 * | ||||||||
| GLU | 0.15 | 0.31 | −0.17 | 0.77 * | 0.16 | 0.10 | −0.10 | |||||||
| AST | −0.01 | 0.35 | −0.01 | 0.28 | 0.10 | 0.46 | 0.37 | 0.30 | ||||||
| ALP | −0.07 | 0.24 | 0.28 | 0.76 * | 0.30 | 0.23 | 0.16 | 0.56 * | 0.13 | |||||
| GGT | 0.32 | 0.20 | 0.15 | 0.17 | 0.02 | 0.29 | 0.13 | 0.05 | 0.00 | 0.37 | ||||
| LDH | −0.29 | 0.33 | −0.22 | 0.11 | −0.05 | 0.27 | 0.36 | 0.06 | 0.40 * | 0.01 | −0.16 | |||
| CK | −0.05 | 0.35 | −0.17 | 0.41 | 0.38 | 0.54 | 0.45 | 0.24 | −0.07 | −0.01 | 0.00 | 0.41 | ||
| GPx | 0.27 | 0.42 | −0.19 | −0.22 | 0.11 | 0.15 | 0.13 | −0.29 | −0.24 | −0.27 | 0.01 | 0.14 | −0.05 | |
| SOD | −0.27 | −0.40 | 0.24 | −0.17 | −0.18 | −0.44 | −0.29 | −0.27 | −0.02 | −0.06 | −0.11 | −0.09 | 0.11 | −0.47 |
| Autumn | ||||||||||||||
| TP | 0.32 | |||||||||||||
| UREA | −0.74 | −0.36 | ||||||||||||
| CREA | 0.05 | 0.77 * | −0.29 | |||||||||||
| CHOL | 0.58 | 0.87 * | −0.68 | 0.48 | ||||||||||
| TRIG | 0.32 | 0.55 | −0.34 | 0.37 | 0.63 | |||||||||
| NEFA | 0.73 * | 0.68 | −0.60 | 0.21 | 0.79 * | 0.79 * | ||||||||
| GLU | −0.05 | 0.53 | −0.11 | 0.73 * | 0.33 | 0.55 | 0.14 | |||||||
| AST | 0.22 | 0.70 * | −0.18 | 0.83 * | 0.45 | 0.38 | 0.19 | 0.90 * | ||||||
| ALP | 0.49 | 0.55 | 0.00 | 0.48 | 0.38 | 0.48 | 0.71 * | 0.28 | 0.55 | |||||
| GGT | 0.10 | 0.68 * | −0.40 | 0.45 | 0.77 * | 0.70 * | 0.50 | 0.15 | −0.03 | 0.28 | ||||
| LDH | 0.08 | 0.60 | −0.02 | 0.82 * | 0.28 | 0.32 | 0.14 | 0.82 * | 0.95 * | 0.59 | 0.02 | |||
| CK | −0.80 | 0.30 | 0.00 | 0.80 | −0.10 | −0.70 | −0.80 | 0.20 | 0.60 | −0.20 | 0.20 | 0.90 * | ||
| GPx | 0.45 | 0.62 | −0.38 | 0.25 | 0.77 * | 0.48 | 0.28 | 0.10 | 0.15 | 0.13 | 0.41 | 0.10 | −0.50 | |
| SOD | −0.54 * | −0.50 | 0.70 | −0.05 | −0.82 * | −0.67 * | −0.53 | −0.05 | 0.05 | −0.11 | −0.36 | 0.13 | 0.60 | −0.71 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferri, J.; Matić-Skoko, S.; Čož-Rakovac, R.; Strunjak-Perović, I.; Beer Ljubić, B.; Topić Popović, N. Assessment of Fish Health: Seasonal Variations in Blood Parameters of the Widely Spread Mediterranean Scorpaenid Species, Scorpaena porcus. Appl. Sci. 2022, 12, 4106. https://doi.org/10.3390/app12094106
Ferri J, Matić-Skoko S, Čož-Rakovac R, Strunjak-Perović I, Beer Ljubić B, Topić Popović N. Assessment of Fish Health: Seasonal Variations in Blood Parameters of the Widely Spread Mediterranean Scorpaenid Species, Scorpaena porcus. Applied Sciences. 2022; 12(9):4106. https://doi.org/10.3390/app12094106
Chicago/Turabian StyleFerri, Josipa, Sanja Matić-Skoko, Rozelindra Čož-Rakovac, Ivančica Strunjak-Perović, Blanka Beer Ljubić, and Natalija Topić Popović. 2022. "Assessment of Fish Health: Seasonal Variations in Blood Parameters of the Widely Spread Mediterranean Scorpaenid Species, Scorpaena porcus" Applied Sciences 12, no. 9: 4106. https://doi.org/10.3390/app12094106
APA StyleFerri, J., Matić-Skoko, S., Čož-Rakovac, R., Strunjak-Perović, I., Beer Ljubić, B., & Topić Popović, N. (2022). Assessment of Fish Health: Seasonal Variations in Blood Parameters of the Widely Spread Mediterranean Scorpaenid Species, Scorpaena porcus. Applied Sciences, 12(9), 4106. https://doi.org/10.3390/app12094106










