Abstract
Blackberries are rich in antioxidants due to their high polyphenol and vitamin content, making them an excellent source of health protection. It is well known that good quality juices and fruit extracts can be obtained only from high quality fruits. The aim of this research is to characterize the antioxidant properties of garden blackberries fruits grown in Turia (Romania). Extracts were made from blackberry fruits with four different solvents, and the antioxidant capacity was studied by applying DPPH and FRAP assay. The total phenolic content (with Folin–Ciocâlteu reagent), total anthocyanin content (with the pH differential method), and total flavonoid content (with aluminum chloride colorimetric method) were also measured. The quercetin and gallic acid content were also determined by HPLC-DAD. As the best results were obtained with 90% v/v acidified acetonitrile, the Hansen parameters analysis was performed for the acetonitrile-water solvent mixture as a solvent and cyanidin-3-O-glucoside as a solute.
1. Introduction
Blackberries belong to the thorny shrubs (genus Rubus) group and are members of the rose family (Rosaceae). Wild species are found on five continents, and low-growing shrubs are common in temperate forests and tropical highlands. Some species prefer cool mountain habitats, others grow better in marshy tundra areas. Researchers have identified hundreds of species, but only the fruits of a few species are commercially available [1]. Blackberries (Rubus fruticosus L.) are widely cultivated edible fruits in Europe and North America. Their composition and nutritional value depend on several agro-geo-climatological factors, such as genotype, environmental conditions, agronomic practices, harvesting time, post-harvest storage, and processing techniques [2,3]. Among the most important bioactive compounds in blackberries—flavonoids, phenolic acids, tannins, anthocyanins, and vitamins—have been found [4,5]. The dietary consumption of blackberries has been associated with several health benefits, such as preventing and treating metabolic syndrome, supporting the digestive and immune systems, preventing inflammatory diseases and cardiovascular diseases, and providing protective effects against gastrointestinal cancers [3,4,6].
Blackberry presents multiple health benefits for food consumers due to its antioxidant activity and the polypharmacological effects of anthocyanins [7]. Blackberries are used as conventional raw materials in obtaining jam, compote, as well as unfermented and fermented beverages, but they may replace synthetic additives from foodstuffs such as colorants, stabilizers, etc. [8,9]. Additionally, the use of blackberries improves the nutritional value and shelf life of finished food. The anthocyanins from blackberries can be applied in food packaging systems and as food colorants, since natural dyes are rarely toxic as well as easy to prepare and pollution free [10,11,12]. The possible applications are shown in Figure 1.
Figure 1.
The main unconventional applications of blackberries in the food industry.
During fruit processing, a large by-product quantity is generated with an important bioactive compound content [3,5,13]. Through food by-products utilization, it is possible to simultaneously recover the valuable bioactive compounds and minimize the waste quantity. This way, the fruit processing industry has become more environmentally friendly by removing phenolic compounds from the waste, which has a bacteriostatic effect on degradation microorganisms [14]. There are also difficulties in recovering bioactive compounds from food industry by-products. Extractions involve the co-extraction of non-phenolic substances, such as sugars, organic acids, and proteins, which require subsequent purification processes. The temperature decreases solvent viscosity and surface tension, enhancing diffusion and extraction efficiency. However, high temperatures may accelerate the degradation of polyphenols, reduce antioxidant capacity, and produce solvent evaporation. Extraction of polyphenols is usually carried out at low pH, as, in acidic environments, these compounds take a neutral form, which is best suited for solubilization. However, excessive acidification may impair extraction, as the profile of native polyphenols may be distorted by the hydrolysis of simple glycosides. Extraction time is also crucial, as prolonged exposure to oxygen/light can degrade phenols. In addition, other polyphenols are susceptible to oxidation or volatility and therefore require short processes or conditions that protect the dissolved fraction from oxygen/light damage. Current extraction methods (ultrasound-assisted extraction, supercritical fluid extraction, hot-pressurized liquid extraction, microwave-assisted extraction, enzyme-assisted extraction) are generally efficient, sustainable, potentially cost-effective, and easier to scale up. Nevertheless, the optimal feasibility of extracting plant by-products is still far from being within reach. Efforts should be made to find the optimal compromise between maximum recovery and minimum disturbance of polyphenol structure [15].
In recent years, more and more studies have been frequently published looking at red berries’ composition and antioxidant properties. Several methods have been adapted to determine antioxidants in plants, foods, dietary supplements, and food supplements. The total antioxidant capacity of food does not necessarily correlate with the value obtained by summing the levels of individual antioxidants from a composite food matrix. Antioxidant activity can be monitored by various assays with different mechanisms, including hydrogen atom transfer (HAT), single electron transfer (SET), reducing power, and metal chelation. Among the methods using the SET reaction mechanism, the FRAP (Ferric reducing antioxidant power), the Folin–Ciocâlteu method, and the DPPH (2,2-diphenylpicrylhydrazyl) radical scavenging method are widely used. Flavonoids and anthocyanins can also be quantified by UV-Vis spectrophotometry.
In parallel, extensive research is taking place worldwide to find the optimal extraction methods to obtain antioxidant-rich products for a range of berries. Although conventional solvent extraction is the most widespread technique for extracting antioxidant compounds from red berries, new non-conventional methods have emerged as environmentally friendly alternatives to the first method, such as ultrasound, microwave, and pressure-assisted extractions, applied alone or in conjunction with the use of solvents, to reduce energy and solvent requirements [16,17].
The traditional method for anthocyanins extraction is Soxhlet extraction with acidified ethanol or methanol. Acid addition is necessary because anthocyanins are reactive compounds and are sensitive to pH changes. Anthocyanins present instability and tend to change their color in response to changes in environmental parameters such as pH, heat, light, oxygen, and co-existing substances [18]. Anthocyanins can be found in different chemical forms that depend on the solution’s pH (Figure 2).
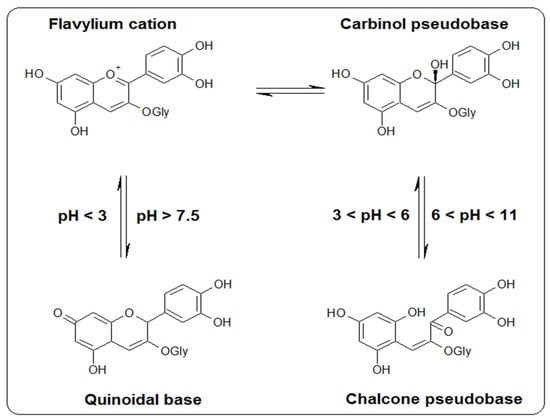
Figure 2.
The structures of cyanidin-3-O-glucoside at different pHs.
The presence of ascorbic acid in an environment containing anthocyanins causes a loss of color, thus suggesting a direct interaction between the two molecules. At the same time, the presence of oxygen in the environment will favor faster degradation of anthocyanins by ascorbic acid, thus determining the formation of the polymeric pigment and the whitening of the anthocyanin pigment. The specific degradation process is unknown; however, adding ascorbic acid to anthocyanins increases the decomposition rate of both molecules. The presumed mechanisms by which this phenomenon would occur are the direct condensation of ascorbic acid with anthocyanins or the formation of hydrogen peroxide and oxidative cleavage [19].
The most commonly used extraction solvents are acidified methanol and ethanol, but in the food industry, ethanol is preferred because of the toxicity of methanol. Among ethanol and methanol extractions, many other extraction solvents have been mentioned in the literature, such as: ethyl acetate, chloroform, acetone/water 10–90% (v/v), n-hexane, isooctane [20]. Solvents with high polarity (ethanol and methanol) are favorable for use in the extraction of polar compounds such as phenolic compounds and flavonoids. Non-polar solvents, such as ether, or solvents with low polarity, such as chloroform and ester, are used in specific cases with a low frequency of use. In addition to the extraction method and solvent selection, one should pay great attention to the extraction temperature and pH. Different extraction temperatures may affect the types of polyphenols extracted. At high extraction temperatures, the formation of new compounds, known as products of the Maillard reaction, also involves the polyphenols extraction, and thermal degradation of the polyphenols may occur [21].
A solvent mixture can display more affinity with the chemical target than a pure solvent and can enhance the extraction yields. The solubility of the different compounds in variable solvents was predicted, initially, by comparing the Hildebrand solubility parameters of the solute and solvent. Still, this method had many discrepancies between the practice and theory. The Hildebrand solubility parameter is the square root of cohesive energy density, and this is mainly applicable for nonpolar (van der Waals) interactions.
Improvement was made by Hansen (2007) by introducing three partial solubility parameters (δD, δP, δH), characterizing the different physical interactions separately (dispersive, polar, hydrogen-bond) that can occur between the solvent and solute molecules [22]. Mathematically, the original Hildebrand parameter was treated, somehow, as a vector in three-dimensional space (having three components), with the value of the Hildebrand parameter representing the length of the vector, given by the square root of the sums of the square of its components, as in Equation (1):
Two components mix easily if their value of Hildebrand parameters differs only by 2 MPa0.5. However, experience shows that it is also important that at least two of the Hansen parameters have close values to ensure good solubility. Therefore, comparison of the Hansen parameters would give more plausible predictions.
The aim of this research is to characterize the antioxidant properties of garden blackberries fruits grown in Turia, Romania. To obtain more accurate and comprehensive results, we used several solvents for extraction. Using Hansen’s solubility parameters, we want to calculate the optimal concentration of a solvent mixture suitable for extracting anthocyanins.
2. Materials and Methods
In this study, frozen garden blackberries were used, collected from own cultivation in Turia, Covasna County, Romania (46°3′51.678″ N and 25°58′40.8828″ E) in September 2020. The village has a temperate continental climate (hot summers, cold winters), with average annual temperatures ranging from 2–7 °C. Annual rainfall in Turia varies between 500 and 1100 mm. The type of soil is eutricambosol soil. The blackberry fruits were hand-harvested at full maturity from randomly selected plants, the fruits without any damage were picked and stored at −20 °C until the analysis.
2.1. Chemicals
Ascorbic acid, Folin–Ciocâlteu’s phenol reagent, 2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ), 2-diphenyl-1-picrylhydrazyl (DPPH), aluminum chloride, quercetin, and gallic acid were purchased from Sigma–Aldrich Chemie GmbH (Hamburg, Germany). The solvents (methanol, ethanol, acetonitrile, acetone, hydrochloric acid, acetic acid, and formic acid) were LiChrosolv HPLC grade, purchased from Merck KGaA (Darmstadt, Germany). Sodium acetate, ferric chloride, sodium carbonate, and potassium chloride were of analytical grade and purchased from VWR International (Batavia, IL, USA).
2.2. Sample Preparation
The frozen garden blackberries were homogenized using a domestic blender, and 20 mL of the following solvents were added to 1 g of sample:
- -
- Et: 80% (v/v) ethanol [23];
- -
- A: 70% (v/v) acetone + 2% (v/v) acetic acid [24,25,26];
- -
- Met: 60% (v/v) methanol + 3% (v/v) formic acid [27];
- -
- ACN: 90% (v/v) acetonitrile + 10% (v/v) 6 molar HCl [28].
The samples were sonicated for 20 min for a better cell wall rupture, centrifuged at 3461 RCF (6000 RPM) for 10 min, and the supernatant was removed and used for further determinations. Each sample was independently extracted in triplicate, and analyses were performed on the same day. Among the utilized solvents, only ethanol and acetone (in some cases) are allowed for food industry purposes. However, our research aimed to map the antioxidant capacity of blackberries and not to produce an extract suitable for human consumption. Therefore, we used some other solvents that do not belong to the GRAS (Generally Recognized As Safe) category.
2.3. Determination of the Dry Matter Content of Blackberries
The dry matter content was determined using 2 g of the sample in an oven at 105 ± 2 °C (Memmert, Memmert Gmbh, Schwabach, Germany) until constant weight.
2.4. Antioxidant Activity Determination
2.4.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
For the measurement, first, a methanolic DPPH solution was prepared (9 mg DPPH dissolved in 100 mL methanol), and then, sample mixtures were prepared. To 1 mL DPPH solution, 20 μL blackberry extract and 980 μL distilled water were added. The mixture was incubated for 30 min at room temperature in the dark, and the absorbance was measured at λ = 517 nm on Varian Cary 50 UV–VIS spectrophotometer (Varian Co., Palo Alto, Santa Clara, CA, USA) [29]. Measurements were performed in triplicate from all samples. The percentage of the DPPH consumed was calculated with the following equation (Equation (2)):
where: A0—the initial absorbance, and A1—the absorbance in the presence of the extract.
E% = 100 × (A0 − A1)/A0
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
The antioxidant potential of blackberries extract was also determined using a FRAP assay measuring the change in absorbance at λ = 593 nm due to the formation of a blue-colored Fe2+-tripyridyl-triazine compound from colorless oxidized Fe3+-form by the action of electron-donating antioxidants [30]. The stock solutions required for the FRAP solution were prepared as follows: acetate buffer: 3.1 g sodium-acetate + 16 mL acetic acid + 1 L distilled water; TPTZ solution: 0.312 g TPTZ + 100 mL distilled water + 336 μL hydrochloric acid (37% v/v); Ferric chloride solution: 0.54 g FeCl3 + 100 mL distilled water. The FRAP solution was prepared as follows: 25 mL acetate buffer + 2.5 mL TPTZ solution + 2.5 mL ferric chloride solution.
The absorbance was measured at λ = 593 nm (Varian Cary 50 UV–VIS spectrophotometer, Varian Co., Palo Alto, Santa Clara, CA, USA). Calibration was performed with ascorbic acid, and results were expressed as milligrams of ascorbic acid equivalents (AAE) per 100 g of the dry weight of berries. Triplicate measurements were performed from all samples.
2.5. Determination of Total Polyphenol Content (TPC)
The total polyphenol content was determined with the Folin–Ciocâlteu reagent, according to the method modified by Singleton [31], using gallic acid as a standard. There were 50 µL of the sample diluted with 200 µL of methanol: water (4:1) solution was mixed with 0.25 mL of Folin–Ciocâlteu reagent, and 1 min later, 1 mL of sodium carbonate (0.7 M) was added. The mixture was heated to 50 °C and kept at this temperature for 5 min. After cooling, the absorbance was measured at λ = 760 nm. Gallic acid was used as standard, and the results were expressed as milligrams of gallic acid equivalents (GAE) per 100 g of dry weight (DW) of blackberries. Measurements were performed in triplicate on each sample.
2.6. Determination of Total Flavonoid Content (TFC)
The aluminum chloride colorimetric method was used to determine the total flavonoid content of the sample. For calibration, the following concentrations of quercetin solution were used: 12.5; 25; 50; 100; 200 µg/mL quercetin.
For the sample mixtures, 1.2 mL of aluminum chloride solution (2% m/v) were added to 0.6 mL of blackberry extract and incubated for 60 min at room temperature. Then, absorbances were measured at λ = 420 nm wavelength on a Varian Cary 50 UV spectrophotometer (Varian Co., Palo Alto, Santa Clara, CA, USA) [32]. Results were expressed as mg quercetin per 100 g dry weight. Measurements were performed in triplicate on each sample.
2.7. Determination of Total Anthocyanin Content (TAC)
Total anthocyanin content was determined according to the pH differential method [31]. The sample mixtures were prepared as follows:
- -
- 0.2 mL blackberry extract + 1.8 mL 0.025 M potassium chloride solution (pH = 1);
- -
- 0.2 mL blackberry extract + 1.8 mL 0.4 M sodium-acetate solution (pH = 4.5).
The sample mixtures were measured serially at λ = 510, and λ = 700 nm wavelengths, and the results were calculated using Equations (3) and (4) [33]. Results are expressed as mg cyanidin-3-glucoside/100 g dry matter.
where: A—absorbance; MW—molecular weight (449.2 g/mol); DF—dilution factor; ε—molar absorption coefficient (26,900 L/(mol·cm)); l = optical path length (cm).
A = (A510 nm − A700 nm)pH1.0 − (A510 nm − A700 nm)pH4.5
TAC (mg/L) = A × MW × DF × 1000/ε × l
2.8. Determination of Gallic Acid and Quercetin by HPLC-DAD
In the first step, 10 g of frozen blackberry sample was poured into 100 mL of methanol and was sonicated in an ultrasonic bath (ultrasound-assisted extraction, UAE) for 30 min. The supernatants were filtered through a 0.45 µm syringe filter. The analyses were operated on an Agilent 1260 HPLC system (Agilent, Santa Clara, CA, USA) with a photodiode array detector, with Betasil C18 (150 mm × 4.6 mm, 5 μm) analytical column (Thermo Scientific®, Waltham, MA, USA ). The mobile phase was a binary solvent mixture of A (0.1% formic acid in water) and B (methanol). The solvents used were filtered through a 0.45 µm filter and degassed. The mobile phase flow rate was 0.6 mL·min−1. The gradient program was: 0–2 min: 15% B; 2–7 min: 15–30% B; 11–15 min: 30–80% B; 15–20 min: 80–15% B (UV absorbance was monitored in the interval λ = 20–400 nm [34,35]. The calibration curve was constructed by plotting standard solutions’ peak area against the concentration (5–100 µg/mL for gallic acid and 2.5–50 µg/mL). The determination coefficient (R2) was 0.9918 for gallic acid and 0.9952 for quercetin. The LOD and LOQ were 0.85 and 2.17 µg/mL for gallic acid and 0.17 and 0.32 µg/mL for quercetin.
2.9. Calculation of a Solvent Mixture Optimal Concentration for Anthocyanin Extraction Using the Hansen Solubility Parameters
The theoretical optimal concentration of a solvent mixture (e.g., acetonitrile-water solution) could be calculated by using the δP-δH coordinate system. In this coordinate, the solvent-water solution is represented by a line, and the flavylium cation is shown by a point (P). The coordinate of the intersection of the perpendicular lines from the point P to the line will give the two Hansen parameter of the optimal solvent composition. The equation of the line for solvent-water mixture is given by:
where: δsP—the polar Hansen parameter of the solvent mixture; δsH—the hydrogen-bond Hansen parameter of the solvent mixture; m-slope; n-intercept. The numerical values for the slope and intercept are determined by linear regression.
δsP = m·δsH + n
The point, representing the flavylium cation, is marked as P(δfH,δfP).
The coordinates of the closest point of the line to the P point, representing the optimal solvent composition (P*(δ*H,δ*P)), are given by:
and
δ*H = (δfH + m(δfP − n))/(1 + m2)
δ*P = m·δ*H + n
As the dependency V%S-δsH is also linear, given by V%S = a·δsH + b (where a-slope, b-intercept, both determined by linear regression), the optimal solvent composition could be expressed as:
Vs% = a·δ*H + b
2.10. Statistical Analysis
Data are expressed as the means ± standard deviation for at least three independent measurements. Statistical analysis and graphical representation were made using Microsoft Excel 2016 and Statistica 8.0 (StatSoft Inc., Tulsa, OK, USA). The normal distribution of the experimental data was tested with Kolmogorov–Smirnov and Shapiro–Wilk tests. The normality test was performed on three variables from three independent measurements. The relationship between bioactive compounds and antioxidant capacity was tested using Pearson correlation in SPPS 19.0 (SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
Ultrasound-assisted extraction allows low concentrations of solvents and lower temperatures to extract anthocyanins [36]. Therefore, we opted for ultrasound-assisted extraction, at room temperature, with different extraction solvents [17].
3.1. Dry Matter Content of Blackberries
The dry matter content of the blackberries was obtained as 13.55 ± 0.92%. Hereafter, the results are expressed in terms of dry matter content.
3.2. Total Anthocyanin, Flavonoid, and Polyphenol Concentrations
The total anthocyanin, flavonoid, and polyphenol concentrations in the blackberries tested are summarized in Table 1. The results show that the total anthocyanin content ranges from 642.96 ± 71.24 to 885 ± 59.81 mg cyanidin-3-glucoside/100 g DW (average 780.13 ± 98.54 mg cyanidin-3-glucoside/100 g DW), and the total flavonoid content ranges from 129.75 ± 9.5 to 240.93 ± 16.95 mg quercetin/100 g DW (average 183.38 ± 14.87 mg quercetin/100 g DW). The total polyphenol concentration varies from 2143 ± 321.21 to 3311.83 ± 54.38 mg gallic acid/100 g DW (average 2872.69 ± 231.65 mg gallic acid/100 g DW) depending on the solvent. The values obtained agree with several literature data points [23,26,27,28,37,38]; total phenolic compounds present in blackberries could vary based on variety, climatic conditions of the year, soil fertility, harvest time, or extraction method. The Folin–Ciocâlteu test for TPC has several advantages, including simplicity, reproducibility, and robustness. However, it has several disadvantages. Firstly, the test is sensitive to pH, temperature, and reaction time, and secondly, overestimation of TPC in the Folin–Ciocâlteu test is a serious concern, as reducing sugars and ascorbic acid can be present in high amounts in fruit extracts and can reduce the effect of the Folin–Ciocâlteu reagent, which distorts the TPC results.

Table 1.
Total anthocyanin, flavonoid, and polyphenol content of blackberry extracts obtained with different solvents.
The use of acetonitrile is common in research and analytical measurements, but it is not allowed in industrial-scale extractions due to its toxicity and flammability. In our study, we also investigated this solvent because it is known to be an excellent HPLC solvent for the analysis of anthocyanins. Even though acetonitrile is rarely used to extract plant bioactive components, our results showed that these extracts had the highest antioxidant activity. Sellappan et al. (2002) also used acetonitrile extraction with acetic acid pickling. In their case, the total anthocyanin concentration of blackberries from the state of Georgia was 116.59 ± 8.58 mg C3G/100 g FW, while the total polyphenol concentration was 486.53 ± 97.13 mg GAE/100 g FW [28].
The least effective solvent was found to be 80 v/v% ethanol even though, in our previous study, 50–70 v/v% ethanol was the most effective solvent for blackcurrant by-products, and other literature data also showed 50 v/v% ethanol to be the most effective [39]. This result can be explained by the fact that the extraction was carried out without acidification. Celant et al. (2016) also used 80% ethanol as solvent, when the total anthocyanin concentration varied between 6.76 ± 0.03 and 9.42 ± 0.03 mg C3G/g FW, the total flavonoid concentration between 0.46 ± 0.02 and 1.14 ± 0.01 mg Q/g FW, while the total polyphenol concentration ranged from 8.23 ± 0.16 to 14.98 ± 0.54 mg GAE/g FW [23]. Cho et al. (2005) used methanolic extraction with formic acid acidification. In their case, the total polyphenol concentration ranged between 292.2 and 446.4 mg GAE/100 g FW, depending on the blackberry ssp., which is very similar to our results [27]. For extracts obtained with acetone extraction, our results and the literature data do not always agree; however, the results of Moyer et al. (2002) (average of blackberry cultivars in the state of Oregon) are pretty close to our values since, in their case, the total anthocyanin content was 164 ± 36 mg C3G/100 g FW, while the total polyphenol concentration was 577 ± 56.1 mg GAE/100 g FW [26].
Despite the anthocyanins belonging to the group of flavonoids, in this case, the detected flavonoid content was lower than that of anthocyanins, although this should be inverse. A similar trend was observed by Celant et al. (2016), indicating that the aluminum chloride colorimetric method cannot detect all flavonoids [23]. This theory is supported by Tabart et al. research. They found that the pH differential method is very specific for anthocyanins, but different anthocyanins respond very differently. In contrast, the aluminum chloride method seems appropriate only for flavanols. Some other flavonoids, such as flavanones, could also be detected, but with lower sensitivity. Anthocyanins were not detected [40].
The standard deviation indicates the reproducibility of the measurements, the average precision of the measured results, and the deviation from the mean. Studying the standard deviations (Figure 3), the highest value was observed for the acetonic extracts, especially for TPC, which could be mainly due to the high volatility of acetone and their poor water miscibility. This latter property may be important because of the high moisture content of the plant samples. Due to the limited immiscibility, the availability of the solute contained in the solvent is highly influenced by the mixing intensity of the suspension, through the variation of the diffusivity of the solvent through the moisture film, around the plant material particles.
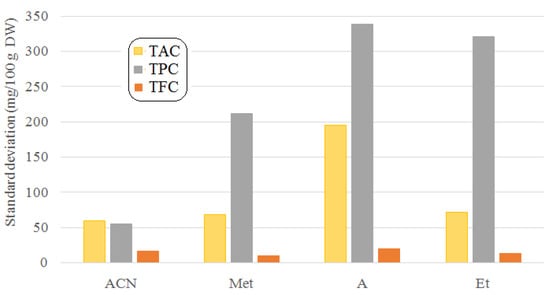
Figure 3.
Comparison of standard deviations for different solvents and methods.
3.3. Antioxidant Capacity
In the determination of the DPPH radical binding activity, it was observed that the antioxidant capacity is also affected by the solvents, with the extract prepared with acetonitrile showing the highest antioxidant capacity by both FRAP and DPPH methods. The results are shown in Table 2. This could explain that components extracted with solvents of higher polarity are less effective at binding the DPPH radical than their lower polarity counterparts. However, changes in the polarity of solvents alter their ability to leach certain antioxidants, thus affecting the measurements [23]. This tendency is also evident in the present case, as acetonitrile has a relatively low polarity (0.460) and was found to be the best solvent. In contrast, ethanol has a higher polarity (0.654) and, therefore, inefficiency [41].

Table 2.
The antioxidant capacity of blackberry extracts, obtained with different solvents, measured by three different methods.
The obtained data suggest that the garden blackberries have excellent antioxidant potential due to their high polyphenol and anthocyanin content. The total antioxidant concentration values agree with Koczka et al.’s results, where the total antioxidant concentration of blackberries averaged 150 μmol ascorbic acid/100 g dry matter (2.64 g ascorbic acid/100 g dry matter) [42].
The standard deviations also follow the trend in the present case. Figure 4 shows that the highest standard deviation is for acetone, which is due to its higher volatility in an aqueous mixture, in comparison to the other used solvents.
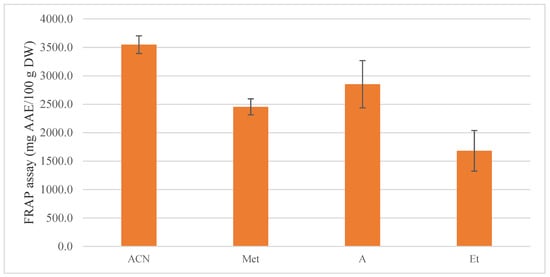
Figure 4.
FRAP assay of blackberries extracts in mg AAE/g DW.
3.4. Statistical Correlations
Blackberries contain many phytochemicals, and to elucidate which components are responsible for the main antioxidant effect, we examined whether the data were normally distributed, and then, we applied the Pearson correlation between the antioxidant capacity and the total anthocyanins, flavonoids, and polyphenols. Table 3 shows that, in all cases, the distribution is normal, the values obtained for the Z-test fall within the 95% confidence interval (−1.96; +1.96), and both the Kolmogorov–Smirnov and Shapiro–Wilk tests also show that the distribution is normal at p = 0.05 significance level. Since the distribution is normal, the Pearson correlation could be applied.

Table 3.
The normality testing results of the measurement error distribution.
In Table 4, the Pearson correlation analysis results are summarized. The result shows that there is a significant positive correlation between DPPH radical scavenging activity and total polyphenol content (r = 0.95; p < 0.01), a similar correlation has been reported in the literature [23,28,37,43,44]. Furthermore, it was shown that DPPH stable radicals are mainly able to be reduced by more reactive components than polyphenolic compounds. They are the main components responsible for the high antioxidant capacity of the extracts. This trend is also characteristic of the FRAP method (r = 0.836; p < 0.01), as pointed out by other authors such as Koczka et al., by the polyphenol’s Fe3+ ion reducing ability [42].

Table 4.
The results of Pearson linear correlation analysis.
The correlation between total anthocyanins-total flavonoids, as well as between the DPPH–FRAP results are also significant. Still, the correlations are looser in comparison to the radical scavenging activity-total polyphenol content, indicating that anthocyanins and flavonoids are only moderately responsible for the antioxidant effect: a phenomenon that has been observed by other researchers [28,42].
Using linear regression in Microsoft Excel, the Figure 5 graphically illustrates the positive linear correlation between total polyphenol content and the DPPH scavenging activity of the extracts (R2 = 0.9026).
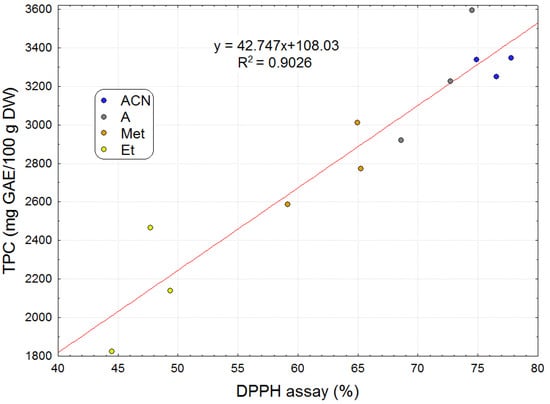
Figure 5.
Relationship between radical-binding activity and total polyphenol content of the extracts obtained with different solvents.
3.5. Determination of Quercetin and Gallic Acid
The quercetin content was 0.465 ± 0.058 mg/100 g fresh weight, while the gallic acid content was 88.25 ± 0.478 mg/100 g fresh weight. The values for quercetin content from the literature are quite variable. Still, Ungureanu et al. (2020) obtained similar values [38], as in their case, the concentration of quercetin is 2.96 ± 0.05 mg/100 g dry weight, while Cho et al. (2005) obtained 0.63 ± 0.01 mg/100 g fresh weight [27]. This can be explained by the result obtained by Ungureanu et al. that higher precipitation and lower temperature led to an increase in the quercetin and gallic acid content of blackberries [38]. For gallic acid, our results were relatively close to the values obtained by Jacques et al. (2010): 113.13 mg/100 g fresh weight [45].
3.6. Theoretical Study of the Effect of Acetonitrile-Water Concentration on Anthocyanin Extraction Based on Hansen Solubility Parameters
Theoretical tools and models are often used to predict the solubility of bioactive molecules. These tools are phase solubility studies, quantitative structure-property relationships, calculation of Hansen solubility parameters by the group contribution method, the Flory–Huggins interaction parameter, the real solvent conductive screening model (COSMO-RS), and the COSMO segmental activity coefficient model. These models can be applied to the extraction process of anthocyanins to predict which solvent is most effective against which molecule. However, these models have varying degrees of complexity, and there is no literature available to evaluate which model would be preferable for this purpose. The Hansen solubility parameters are the most suitable for finding the most optimal organic solvent-water mixture ratio. Finding the most optimal ratio of organic solvent to water is important because it gives a higher dissolution rate. Using this mixture will shorten the analysis time, and the amount of anthocyanins extracted will be as high as possible, giving the closest value to the true antioxidant content. Due to the good solubility, the amount of solvent used can also be significantly reduced.
The difference in solvent efficiency could be explained by the terms of Hansen and Hildebrand solubility parameters of the solvents and solutes, as summarized in Table 5. Cyanidin and cyanidin-3-O-glucoside were chosen for solutes because they are the dominant anthocyanins in blackberry [46].

Table 5.
Hansen and Hildebrand parameters of the main bioactive components of blackberry [47,48] and the used solvents [21].
Both acetone and acetonitrile are polar solvents (Table 5), and their intermolecular hydrogen bond forming ability is low. Acetonitrile has the highest polar Hansen parameter among the used solvents and, therefore, is better able to extract more polar active compounds, some of which may have strong antioxidant activity, although, more precise correlations could be made only when the individual components in the extracts are known. In the case of aliphatic alcohols, strong hydrogen bonds are formed between the solvent molecules (polar protic solvents), making them suitable solvents, but in the case of alcohols, solubility decreases with increasing the carbon chain length, which explains why methanol is a better solvent than ethanol [49].
As the best results were obtained with 90% v/v acidified acetonitrile, the Hansen parameters analysis was performed for the acidified acetonitrile-water solvent mixture as a solvent and cyanidin-3-O-glucoside as a solute. The addition of acid shifts the equilibrium towards the more stable flavylium form and conforms to Figure 2, changing the δH value of cyanidin-3-O-glucoside.
Similar to malvidin, the difference between the Hansen solubility parameters of the flavylium and carbinol pseudobase forms of cyanidin is expected to be equal, as the structural changes between the tautomers don’t affect the side structures that differentiate the two anthocyanidins [48]. Accordingly, the decrease in the HSPs during the carbinol-flavylium transformation, caused by acidification, could be approximately:
ΔδP ≈ 0 MPa0.5, ΔδD = 1.85 MPa0.5, and ΔδH = 6 MPa0.5, respectively.
The Hansen solubility parameters (HSP) of solvent mixtures differ from pure solvents. Still, they could be the volumetric fraction-weighted average of the components’ HSPs, even for solvents in a supercritical state, as seen in Equation (9) [50].
where: δj,mix the HSPs of the solvent mixture; Φi the volumetric fraction of i-th component; n is the number of components of the mixture, and the j index denoting the different solubility parameters as follows: j = D, P, H− dispersion, polar and hydrogen bond partial HSPs.
δj,mix = Σni = 1Φiδj,i
Knowing the HSPs of water and acetonitrile, the solubility parameters of the mixture were calculated for the whole concentration range, as in Equation (10):
δj,mix = ΦWδW + ΦAcNδAcN
In water-acetonitrile mixtures, the dispersion Hansen parameter varies minimally (DδD,mix ≈ 0 MPa0.5), as changing the acetonitrile concentration would change the solubility of cyanidin-3-O-glucoside only through the other two members. The δP values of water and acetonitrile are also quite close (ΔδP,mix ≤ 2 MPa0.5), but the variation of δH had a wide variation range (ΔδH,mix ≤ 36.2 MPa0.5).
The value of the polar component of the flavylium cation (δP = 19.48 MPa0.5) doesn’t change in comparison to the natural cyanidin-3-O-glucoside before the acidification, but this value is near to the values of the acetonitrile-water mixtures (δP ≈ 16–18 MPa0.5). The value of the dispersive component of the flavylium cation (δD = 23.85 MPa0.5) is closer than of the original form (δD = 25.7 MPa0.5) to the values of the acetonitrile-water mixtures (δD ≈ 15.4 MPa0.5), but the difference remains considerable. In this situation, the solvent optimization task is, mainly, to find the acetonitrile-water ratio with the δH value closest to the δH value of the flavylium ion (δH = 22.08 MPa0.5). As for the acetonitrile, the hydrogen-bond component had a low value (δH = 6.1 MPa0.5), and for water, it had a very high value (δH = 42.3 MPa0.5). Obviously, with the increase in the water amount, the optimum could be reached. In Figure 6, it could be observed that acidification decreases the cyanidin-3-O-glucoside point distance, substantially, to the acetonitrile-water curve. The hydrogen-bond component of the solvent mixture increases significantly with water content increase, but the polar component decreases slightly and has an opposite effect on distance. Therefore, the optimum concentration could differ from c ≈ 56% v/v acetonitrile (δH ≈ 22 MPa0.5) and should be calculated by an exact mathematical method. From Figure 6, we obtained data that, for the extraction of cyanidin-3-O-glucoside, predicted the optimal theoretical concentration of acetonitrile-water mixture will be copt = 56.2% v/v, which is in good agreement with the previous estimation.
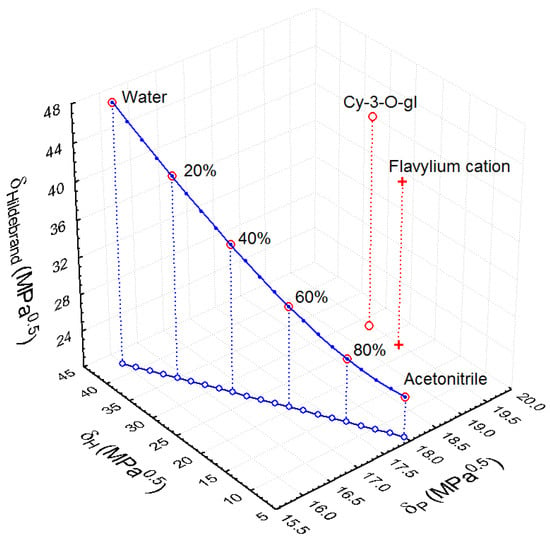
Figure 6.
The Hansen space representation of solubility parameters of the acetonitrile-water mixture (solvent) and cyanidin-3-O-glucoside in natural and protonated form (solute).
For extractions at a higher temperature, such as Soxhlet and, especially, for pressurized liquid extraction (PLE), the Hansen parameters should be calculated, taking into account the temperature dependence of these parameters [50,51].
4. Conclusions
The results obtained in our study show that the garden blackberry from Turia, Romania presented high antioxidant capacity. With our results, we would like to emphasize the nutritional role of blackberries. The obtained data suggest that the blackberries growing and harvested in our region are suitable for the production of premium functional foods. With modern and efficient extraction methods, pure blackberry anthocyanin extracts can be produced. Agricultural and food processing wastes from the blackberry industry are potential sources of anthocyanins. Blackberry has been used as a beneficial food and food ingredient, and it can contribute even more through modern extraction methods.
Our measurements showed that the best solvent is the acidified acetonitrile-water mixture. Still, it is known that it is only suitable for analytical tests and is not approved for use in the food industry. Based on theoretical calculations, 56.2% v/v acetonitrile would be the optimal solvent for the main anthocyanin. Acetonitrile is a widely used solvent in chromatography, so data on the solubility of different bioactive substances in this solvent can be very useful. The resulting extract can be used for quantitative chromatographic analysis without any other sample preparation
The Hansen parameters for different anthocyanidins show quite similar values, so our method can be further developed and extended to other solvent mixtures and other anthocyanins.
Author Contributions
Conceptualization, C.A., M.H. and C.D.A.; methodology, C.A.; software, A.C.; validation, C.A., G.G.C. and A.D.; formal analysis, C.A. and M.H.; investigation, C.A., M.H. and C.D.A.; resources, C.A., M.H. and C.D.A.; data curation, A.C.; writing—original draft preparation, C.A. and A.C.; writing—review and editing, C.A. and A.C.; visualization, C.A., G.G.C. and A.D.; supervision, G.G.C. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bushway, L.; Pritts, M.; Handley, D. Raspberry and Blackberry Production Guide for the Northeast, Midwest, and Eastern Canada (NRAES-35). Natural Resource, Agriculture, and Engineering Service (NRAES). 2008. Available online: https://hdl.handle.net/1813/66930 (accessed on 15 February 2022).
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Riaz, M.; de Feo, V.; Jaafar, H.Z.E.; Moga, M. Rubus fruticosus L.: Constituents, biological activities and health related uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Rai, D.K.; Tzima, K. A Review on Chromatography–Mass Spectrometry Applications on Anthocyanin and Ellagitannin Metabolites of Blackberries and Raspberries. Foods 2021, 10, 2150. [Google Scholar] [CrossRef]
- Ravichandran, K.S.; Krishnaswamy, K. Sustainable food processing of selected North American native berries to support agroforestry. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Santhi, V.P.; Sriramavaratharajan, V.; Murugan, R.; Masilamani, P.; Gurav, S.S.; Sarasu, V.P.; Ayyanar, M. Edible fruit extracts and fruit juices as potential source of antiviral agents: A review. J. Food Meas. Charact. 2021, 15, 5181–5190. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Dormán, G.; Flachner, B.; Hajdú, I.; András, D.C. Target identification and polypharmacology of nutraceuticals. In Nutraceuticals: Efficacy, Safety and Toxicity; Gupta, R.C., Lall, R., Srivastava, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 315–343. [Google Scholar]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, G.I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, S.; Zhao, G.; Ye, F. Destabilisation and stabilisation of anthocyanins in purple-fleshed sweet potatoes: A review. Trends Food Sci. Technol. 2021, 116, 1141–1154. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1845. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Celant, V.M.; Braga, G.C.; Vorpagel, J.A.; Salibe, A.B. Composição fenólica e atividade antioxidante dos extratos aquoso e etanólico de amora-preta. Rev. Bras. De Frutic. 2016, 38. [Google Scholar] [CrossRef][Green Version]
- Boeing, J.S.; Barizão, É.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.; Crozier, A.; Duthie, G.G. Potential health benefits of berries. Curr. Nutr. Food Sci. 2005, 1, 71–86. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonol glycosides and antioxidant capacity of various blackberry and blueberry genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2005, 85, 2149–2158. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Antioxidant capacity, phenolic compounds and minerals content of blackcurrant (Ribes nigrum L.) leaves as influenced by harvesting date and extraction method. Ind. Crop. Prod. 2014, 53, 133–139. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; p. 299. [Google Scholar]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid.-Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Aye, T.; Onoomar, T.; Malai, S. Simultaneous determination of gallic acid and quercetin in leaf extract of Madhuca longifolia from Myanmar by HPLC-DAD: Method development and validation. Key Eng. Mater. 2020, 859, 51–56. [Google Scholar]
- Shirazi, O.U.; Khattak, M.M.A.K.; Shukri, N.A.M. Chromatographic evaluation of gallic acid, catechin and quercetin in methanolic extracts of selected formulations of spices and herbs. Prog. Nutr. 2019, 21, 236–251. [Google Scholar]
- Wang, W.; Jung, J.; Tomasino, E.; Zhao, Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT-Food Sci. Technol. 2016, 72, 229–238. [Google Scholar] [CrossRef]
- Croge, C.P.; Cuquel, F.L.; Pintro, P.T.M.; Biasi, L.A.; de Bona, C.M. Antioxidant capacity and polyphenolic compounds of blackberries produced in different climates. HortScience 2019, 54, 2209–2213. [Google Scholar] [CrossRef]
- Ungureanu, C.R.M.; Lupitu, A.I.; Moisa, C.; Rivis, A.; Copolovici, L.O.; Poiana, M.A. Investigation on high-value bioactive compounds and antioxidant properties of blackberries and their fractions obtained by home-scale juice processing. Sustainability 2020, 12, 5681. [Google Scholar] [CrossRef]
- Bae, I.Y.; An, J.S.; Oh, I.K.; Lee, H.G. Optimized preparation of anthocyanin-rich extract from black rice and its effects on in vitro digestibility. Food Sci. Biotechnol. 2017, 26, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Evaluation of spectrophotometric methods for antioxidant compound measurement in relation to total antioxidant capacity in beverages. Food Chem. 2010, 120, 607–614. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weincheim, Germany, 2005. [Google Scholar]
- Koczka, N.; Stefanovits-Bányai, É.; Prokaj, E. Element composition, total phenolics and antioxidant activity of wild and cultivated blackberry (Rubus fruticosus L.) fruits and leaves during the harvest time. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 538. [Google Scholar] [CrossRef]
- Koca, I.; Karadeniz, B. Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea Region of Turkey. Sci. Hortic. 2009, 121, 447–450. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Jacques, A.C.; Pertuzatti, P.B.; Barcia, M.T.; Zambiazi, R.C.; Chim, J.F. Estabilidade de compostos bioativos em polpa congelada de amora-preta (Rubus fruticosus) cv. Tupy. Química Nova 2010, 33, 1720–1725. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E.; Kwolek, M.; Średnicka-Tober, D.; Kazimierczak, R. Genetic differentiation in anthocyanin content among berry fruits. Curr. Issues Mol. Biol. 2021, 43, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Van Den Broeck, E.; Raes, K.; De Clerck, K.; Van Speybroeck, V.; De Meester, S. A comparative theoretical study on the solvent dependency of anthocyanin extraction profiles. J. Mol. Liq. 2022, 351, 118606. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Monrad, J.K.; Howard, L.R.; Hansen, C.M. Optimization of subcritical fluid extraction of bioactive compounds using Hansen solubility parameters. J. Food Sci. 2009, 74, E342–E354. [Google Scholar] [CrossRef] [PubMed]
- Kumoro, A.C.; Retnowati, D.S.; Budiyati, C.S. Solubility of delphinidin in water and various organic solvents between (298.15 and 343.15) K. J. Chem. Eng. Data 2010, 55, 2603–2606. [Google Scholar] [CrossRef]
- András, C.D.; Mátyás, L.; Ráduly, B.; Salamon, R.V. Increasing the prediction efficiency of Hansen solubility parameters in supercritical fluids. Period. Polytech. Chem. Eng. 2019, 63, 286–293. [Google Scholar] [CrossRef]
- Mihalovits, M.; Körösi, M.; Székely, E. New formula for the hydrogen-bonding Hansen component of methanol, ethanol, and n-propanol for non-ambient conditions—Application in gas antisolvent fractionation-based optical resolution. ACS Omega 2021, 6, 18964–18974. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).