Multimycotoxin Analysis in Oat, Rice, Almond and Soy Beverages by Liquid Chromatography-Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples
2.3. Multi-Mycotoxin Extraction Method in Vegetable Drinks
2.4. Determination by Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS)
3. Results and Discussion
3.1. Validation of the Multi-Mycotoxin Determination Method in Vegetable Drinks
3.1.1. Selectivity
3.1.2. Linearity
3.1.3. Sensitivity
3.1.4. Matrix Effect
3.1.5. Repeatability and Reproducibility
3.2. Analysis of Vegetable Drinks
3.2.1. Presence of Mycotoxins
3.2.2. Mycotoxin Co-Presence
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Commission implementing regulation (EU) 2019/1715 of 30 September 2019 laying down rules for the functioning of the information management system for official controls and its system components. Off. J. Eur. Union 2019, L261, 37–96.
- RASFF-Preliminary Annual Report. 2016. Available online: http://ec.europa.eu/food/food/rapidalert/index_en.htm (accessed on 2 September 2017).
- Codex Alimentarius. International Food Standards. In Code of Practice to Prevent and Reduce Mycotoxin Contamination of Cereals CAC/RCP 51-2003, Adopted in 2003; Codex Alimentarius Commission: Rome, Italy, 2003; Amendments 2014.
- REGULATION (EU) No. 1308/2013 of the European Parliament and of the Council of December 17, 2013 Creating the Common Organization of the Markets for Agricultural Products and Repealing Regulations (EEC) No. 922/72, (EEC) No. 234/79, (EC) No. 1037/2001 and (EC) No. 1234/2007. Off. J. Eur. Union 2013, L347, 671–854.
- Directive 2000/13/EC of the European Parliament and of the Council of March 20, 2000 on the approximation of the laws of the Member States regarding the labeling, presentation and advertising of food products. Off. J. Eur. Communities 2000, L109, 29–42.
- REGULATION (EU) No. 1169/2011 of the European Parliament and of the Council of October 25, 2011 on Food Information Provided to the Consumer and by which Regulations (EC) No. 1924/2006 and (EC) No. 1925/2006 are amended of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Directives 2002/67/EC, and 2008/5/EC of the Commission, and Commission Regulation (EC) No. 608/2004. Off. J. Eur. Union 2011, L304, 18–63.
- Directive 2006/114/EC of the European Parliament and of the Council of 12 December 2006 on misleading advertising and comparative advertising. Off. J. Eur. Union 2006, L376, 21–27.
- Miró-Abella, E.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Determination of mycotoxins in plant-based beverages using QuEChERS and liquid chromatography–tandem mass spectrometry. Food Chem. 2017, 229, 366–372. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef]
- Yang, Y.; Guoliang, L.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Hamed, A.M.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Determination of Fusarium toxins in functional vegetable milks applying salting-out-assisted liquid–liquid extraction combined with ultra-high-performance liquid chromatography tandem mass spectrometry. Food Addit. Contam. Part A 2017, 34, 2033–2041. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Hamed, A.M.; García-Campaña, A.M.; Gámiz-Gracia, L. Plant-based milks: Unexplored source of emerging mycotoxins. A proposal for the control of enniatins and beauvericin using UHPLC-MS/MS. Food Addit. Contam. Part B 2019, 12, 296–302. [Google Scholar] [CrossRef]
- Hamed, A.M.; Abdel-Hamid, M.; Gámiz-Gracia, L.; García-Campaña, A.M.; Arroyo-Manzanares, N. Determination of aflatoxins in plant-based milk and dairy products by dispersive liquid–liquid microextraction and high- performance liquid chromatography with fluorescence detection. Anal. Lett. 2019, 52, 363–372. [Google Scholar] [CrossRef]
- Romero-González, R.; Martinez, J.L.; Aguilera-Luiz, M.M.; Garrido, A. Application of Conventional Solid-Phase Extraction for Multimycotoxin Analysis in Beers by Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2009, 57, 9385–9392. [Google Scholar] [CrossRef]
- Mariño-Repizo, L.; Kero, F.; Vandell, V.; Senior, A.; Sanz-Ferramola, M.I.; Cerutti, S.; Raba, J. A novel solid phase extraction-Ultra high performance liquid chromatography–tandem mass spectrometry method for the quantification of ochratoxin A in red wines. Food Chem. 2015, 172, 663–668. [Google Scholar] [CrossRef]
- Delgado, T.; Gómez-Cordovés, C.; Scott, P.M. Determination of alternariol and alternariol methyl ether in apple juice using solid-phase extraction and high performance liquid chromatography. J. Chromatogr. A 1996, 731, 109–114. [Google Scholar] [CrossRef]
- Vendl, O.; Berthiller, F.; Crews, C.; Krska, R. Simultaneous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal-based food by LC-MS-MS. Anal. Bioanal. Chem. 2009, 395, 1347–1354. [Google Scholar] [CrossRef]
- García-Moraleja, A.; Font, G.; Mañes, J.; Ferrer, E. Simultaneous determination of mycotoxin in commercial coffee. Food Control 2015, 57, 282–292. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Rocha, L.O.; Fontes, L.C.; Carnielli, L.; Reis, T.A.; Corrêa, B. Mycotoxin analysis of industrial beers from Brazil: The influence of fumonisin B1 and deoxynivalenol in beer quality. Food Chem. 2017, 218, 64–69. [Google Scholar] [CrossRef]
- Juan, C.; Oueslati, S.; Mañes, J.; Berrada, H. Multimycotoxin Determination in Tunisian Farm Animal Feed. J. Food Sci. 2019, 84, 3885–3893. [Google Scholar] [CrossRef]
- Commission Decision No. 2002/657/EC of August 12, 2002 applying Council Directive 96/23/EC regarding the operation of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, L221, 8–39.
- Commission Recommendation No. 2013/165/EU of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, L91, 12–15.
- Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (including amendments as of 01 September 2014). Off. J. Eur. Union 2006, L364, 5–24.
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef]
- Juan, C.; Raiola, A.; Mañes, J.; Ritieni, A. Presence of mycotoxin in commercial infant formulas and baby foods from Italian market. Food Control 2014, 39, 227–236. [Google Scholar] [CrossRef]
- Juan-García, A.; Juan, C.; Manyes, L.; Ruiz, M.-J. Binary and tertiary combination of alternariol, 3-acetyl-deoxynivalenol and 15-acetyl-deoxynivalenol on HepG2 cells: Toxic effects and evaluation of degradation products. Toxicol. In Vitro 2016, 34, 264–273. [Google Scholar] [CrossRef]
- Zouaoui, N.; Mallebrera, B.; Berrada, H.; Abid-Essefi, S.; Ruiz, M.J. Cytotoxic effects induced by patulin, sterigmatocystin and beauvericin on CHO–K1 cells. Food Chem. Toxicol. 2016, 89, 92–103. [Google Scholar] [CrossRef]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Mañes, J. Presence of Enniatins and Beauvericin in Romanian Wheat Samples: From Raw Material to Products for Direct Human Consumption. Toxins 2017, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Juan, C.; Berrada, H.; Mañes, J.; Oueslati, S. Multi-mycotoxin determination in barley and derived products from Tunisia and estimation of their dietary intake. Food Chem. Toxicol. 2016, 89, 92–103. [Google Scholar] [CrossRef]
- El-Badry, S. Determination of ochratoxin A residues in some animal and plant milk products. Zagazig Vet. J. 2016, 44, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Roselló-Soto, E.; García, C.; Fessard, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F. Nutritional and microbiological quality of tiger nut tubers (Cyperus esculentus) derived plant-based and lactic fermented beverages. Fermentation 2018, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Sebastia, N.; Soler, C.; Soriano, J.M.; Mañes, J. Occurrence of aflatoxins in tigernuts and their beverages commercialized in Spain. J. Agric. Food Chem. 2010, 58, 2609–2612. [Google Scholar] [CrossRef]
- Rubert, J.; Sebastia, N.; Soriano, J.M.; Soler, C.; Mañes, J. One-year monitoring of aflatoxins and ochratoxin A in tiger-nuts and their beverages. Food Chem. 2011, 127, 822–826. [Google Scholar] [CrossRef] [PubMed]
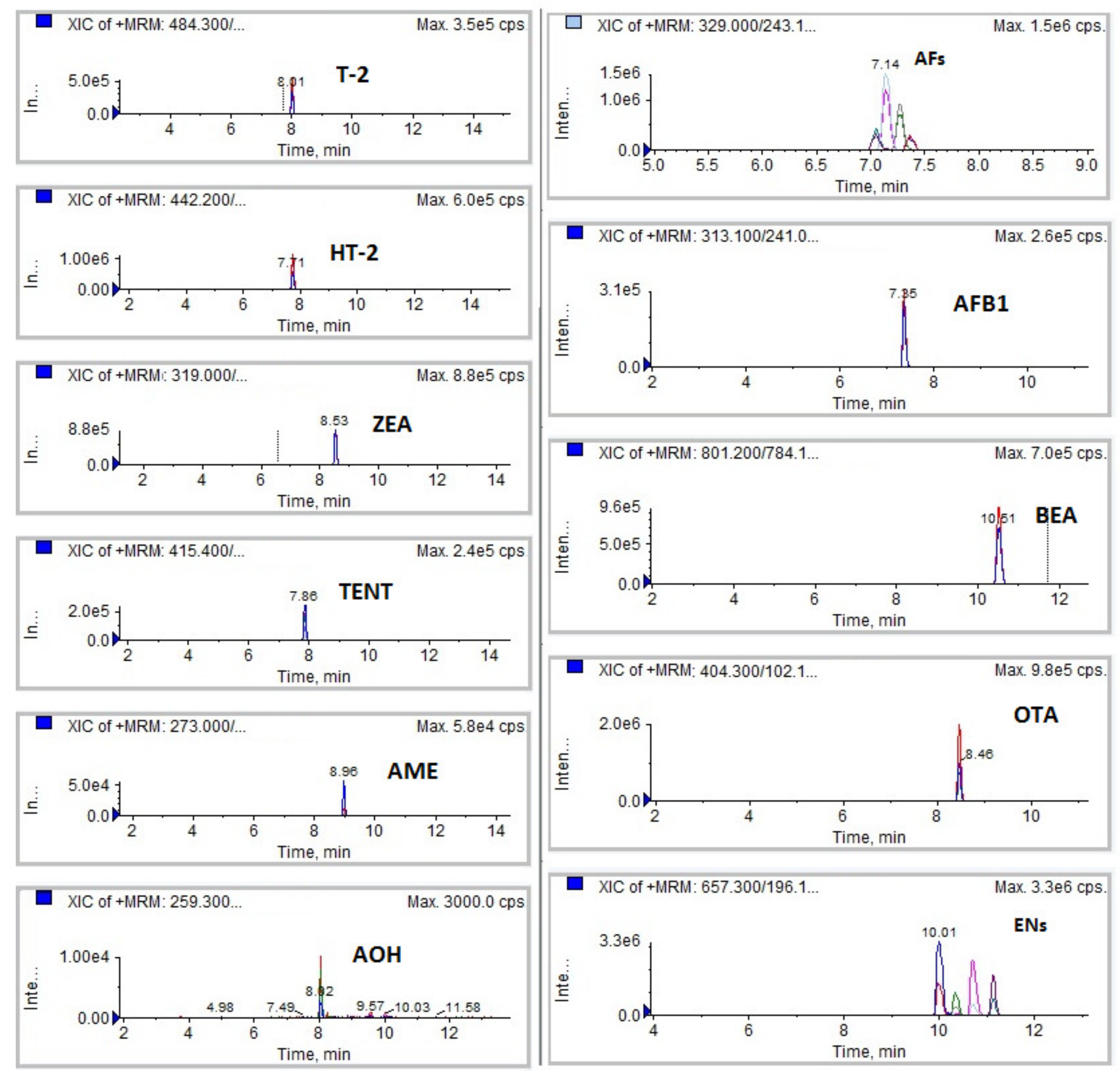
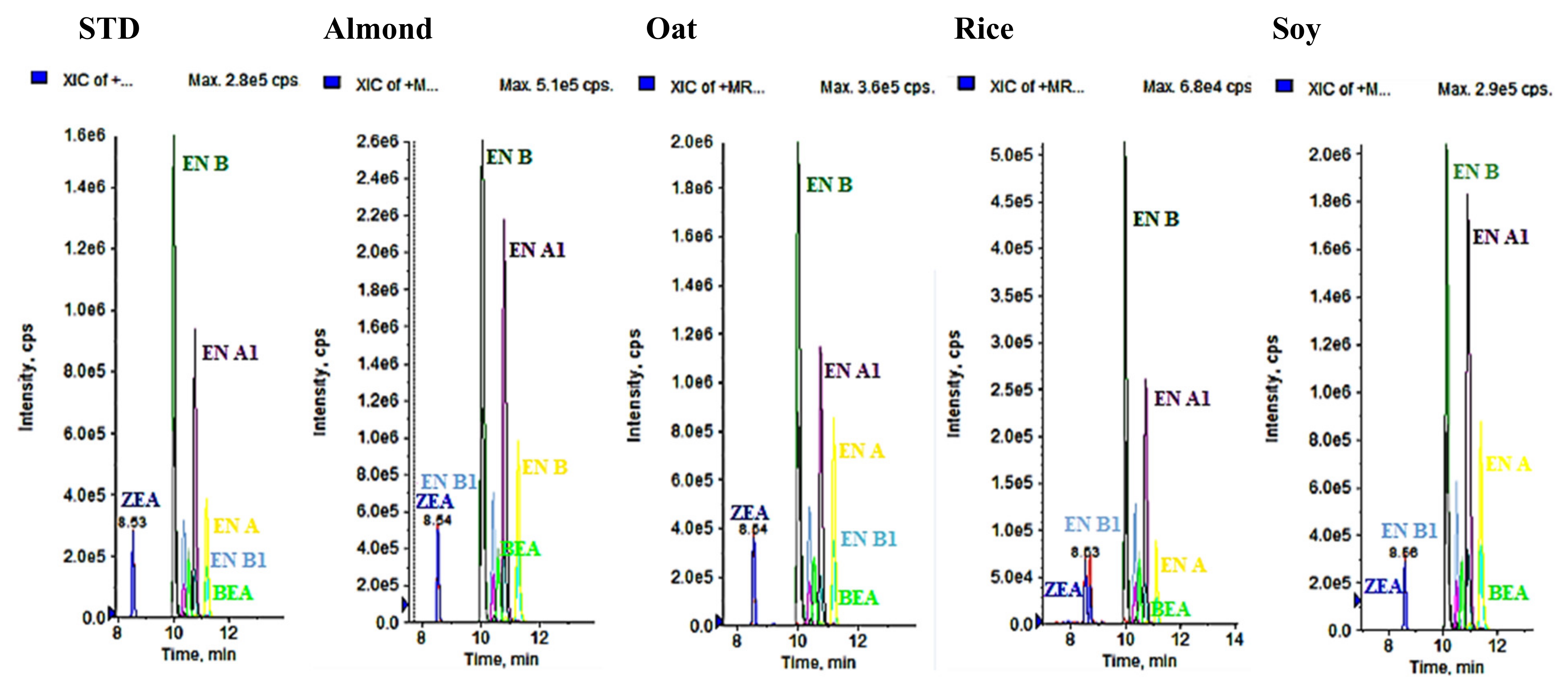

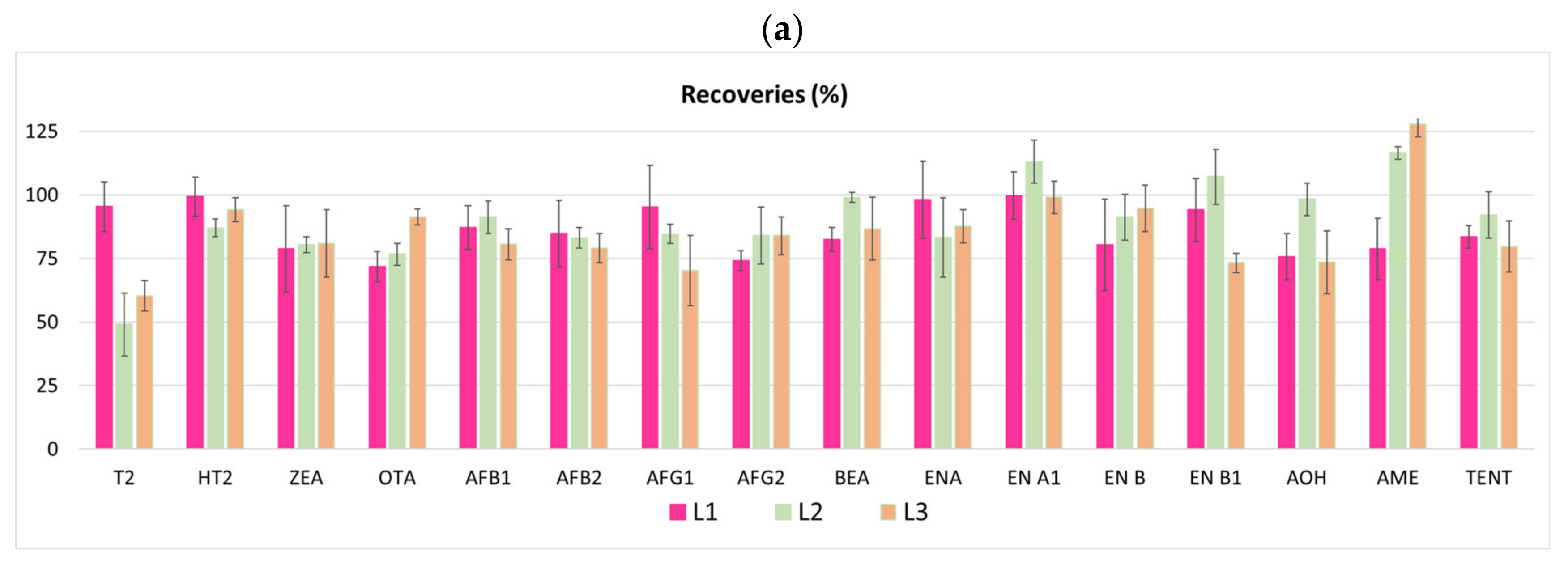

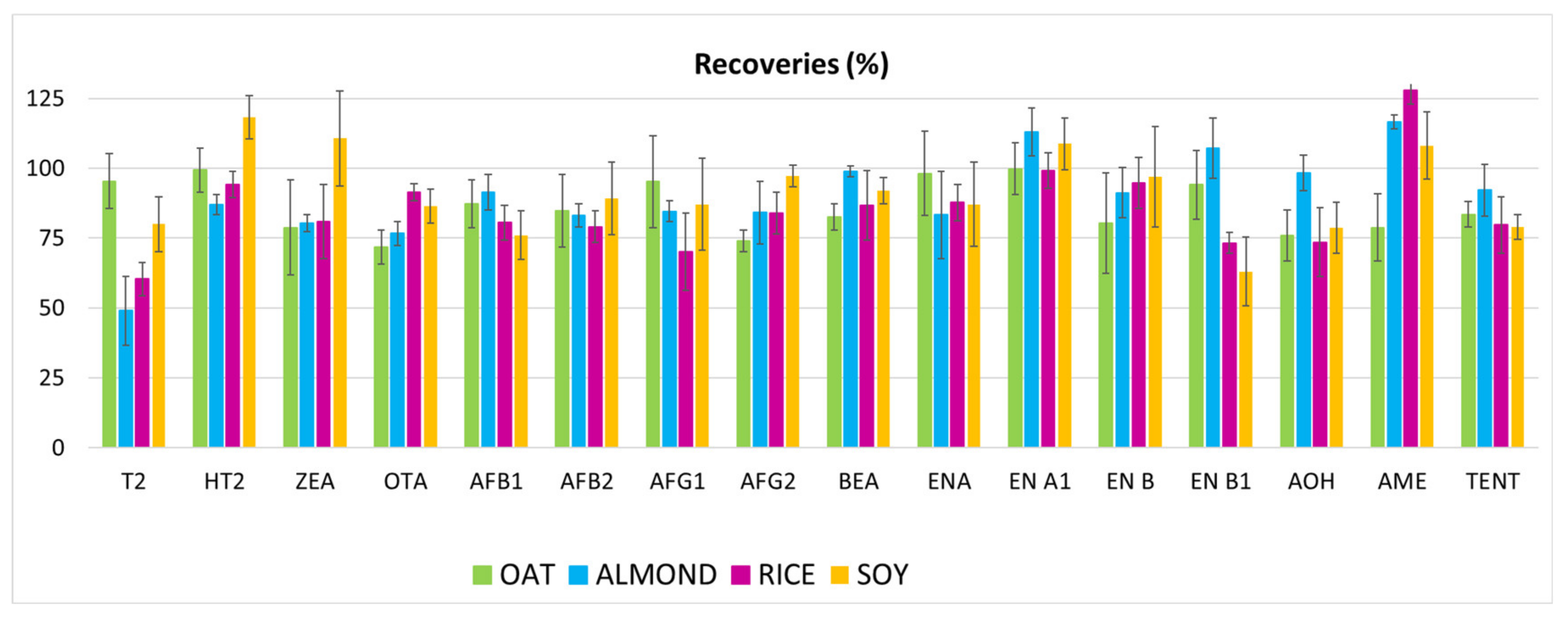


| Analytes | Matrix | SPE Extraction Technique | References |
|---|---|---|---|
| AOH, AME | Apple juice | SPE C18 cartridges | [16] |
| DON, ZEA | Cereal-based foods | SPE C18 cartridges Oasis HLB | [17] |
| 21 mycotoxins: OTA, AFs (AFB1, AFB2, AFG1, AFG2) NIV, STG, DON, 3AcDON, 15AcDON, NEO, HT-2, T-2, FB1, FB2, ENA, ENA1, ENB, ENB1, BEA | Coffee drinks | SPE C18 cartridges | [18] |
| OTA | Red wine | SPE C18 cartridges | [15] |
| Analyte | MS/MS Detection Parameters | |||||
|---|---|---|---|---|---|---|
| Precursor Ion Q1 (m/z) | Product Ion Q3 (m/z) | DP (V) | CEP (V) | EC (V) | CXP (V) | |
| HT-2 | 442.2 [M + NH4] + | 262.8 Q 215.4 | 21 | 22 | 19 19 | 4 8 |
| T-2 | 484.3 [M + NH4] + | 185.1 Q 215.1 | 21 | 23 | 22 29 | 4 4 |
| ZEA | 319.0 [M + H] + | 301.0 Q 282.9 | 26 | 18 | 15 19 | 10 4 |
| BEA | 801.2 [M + NH4] + | 784.1 Q 244.1 | 116 | 33 | 27 39 | 10 6 |
| ENA | 699.4 [M + NH4] + | 210.1 Q 228.2 | 76 | 30 | 35 59 | 14 16 |
| ENA1 | 685.4 [M + NH4] + | 210.2 Q 214.2 | 66 | 29 | 37 59 | 8 10 |
| ENB | 657.3 [M + NH4] + | 196.1 Q 214.0 | 51 | 28 | 39 59 | 8 10 |
| ENB1 | 671.2 [M + NH4] + | 214.1 Q 228.1 | 66 | 29 | 61 57 | 10 12 |
| AF B1 | 313.1 [M + H] + | 284.9 Q 241.1 | 46 | 18 | 39 41 | 4 4 |
| AF B2 | 315.1 [M + H] + | 286.9 Q 259.6 | 81 | 18 | 33 39 | 6 6 |
| AF G1 | 329.1 [M + H] + | 243.1 Q 311.1 | 76 | 18 | 39 29 | 6 6 |
| AF G2 | 329.1 [M + H] + | 313.1 Q 245.1 | 61 | 18 | 27 39 | 6 6 |
| OTA | 404.1 [M + H] + | 239.0 Q 102.1 | 55 | 21 | 27 97 | 6 6 |
| AME | 273 [M + H] + | 128 Q 228 | 32 | 16 | 60 40 | 3 3 |
| AOH | 259 [M + H] + | 128 Q 184 | 39 | 17 | 65 42 | 13 13 |
| TENT | 415 [M + H] + | 312 Q 256 | 55 | 21 | 29 39 | 2 2 |
| Mycotoxin | Maximum Limits * (ng/g) | OAT | ALMOND | RICE | SOY | ||||
|---|---|---|---|---|---|---|---|---|---|
| LQ (ng/mL) | LD (ng/mL) | LQ (ng/mL) | LD (ng/mL) | LQ (ng/mL) | LD (ng/mL) | LQ (ng/mL) | LD (ng/mL) | ||
| HT-2 | * Sum HT-2 + T-2: 50 a | 5.3 | 1.1 | 7.3 | 1.5 | 18.0 | 3.6 | 6.5 | 1.3 |
| T-2 | 4.2 | 0.8 | 5.8 | 1.2 | 14.2 | 2.8 | 5.1 | 1.0 | |
| ZEA | 20 b | 0.7 | 0.1 | 0.9 | 0.2 | 2.3 | 0.5 | 0.8 | 0.2 |
| AFB1 | 8 c; 2 b | 0.3 | 0.1 | 0.5 | 0.1 | 1.1 | 0.2 | 0.4 | 0.1 |
| AFB2 | Sum AFs: 10 c; 4 b | 0.3 | 0.1 | 0.5 | 0.1 | 1.1 | 0.2 | 0.4 | 0.1 |
| AFG1 | 0.3 | 0.1 | 0.5 | 0.1 | 1.1 | 0.2 | 0.4 | 0.1 | |
| AFG2 | 0.3 | 0.1 | 0.5 | 0.1 | 1.1 | 0.2 | 0.4 | 0.1 | |
| OTA | 3 d,e | 2.0 | 0.4 | 2.8 | 0.6 | 2.6 | 1.1 | 1.6 | 0.3 |
| BEA | - | 1.0 | 0.2 | 1.4 | 0.3 | 3.4 | 0.7 | 1.2 | 0.2 |
| ENA | - | 1.0 | 0.2 | 1.4 | 0.3 | 3.4 | 0.7 | 1.2 | 0.2 |
| ENA1 | - | 1.0 | 0.2 | 1.4 | 0.3 | 3.4 | 0.7 | 1.2 | 0.2 |
| ENB | - | 1.0 | 0.2 | 1.4 | 0.3 | 3.4 | 0.7 | 1.2 | 0.2 |
| ENB1 | - | 1.0 | 0.2 | 1.4 | 0.3 | 3.4 | 0.7 | 1.2 | 0.2 |
| AOH | - | 2.3 | 0.5 | 2.8 | 0.6 | 7.9 | 1.6 | 2.9 | 0.6 |
| AME | - | 2.3 | 0.5 | 4.6 | 0.9 | 6.8 | 1.4 | 2.5 | 0.5 |
| TENT | - | 1.0 | 0.2 | 1.4 | 0.3 | 3.4 | 0.7 | 1.2 | 0.2 |
| Mycotoxin | Oat Beverages (n = 17) | Almond Beverages (n = 11) | Rice Beverages (n = 10) | Soy Beverages (n = 18) | Total Beverages (n = 56) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | Mp | Mx | M | Mp | Mx | M | Mp | Mx | M | Mp | Mx | M | Mp | Mx | |
| HT-2 | 2.68 ± 5.3 | 9.10 ± 0.8 | 10.21 | 0.33 ± 2.3 | 3.60 | 3.60 | n.d. | n.d. | n.d. | 4.56 ± 55 | 82.09 | 82.09 | 2.34 ± 56 | 29.86 ± 37 | 82.09 |
| T-2 | 0.49 ± 2.9 | 4.18 ± 0.3 | 4.55 | 0.20 ± 1.4 | 2.20 | 2.20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.19 ± 3 | 1.70 ± 2 | 4.55 |
| ZEA | 0.33 ± 3.8 | 5.68 | 5.68 | 0.57 ± 1.8 | 3.13 | 3.17 | 6.24 ± 8.4 | 15.61 ± 1.8 | 18.14 | 1.80 ± 6.5 | 10.79 ± 0.2 | 11.02 | 1.91 ± 12 | 8.59 ± 7 | 18.14 |
| AFB1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| AFB2 | n.d. | n.d. | n.d. | 0.07 ± 0.4 | 0.7 | 0.7 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.14 ± 04 | 0.7 |
| AFG1 | 0.01 ± 0.1 | 0.10 | 0.10 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.03 ± 0.05 | 0.10 |
| AFG2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| OTA | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| BEA | 1.19 ± 0.7 | 1.35 ± 0.5 | 2.12 | 0.15 ± 1 | 1.60 | 1.60 | 0.50 ± 1.2 | 1.67 ± 0.35 | 2.17 | 0.30 ± 1.1 | 1.81 ± 0.01 | 1.82 | 0.58 ± 1.1 | 1.60 ± 04 | 2.17 |
| ENA | 0.08 ± 04 | 0.65 | 0.65 | 0.37 ± 1.3 | 2.01 ± 0.2 | 2.25 | 0.00 | n.d. | 0.00 | 0.00 | n.d. | 0.00 | 0.09 ± 1.5 | 0.59 ± 1.2 | 2.25 |
| ENA1 | 1.67 ± 2.4 | 2.59 ± 1.7 | 5.02 | 0.47 ± 2.3 | 2.57 ± 0.8 | 3.66 | 0.00 | n.d. | 0.00 | 0.30 ± 1.7 | 2.74 | 2.74 | 0.70 ± 3 | 2.17 ± 2 | 5.02 |
| ENB | 2.83 ± 2.6 | 3.21 ± 2.3 | 6.52 | 10.1 ± 69 | 55.42 ± 37 | 108.85 | 0.82 ± 2.5 | 4.08 ± 0.16 | 4.30 | 1.52 ± 1.7 | 2.29 ± 1.14 | 3.90 | 3.47 ± 74 | 13.33 ± 68 | 108.85 |
| ENB1 | 1.02 ± 3.9 | 1.16 ± 3.8 | 6.52 | 5.79 ± 38 | 15.92 ± 31 | 60.03 | 0.17 ± 1.1 | 0.83 ± 0.6 | 1.65 | 0.72 ± 1.3 | 2.59 ± 0.02 | 2.62 | 1.71 ± 41 | 4.46 ± 39 | 60.03 |
| AOH | 0.16 ± 0.8 | 0.70 ± 0.5 | 1.35 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.05 ± 0.9 | 0.21 ± 0.8 | 1.35 |
| AME | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| TENT | 2.93 ± 19 | 16.62 ± 10 | 30.60 | 15.1 ± 59 | 82.81 ± 11 | 98.08 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.85 ± 67 | 21.30 ± 54 | 98.08 |
| Combinations of Mycotoxins Present in the Same Sample (Incidence) | |||
|---|---|---|---|
| 2 Mycotoxins | 3 Mycotoxins | 4 Mycotoxins | >5 Mycotoxins |
| TENT + ZEA | ENA1 + B + B1 (3) | BEA + ENA1 + B + B1 (3) | BEA + ENA + A1 + B + B1 + HT-2 + TENT + ZEA |
| ENB + HT2 | ENB + B1 + ZEA | BEA + AOH + ENB + B1 | BEA+ EN A1 + B + B2 + HT-2 + TENT |
| ENB + B1 | BEA + ENB + ZEA | BEA + ENA1 + B + B1 (2) | BEA + AOH + ENA + A1 + B + B1 + HT-2 + TENT |
| ENB + ZEA | BEA + EN B + B1 | BEA + ENA1 + B + B1 + HT-2 + T-2 + TENT + ZEA | |
| ENB1 + ZEA | BEA + ENB + ZEA | BEA + ENA + A1 + B + B1 + HT-2 | |
| BEA + ENB + B1 (3) | |||
| BEA + ENA1 + B | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan, C.; Mañes, J.; Juan-García, A.; Moltó, J.C. Multimycotoxin Analysis in Oat, Rice, Almond and Soy Beverages by Liquid Chromatography-Tandem Mass Spectrometry. Appl. Sci. 2022, 12, 3942. https://doi.org/10.3390/app12083942
Juan C, Mañes J, Juan-García A, Moltó JC. Multimycotoxin Analysis in Oat, Rice, Almond and Soy Beverages by Liquid Chromatography-Tandem Mass Spectrometry. Applied Sciences. 2022; 12(8):3942. https://doi.org/10.3390/app12083942
Chicago/Turabian StyleJuan, Cristina, Jordi Mañes, Ana Juan-García, and Juan Carlos Moltó. 2022. "Multimycotoxin Analysis in Oat, Rice, Almond and Soy Beverages by Liquid Chromatography-Tandem Mass Spectrometry" Applied Sciences 12, no. 8: 3942. https://doi.org/10.3390/app12083942
APA StyleJuan, C., Mañes, J., Juan-García, A., & Moltó, J. C. (2022). Multimycotoxin Analysis in Oat, Rice, Almond and Soy Beverages by Liquid Chromatography-Tandem Mass Spectrometry. Applied Sciences, 12(8), 3942. https://doi.org/10.3390/app12083942








