Clinical-Pathological Study on Expressions β-APP, GFAP, NFL, Spectrin II, CD68 to Verify Diffuse Axonal Injury Diagnosis, Grade and Survival Interval
Abstract
:1. Introduction
1.1. Aims and Objectives
1.2. Biomechanics and Pathophysiology of Axonal Injury
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Working Technique Used
2.3. Immunohistochemical Staining Technique of Sections
2.4. Microscopic Examination
2.5. Immunohistochemical Staining
2.6. Statistical Analysis
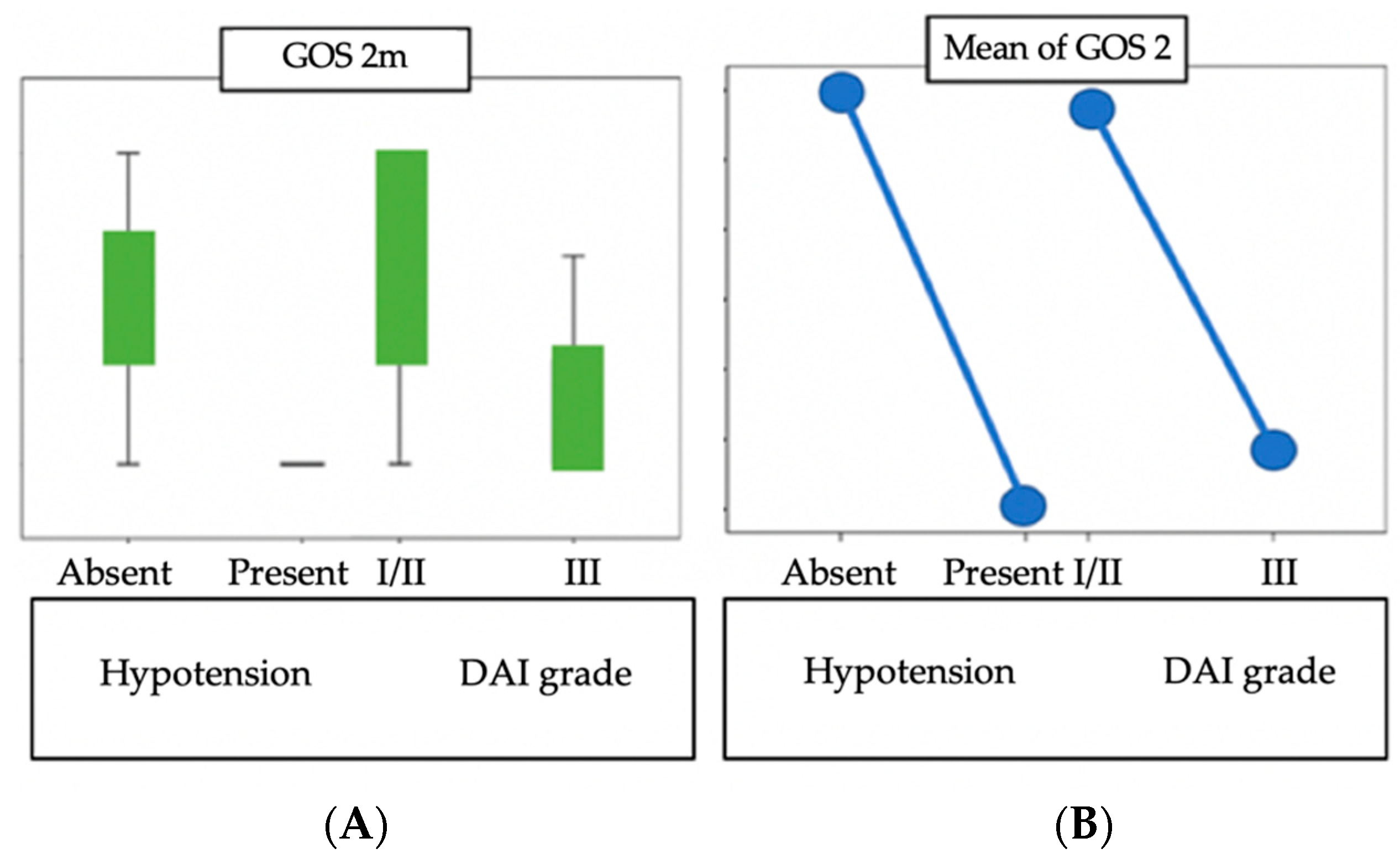
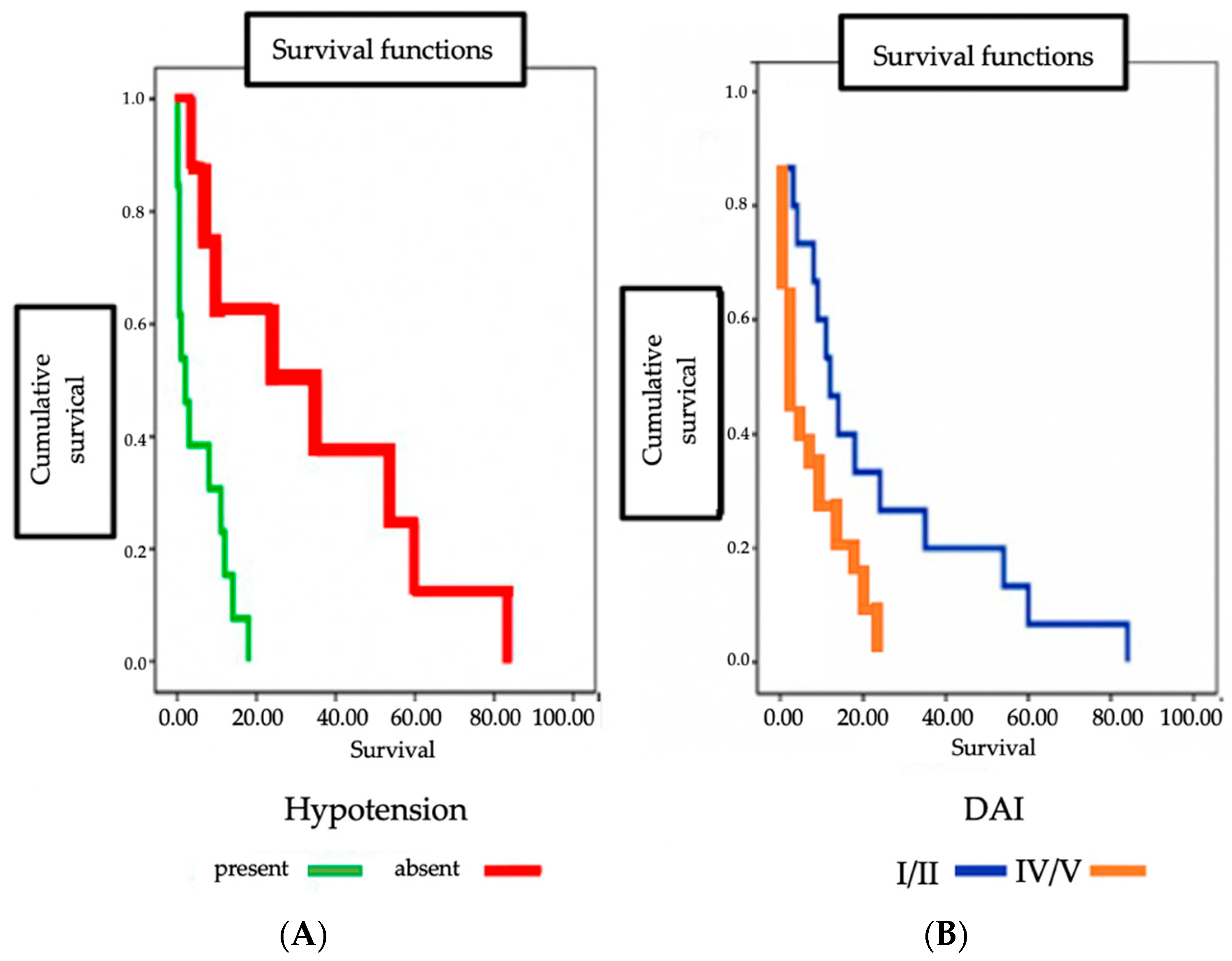
3. Results
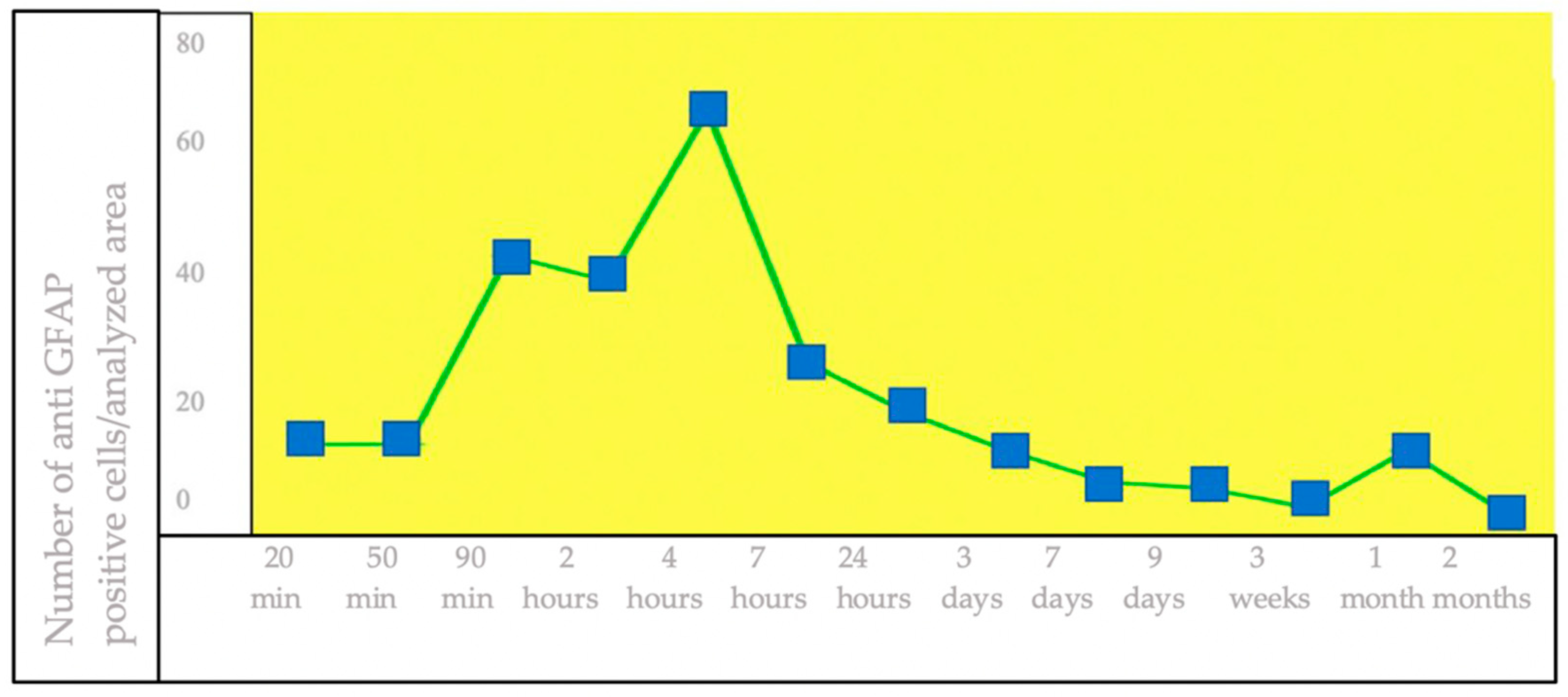
3.1. IHC Staining for the Detection of GFAP+ Brain Cells
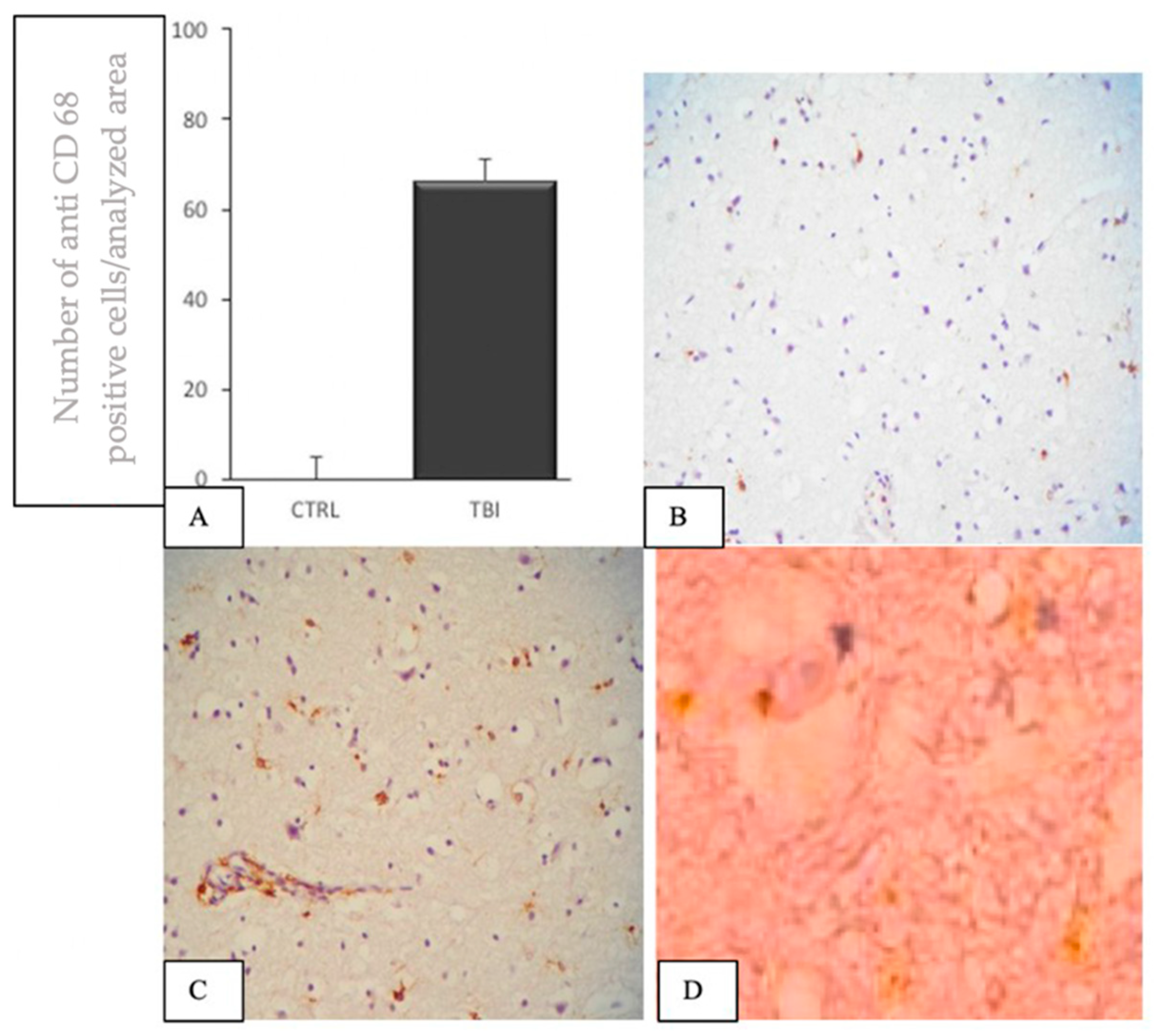
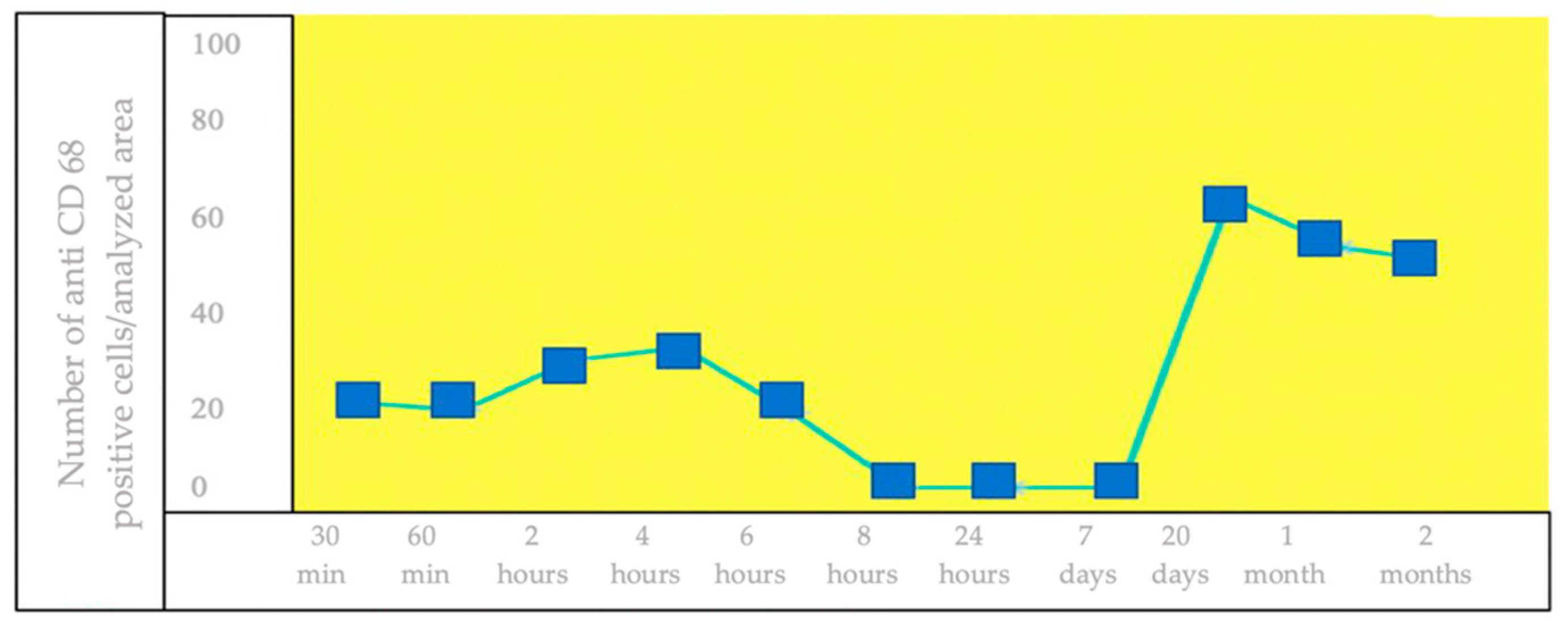
3.2. IHC Staining for the Detection of CD68+ Brain Cells
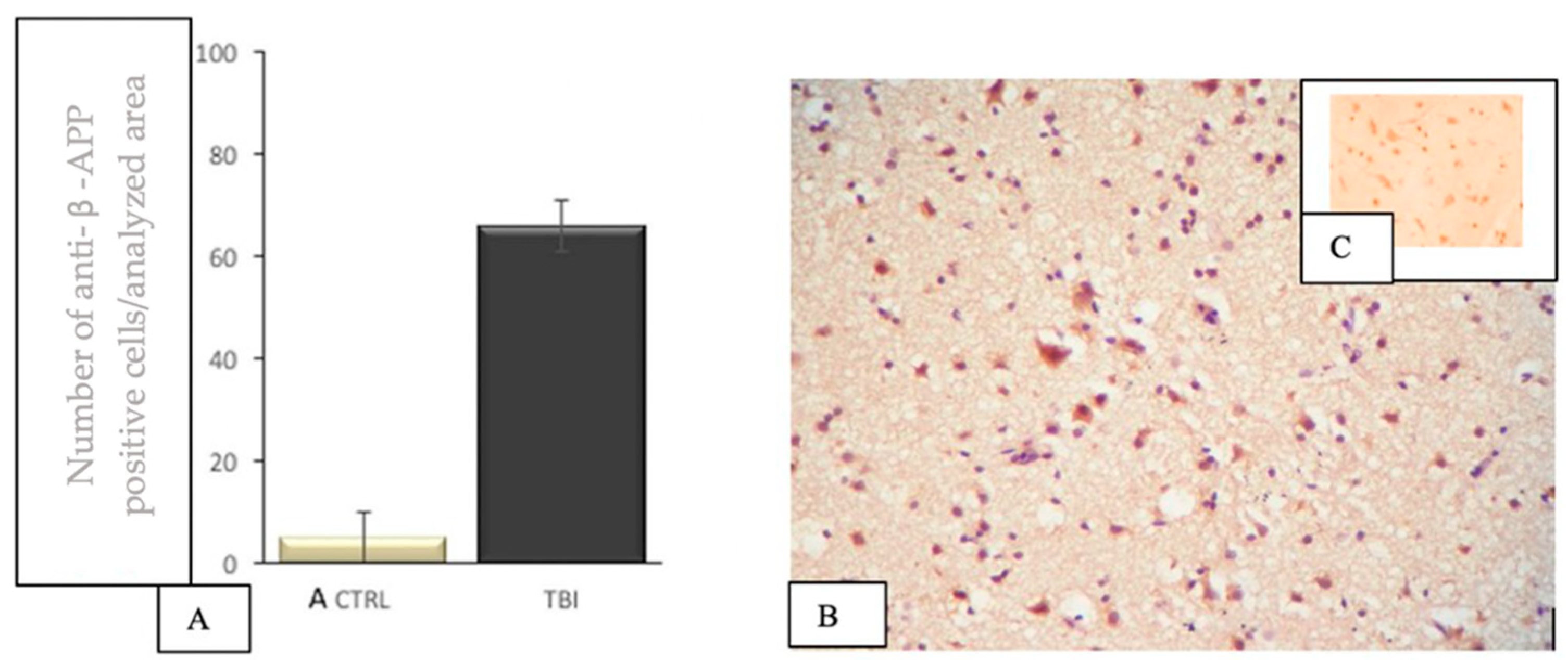
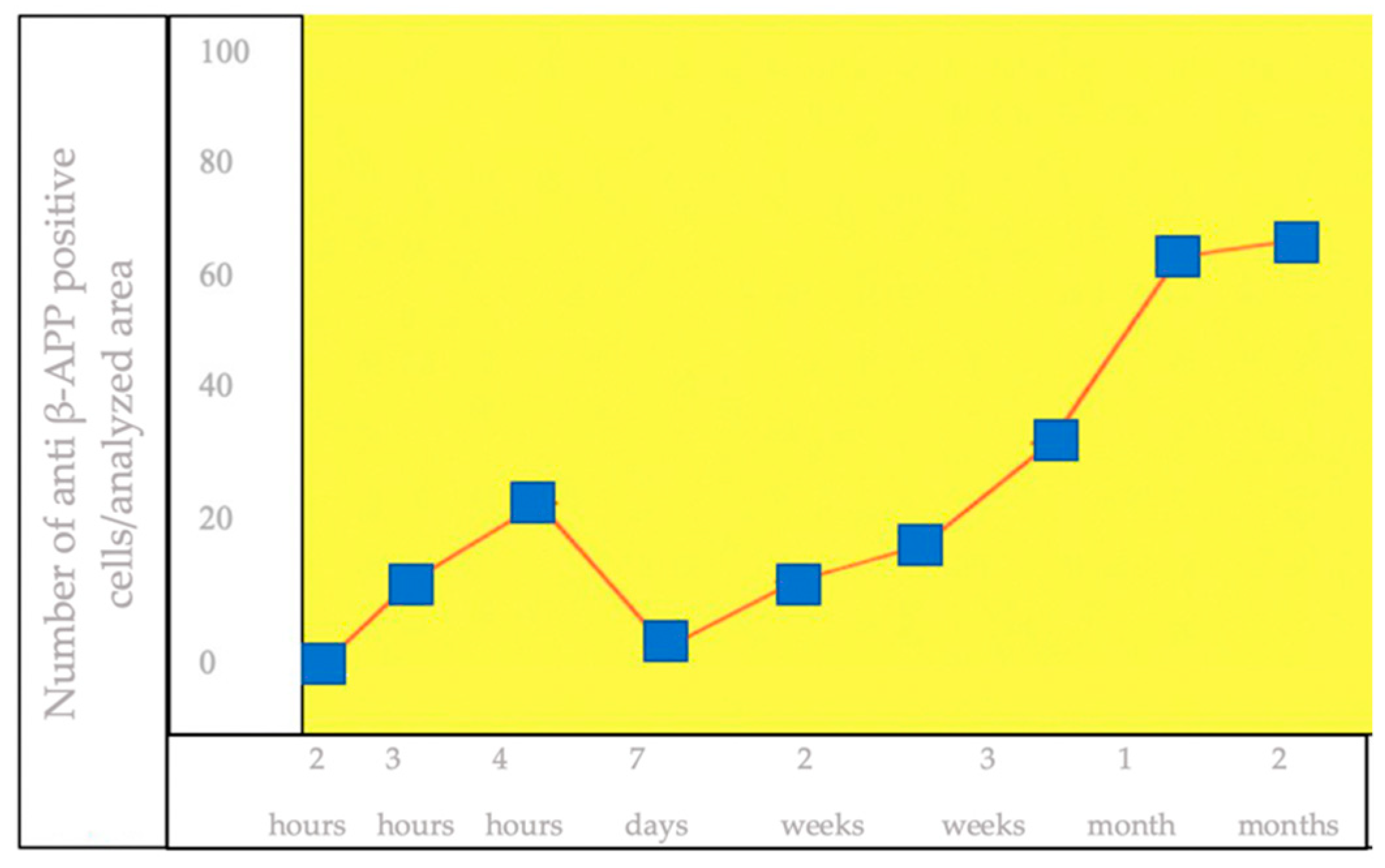
3.3. IHC Staining for the Detection of β-APP+ Brain Cells
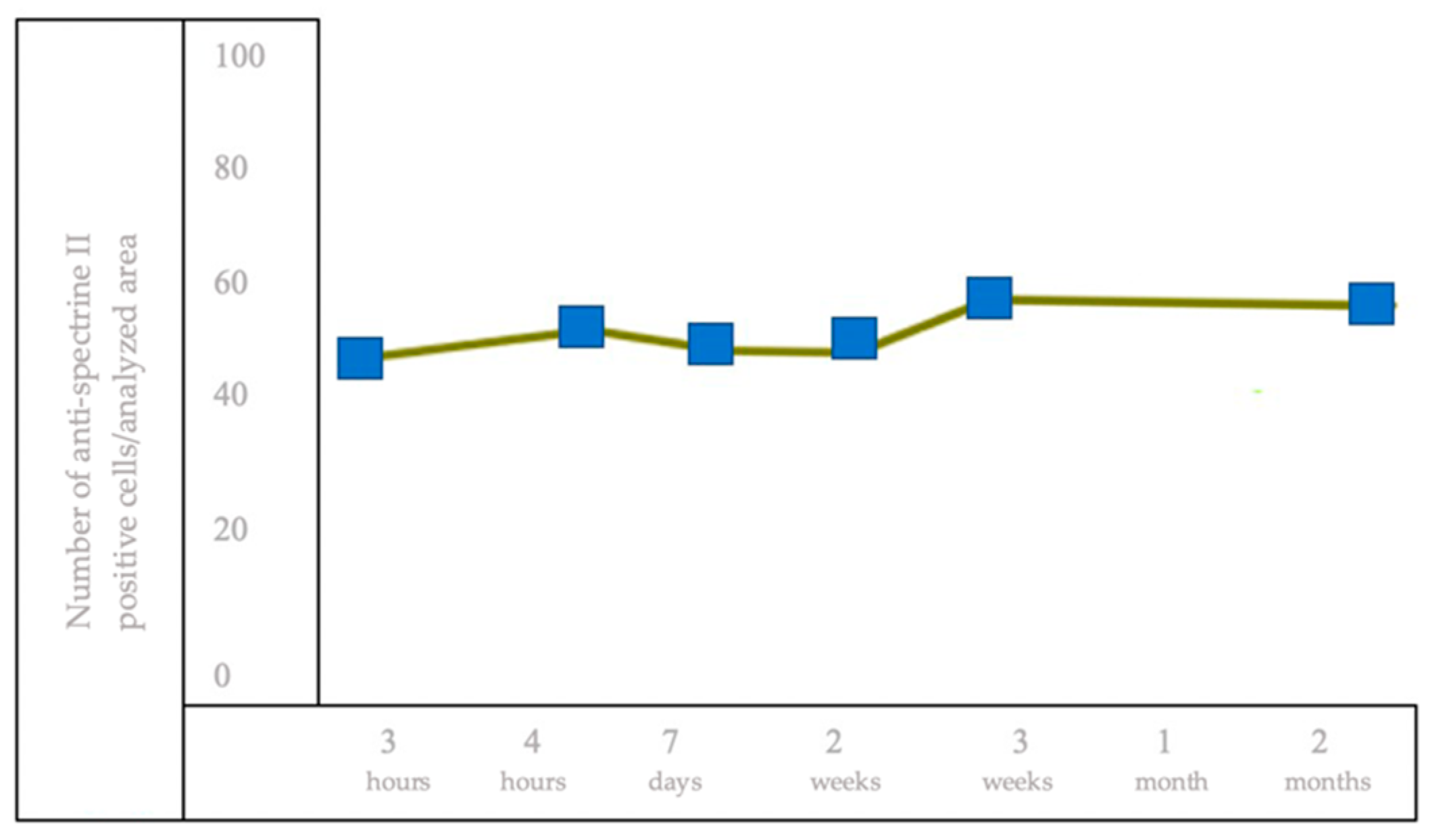
3.4. IHC Staining for the Detection of Brain Cells of Anti-Spectrin II+
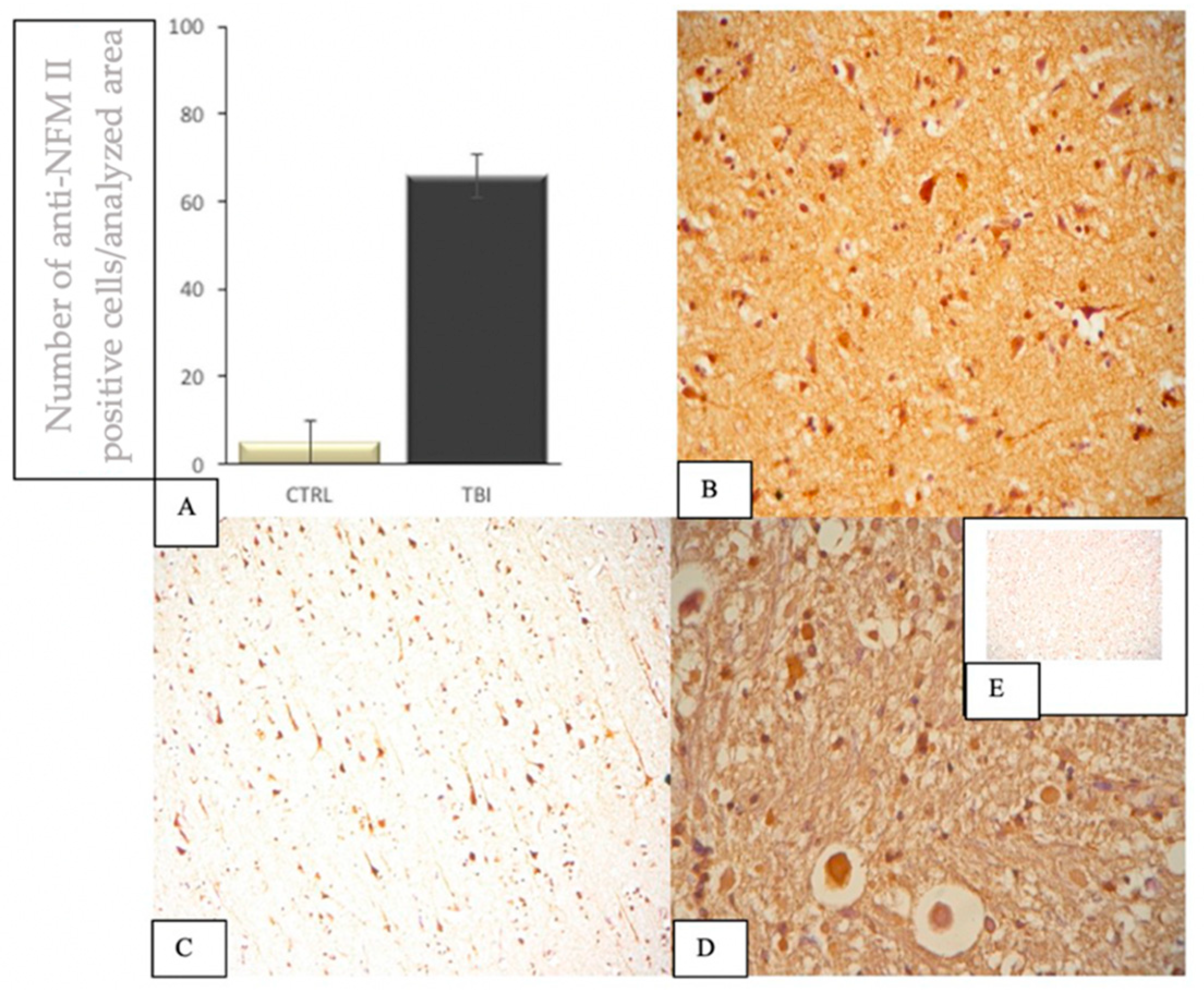
3.5. IHC Staining to Highlight Brain Cells of NFM II+
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mata-Mbemba, D.; Mugikura, S.; Nakagawa, A.; Murata, T.; Kato, Y.; Tatewaki, Y.; Li, L.; Takase, K.; Ishii, K.; Kushimoto, S.; et al. Intraventricular hemorrhage on initial computed tomography as marker of diffuse axonal injury after traumatic brain injury. J. Neurotrauma 2015, 32, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hunea, I.; Damian, S.I.; David, S.; Diac, M.M.; Iliescu, D.B.; Ciocoiu, M. Brief literature Review of the Mechanism involved in producing diffuse axonal injury. Brain 2017, 8, 91–97. [Google Scholar]
- Currie, S.; Saleem, N.; Straiton, J.A.; Macmullen-Price, J.; Warren, D.J.; Craven, I.J. Imaging assessment of traumatic brain injury. Postgrad. Med. J. 2016, 92, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.P.; Nelson, D.W.; Vehviläinen, J.; Nyström, H.; Kivisaari, R.; Siironen, J.; Svensson, M.; Skrifvars, M.B.; Bellander, B.M.; Raj, R. Evaluation of novel computerized tomography scoring systems in human traumaticbrain injury: An observational, multicenter study. PLoS Med. 2017, 14, e1002368. [Google Scholar] [CrossRef]
- Ripoll, M.A.; Siösteen, B.; Hartman, M.; Raininko, R. MR detectability and appearance of small experimental intracranial hematomas at 1.5 T and 0.5 T. A 6–7-month follow-up study. Acta Radiol. 2003, 44, 199–205. [Google Scholar] [CrossRef]
- Schiavone, S.; Neri, M.; Trabace, L.; Turillazzi, E. NADPH oxidase NOX2 mediates loss of parvalbumin interneurons in traumatic brain injury: Human autoptic immunohistochemical evidence. Sci. Rep. 2017, 7, 8752. [Google Scholar] [CrossRef] [Green Version]
- Yokobori, S.; Hosein, K.; Burks, S.; Sharma, I.; Gajavelli, S.; Bullock, R. Biomarkers for the Clinical Diferential Diagnosis in Traumatic Brain Injury—A Systematic Review. CNS Neurosci. Ther. 2013, 19, 556–565. [Google Scholar] [CrossRef]
- Graham, E.M.; Burd, I.; Everett, A.D.; Northington, F.J. Blood Biomarkers for Evaluation of Perinatal Encephalopathy. Front. Pharmacol. 2016, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Redell, J.B.; Zhao, J.; Dash, P.K. Altered Expression of miRNA-21 and Its Targets in the Hippocampus Afer Traumatic Brain Injury. J. Neurosci. Res. 2011, 89, 212–222. [Google Scholar] [CrossRef]
- Valiyaveettil, M.; Alamneh, Y.A.; Miller, S.A.; Hammamieh, R.; Arun, P.; Wang, Y.; Wei, Y.; Oguntayo, S.; Long, J.B.; Nambiar, N.P. Modulation of cholinergic pathways and infammatory mediators in blast-induced traumatic brain injury. Chem. Biol. Interact. 2013, 203, 371–375. [Google Scholar] [CrossRef]
- Sun, T.Y.; Chen, X.R.; Liu, Z.L.; Zhao, L.L.; Jiang, Y.X.; QU, G.Q.; Wang, R.S.; Huang, S.Z.; Liu, L. Expression Profling of MicroRNAs in Hippocampus of Rats Following Traumatic Brain Injury. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Farkas, O.; Lifshitz, J.; Povlishock, J.T. Mechanoporation induced by diffuse traumatic brain injury: An irreversible or reversible response to injury? J. Neurosci. 2006, 26, 3130–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celeghin, A.; Galetto, V.; Tamietto, M.; Zettin, M. Emotion Recognition in Low-Spatial Frequencies Is Partly Preserved following Traumatic Brain Injury. Biomed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacono, D.; Lee, P.; Hallett, M.; Perl, D. Possible Post-Traumatic Focal Dystonia Associated with Tau Pathology Localized to Putamen-Globus Pallidus. Mov. Disord. Clin. Pract. 2018, 5, 492–498. [Google Scholar] [CrossRef]
- Hendricks, H.T.; Heeren, A.H.; Vos, P.E. Dysautonomia after severe traumatic brain injury. Eur. J. Neurol. 2010, 17, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Van Eijck, M.M.; Schoonman, G.G.; Van der Naalt, J.; De Vries, J.; Roks, G. Diffuse axonal injury after traumatic brain injury is a prognostic factor for functional outcome: A systematic review and meta-analysis. Brain Inj. 2018, 32, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.T.; Arena, J.D.; Xiao, R.; Wolf, J.A.; Johnson, V.E. Clarity reveals a more protracted temporal course of axon swelling and disconnection than previously described following traumatic brain injury. Brain Pathol. 2019, 29, 437–450. [Google Scholar] [CrossRef]
- Williams, A.F.; McCartt, A.T.; Sims, L.B. History and current status of state graduated driver licensing (GDL) laws in the United States. J. Saf. Res. 2016, 56, 9–15. [Google Scholar] [CrossRef]
- Curtin, S.C.; Warner, M.; Hedegaard, H. Increase in suicide in the United States, 1999–2014. NCHS Data Briefs 2016, 241, 1–8. [Google Scholar]
- Davceva, N.; Sivevski, A.; Basheska, N. Traumatic axonal injury, a clinical-pathological correlation. J. Forensic Leg. Med. 2017, 48, 35–40. [Google Scholar] [CrossRef]
- Humble, S.S.; Wilson, L.D.; Wang, L.; Long, D.A.; Smith, M.A.; Siktberg, J.C.; Mirhoseini, M.F.; Bhatia, A.; Pruthi, S.; Day, M.A.; et al. Prognosis of diffuse axonal injury with traumatic brain injury. J. Trauma Acute Care Surg. 2018, 85, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, K.; Wang, Z.; Chen, G. Progress of Research on Diffuse Axonal Injury after Traumatic Brain Injury. Neural Plast. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]



| Antibody | Control Group | TBI Group | Thalamus | Hypothalamus | Striatum | Statistical Value TBI vs. Control |
|---|---|---|---|---|---|---|
| anti-β-APP | −/+ | +++ | +++ | +++ | +++ | p < 0.04 |
| anti-GFAP | + | +++ | +++ | +++ | +++ | p < 0.03 |
| anti-NFL | −/+ | +++ | +++ | +++ | +++ | p < 0.03 |
| anti-spectrin II | −/+ | +++ | +++ | +++ | +++ | p < 0.02 |
| anti-CD68 | −/+ | +++ | +++ | +++ | +++ | p < 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunea, I.; Riscanu, L.; Girlescu, N.; Diac, M.; Knieling, A.; David, S.; Furnica, C.; Lucasevici, C.; Dragomir, I.C.; Iliescu, D.B.; et al. Clinical-Pathological Study on Expressions β-APP, GFAP, NFL, Spectrin II, CD68 to Verify Diffuse Axonal Injury Diagnosis, Grade and Survival Interval. Appl. Sci. 2022, 12, 3638. https://doi.org/10.3390/app12073638
Hunea I, Riscanu L, Girlescu N, Diac M, Knieling A, David S, Furnica C, Lucasevici C, Dragomir IC, Iliescu DB, et al. Clinical-Pathological Study on Expressions β-APP, GFAP, NFL, Spectrin II, CD68 to Verify Diffuse Axonal Injury Diagnosis, Grade and Survival Interval. Applied Sciences. 2022; 12(7):3638. https://doi.org/10.3390/app12073638
Chicago/Turabian StyleHunea, Iuliana, Laura Riscanu, Nona Girlescu, Madalina Diac, Anton Knieling, Sofia David, Cristina Furnica, Codrin Lucasevici, Irina Catrinel Dragomir, Diana Bulgaru Iliescu, and et al. 2022. "Clinical-Pathological Study on Expressions β-APP, GFAP, NFL, Spectrin II, CD68 to Verify Diffuse Axonal Injury Diagnosis, Grade and Survival Interval" Applied Sciences 12, no. 7: 3638. https://doi.org/10.3390/app12073638
APA StyleHunea, I., Riscanu, L., Girlescu, N., Diac, M., Knieling, A., David, S., Furnica, C., Lucasevici, C., Dragomir, I. C., Iliescu, D. B., & Ciocoiu, M. (2022). Clinical-Pathological Study on Expressions β-APP, GFAP, NFL, Spectrin II, CD68 to Verify Diffuse Axonal Injury Diagnosis, Grade and Survival Interval. Applied Sciences, 12(7), 3638. https://doi.org/10.3390/app12073638







