Evaluation of Hydrogen Cyanide in the Blood of Fire Victims Based on the Kinetics of the Reaction with Ninhydrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Sampling
2.4. Distillation (HCN Capture)
2.5. The Hydrogen Cyanide Determination
3. Results
3.1. Spectrophotometric Study of the Reaction between Ninhydrin and CN−
3.2. Analytical Performance
3.3. Reproducibility
3.4. Headspace Extraction for Determination of Hydrogen Cyanide
3.5. Hydrogen Cyanide Recovery
3.6. The Determination HCN at Different Fixed Times
3.7. Chloramine-T/Barbituric Acid-Pyridine Method
4. Discussion
4.1. Basis of the Process
4.2. Micromethod Performance
4.3. Applications for Real Samples
4.4. The Toxicity of HCN
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Son, M.H.; Kim, Y.; Jo, Y.H.; Kwon, M. Assessment of chemical asphyxia caused by toxic gases generated from rigid polyurethane foam (RPUF) fires. Forensic Sci. Int. 2021, 328, 111011. [Google Scholar] [CrossRef] [PubMed]
- Manila, D.P. Hydrogen cyanide: Risk assessment, environmental, and health hazard. In Hazardous Gases Risk Assessment on the Environment and Human Health; Academic Press: Cambridge, MA, USA, 2021; pp. 183–195. [Google Scholar]
- Lulea, D.M.; Iordache, V.; Nastase, I. Numerical model development of the air temperature variation in a room set on fire for different ventilation scenarios. Appl. Sci. 2021, 11, 11698. [Google Scholar] [CrossRef]
- Ma, K.H.; Lippner, D.S.; Basi, K.A.; DeLeon, S.M.; Cappuccio, W.R.; Rhoomes, M.O.; Hildenberger, D.M.; Hoard-Fruchey, H.M.; Rockwood, G.A. Cyanide poisoning compromises gene pathways modulating cardiac injury in vivo. Chem. Res. Toxicol. 2021, 34, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Wyman, J.L.; Understanding Cyanide Toxicity in Victims of Smoke Inhalation. Doctor of Nursing Practice Capstone Projects 56. 2021. Available online: https://encompass.eku.edu/dnpcapstones/59/ (accessed on 30 December 2021).
- McAllister, J.; Kunsman, G.W.; Levine, B.S. Carbon monoxide/cyanide. In Principles of Forensic Toxicology; Springer: Cham, Switzerland, 2020; pp. 545–560. [Google Scholar]
- Loke, J.; Matthay, R.A.; Smith, G.W. The toxic environment and its medical implications with special emphasis on smoke inhalation. In Pathophysiology and Treatment of Inhalation Injuries; CRC Press: Boca Raton, FL, USA, 2020; pp. 453–504. [Google Scholar]
- Stoll, S.; Roider, G.; Keil, W. Concentrations of cyanide in blood samples of corpses after smoke inhalation of varying origin. Int. J. Legal Med. 2017, 131, 123–129. [Google Scholar] [CrossRef]
- Lawson-Smith, P.; Jansen, E.C.; Hyldegaard, O. Cyanide intoxication as part of smoke inhalation-a review on diagnosis and treatment from the emergency perspective. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 14. [Google Scholar] [CrossRef]
- Haikonen, K. Fire-Related Injuries and Their Burden in Finland 2000–2010. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2020. [Google Scholar]
- Anseeuw, K.; Delvau, N.; Burillo-Putze, G.; De Iaco, F.; Geldner, G.; Holmström, P.; Yves, L.; Sabbe, M. Cyanide poisoning by fire smoke inhalation: A European expert consensus. Eur. J. Emerg. Med. 2013, 20, 2–9. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Rużycka, M.; Wroczyński, P.; Purser, D.A.; Stec, A.A. Analysis of fire deaths in Poland and influence of smoke toxicity. Forensic Sci. Int. 2017, 277, 77–87. [Google Scholar] [CrossRef]
- Kaita, Y.; Tarui, T.; Shoji, T.; Miyauchi, H.; Yamaguchi, Y. Cyanide poisoning is a possible cause of cardiac arrest among fire victims, and empiric antidote treatment may improve outcomes. Am. J. Emerg. Med. 2018, 36, 851–853. [Google Scholar] [CrossRef]
- Tabian, D.; Drochioiu, G.; Damian, S.I.; Girlescu, N.; Toma Gradinaru, O.; Toma, S.I.; Bulgaru Iliescu, D. Toxic blood hydrogen cyanide concentration as a vital sign of a deceased room fire victim—Case report. Toxics 2021, 9, 36. [Google Scholar] [CrossRef]
- Haque, M.R.; Bradbury, J.H. Simple method for determination of thiocyanate in urine. Clin. Chem. 1999, 45, 1459–1464. [Google Scholar] [CrossRef]
- Fonollosa, J.; Solórzano, A.; Marco, S. Chemical sensor systems and associated algorithms for fire detection: A review. Sensors 2018, 18, 553. [Google Scholar] [CrossRef]
- Lindsay, A.E.; Greenbaum, A.R.; O’Hare, D. Analytical techniques for cyanide in blood and published blood cyanide concentrations from healthy subjects and fire victims. Anal. Chim. Acta 2004, 511, 185–195. [Google Scholar] [CrossRef]
- Odoul, M.; Fouillet, B.; Nouri, B.; Chambon, R.; Chambon, P. Specific determination of cyanide in blood by headspace gas chromatography. J. Anal. Toxicol. 1994, 18, 205–207. [Google Scholar] [CrossRef]
- Darr, R.W.; Capson, T.L.; Hileman, F.D. Determination of hydrogen cyanide in blood using gas chromatography with alkali thermionic detection. Anal. Chem. 1980, 52, 1379–1381. [Google Scholar] [CrossRef]
- Kaur, P.; Upadhyay, S.; Gupta, V.K. Spectrophotometric determination of hydrogen cyanide in air and biological fluids. Analyst 1987, 112, 1681–1683. [Google Scholar] [CrossRef]
- Ma, J.; Dasgupta, P.K. Recent developments in cyanide detection: A review. Anal. Chim. Acta 2010, 673, 117–125. [Google Scholar] [CrossRef]
- Lindsay, A.E.; O’Hare, D. The development of an electrochemical sensor for the determination of cyanide in physiological solutions. Anal. Chim. Acta 2006, 558, 158–163. [Google Scholar] [CrossRef]
- Sundermann, T.R.; Schmitt, G.; Bartel, M. Modified congener analysis: Quantification of cyanide in whole blood, other body fluids, and diverse beverages. Drug Test. Anal. 2021, 13, 412–418. [Google Scholar] [CrossRef]
- Frison, G.; Zancanaro, F.; Favretto, D.; Ferrara, S.D. An improved method for cyanide determination in blood using solid-phase microextraction and gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2932–2938. [Google Scholar] [CrossRef]
- Murphy, K.E.; Schantz, M.M.; Butler, T.A.; Benner, B.A., Jr.; Wood, L.J.; Turk, G.C. Determination of cyanide in blood by isotope-dilution gas chromatography–mass spectrometry. Clin. Chem. 2006, 52, 458–467. [Google Scholar] [CrossRef][Green Version]
- Meng, L.; Liu, X.; Wang, B.; Shen, G.; Wang, Z.; Guo, M. Simultaneous derivatization and extraction of free cyanide in biological samples with home-made hollow fiber-protected headspace liquid-phase microextraction followed by capillary electrophoresis with UV detection. J. Chromatogr. B 2009, 877, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Silva, M.; Valcarcel, M. Kinetic spectrophotometric determination of nanogram amounts of cyanide. Talanta 1991, 38, 303–308. [Google Scholar] [CrossRef]
- Abbasi, S.; Valinezhad, R.; Khani, H. A novel kinetic spectrophotometric method for the determination of ultra-trace amount of cyanide. Spectrochim. Acta A 2010, 77, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Themelis, D.G.; Karastogianni, S.C.; Tzanavaras, P.D. Selective determination of cyanides by gas diffusion-stopped flow-sequential injection analysis and an on-line standard addition approach. Anal. Chim. Acta 2009, 632, 93–100. [Google Scholar] [CrossRef]
- Jain, A.; Pillai, A.K.; Sharma, N.; Verma, K.K. Headspace single-drop microextraction and cuvetteless microspectrophotometry for the selective determination of free and total cyanide involving reaction with ninhydrin. Talanta 2010, 82, 758–765. [Google Scholar] [CrossRef]
- Santelli, R.E.; Micelli, A.S.; de Carvalho, M.D.F.B. Automated flow injection method for monitoring total cyanide concentration in petroleum refinery effluents using ninhydrin as color reagent. Spectrosc. Lett. 2006, 39, 605–618. [Google Scholar] [CrossRef]
- Surleva, A.; Zaharia, M.; Ion, L.; Gradinaru, R.V.; Drochioiu, G.; Mangalagiu, I. Ninhydrin-based spectrophotometric assays of trace cyanide. Acta Chem. IASI 2013, 21, 57–70. [Google Scholar] [CrossRef][Green Version]
- Mihaescu, I.M.; Drochioiu, G. Cyanide reaction with ninhydrin: The effect of pH changes and UV-Vis radiation upon the analytical results. Rev. Roum. Chim. 2009, 54, 841–945. [Google Scholar]
- Mihaescu, I. Cyanide determination with a modified ninhydrin reagent using N-acetyl cysteine in the presence of heavy metal ions. J. Chil. Chem. Soc. 2010, 55, 467–468. [Google Scholar] [CrossRef][Green Version]
- Nagaraja, P.; Kumar, M.S.H.; Yathirajan, H.S.; Prakash, J.S. Novel sensitive spectrophotometric method for the trace determination of cyanide in industrial effluent. Anal. Sci. 2002, 18, 1027–1030. [Google Scholar] [CrossRef]
- Sulistyarti, H.; Mulyasuryani, A.; Sabarudin, A.; Rumhayati, B.; Cahyani, Y.D.; Atikaningsih, R.A. Thiocyanate determination using technique of flow injection-gas diffusion spectrophotometry with cerium (IV)-ninhydrin reagent. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 032030. [Google Scholar] [CrossRef]
- Tabian, D.; Bulgaru Iliescu, D.; Iov, T.; Barna, B.; Toma, S.I.; Drochioiu, G. Hydrogen cyanide and carboxyhemoglobin assessment in an open space fire-related fatality. J. Forensic Sci. 2021, 66, 1171–1175. [Google Scholar] [CrossRef]
- Surleva, A.; Drochioiu, G. A modified ninhydrin micro-assay for determination of total cyanogens in plants. Food Chem. 2013, 141, 2788–2794. [Google Scholar] [CrossRef]
- McAllister, J.L.; Roby, R.J.; Levine, B.; Purser, D. Stability of cyanide in cadavers and in postmortem stored tissue specimens: A review. J. Anal. Toxicol. 2008, 32, 612–620. [Google Scholar] [CrossRef][Green Version]
- McAllister, J.L.; Roby, R.J.; Levine, B.; Purser, D. The effect of sodium fluoride on the stability of cyanide in postmortem blood samples from fire victims. Forensic Sci. Int. 2011, 209, 29–33. [Google Scholar] [CrossRef]
- Afkhami, A.; Sarlak, N.; Zarei, A.R. Simultaneous kinetic spectrophotometric determination of cyanide and thiocyanate using the partial least squares (PLS) regression. Talanta 2007, 71, 893–899. [Google Scholar] [CrossRef]
- Khatha, P.; Phutthaphongloet, T.; Timpa, P.; Ninwong, B.; Income, K.; Ratnarathorn, N.; Dungchai, W. Distance-based paper device combined with headspace extraction for determination of cyanide. Sensors 2019, 19, 2340. [Google Scholar] [CrossRef]
- Tracqui, A.; Raul, J.S.; Geraut, A.; Berthelon, L.; Ludes, B. Determination of blood cyanide by HPLC-MS. J. Anal. Toxicol. 2002, 26, 144–148. [Google Scholar] [CrossRef]
- Volmer, W.; Giesselmann, G. Simplifying cyanide analysis with microdistillation. Am. Lab. 2000, 32, 18–22. [Google Scholar]
- Parmar, P.; Pillai, A.K.; Gupta, V.K. Rapid spectrophotometric determination of trace amounts of cyanide and hydrogen cyanide using p-aminoacetanilide in various samples. J. Korean Chem. Soc. 2010, 54, 165–168. [Google Scholar] [CrossRef]
- Tucker, R.B.; Graham, B.F.; Mason, V.A. Cyanide levels in fire victims as determined by a simple microdiffusion procedure. Can. Soc. Forensic Sci. J. 1978, 11, 251–256. [Google Scholar] [CrossRef]
- Dumas, P.; Gingras, G.; LeBlanc, A. Isotope dilution-mass spectrometry determination of blood cyanide by headspace gas chromatography. J. Anal. Toxicol. 2005, 29, 71–75. [Google Scholar] [CrossRef][Green Version]
- Seto, Y.; Tsunoda, N.; Ohta, H.; Shinohara, T. Determination of blood cyanide by headspace gas chromatography with nitrogen-phosphorus detection and using a megabore capillary column. Anal. Chim. Acta 1993, 276, 247–259. [Google Scholar] [CrossRef]
- Gambaro, V.; Arnoldi, S.; Casagni, E.; Dell’Acqua, L.; Pecoraro, C.; Froldi, R. Blood cyanide determination in two cases of fatal intoxication: Comparison between headspace gas chromatography and a spectrophotometric method. J. Forensic Sci. 2007, 52, 1401–1404. [Google Scholar] [CrossRef]
- Sim, J.; Kim, M.; Kim, S.; Yang, W. A novel method for cyanide quantification in human whole blood using ion chromatography with amperometric detection and its application to cyanide intoxication cases. J. Forensic Sci. 2021, 67, 353–357. [Google Scholar] [CrossRef]
- Swezey, R.; Shinn, W.; Green, C.; Drover, D.R.; Hammer, G.B.; Schulman, S.R.; Zajicek, A.; Jett, D.A.; Boss, G.R. Comparison of a new cobinamide-based method to a standard laboratory method for measuring cyanide in human blood. J. Anal. Toxicol. 2013, 37, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Wachełko, O.; Chłopaś-Konowałek, A.; Zawadzki, M.; Szpot, P. Old poison, new problem: Cyanide fatal intoxications associated with internet shopping. J. Anal. Toxicol. 2021, 2021, bkab039. [Google Scholar] [CrossRef]
- Silverman, S.H.; Purdue, G.F.; Hunt, J.L.; Bost, R.O. Cyanide toxicity in burned patients. J. Trauma 1988, 28, 171–176. [Google Scholar] [CrossRef]
- Drochioiu, G.; Mangalagiu, I.; Avram, E.; Popa, K.; Dirtu, A.C.; Druta, I. Cyanide reaction with ninhydrin: Evaluation of interference and mechanisms. Anal. Sci. 2004, 20, 1443–1447. [Google Scholar] [CrossRef]
- Verma, A.K.; Gupta, V.K. Spectrophotometric determination of cyanide and hydrogen cyanide using p-amino benzoic acid as a new reagent and its application in steel industries. Asian J. Chem. 1996, 8, 613. [Google Scholar]
- Harris, D.; Davis, A.; Ryan, P.B.; Cohen, J.; Gandhi, P.; Dubiel, D.; Black, M. Chemical exposure and flammability risks of upholstered furniture. Fire Mater. 2021, 45, 167–180. [Google Scholar] [CrossRef]
- Jackson, R.; Logue, B.A. A review of rapid and field-portable analytical techniques for the diagnosis of cyanide exposure. Anal. Chim. Acta 2017, 960, 18–39. [Google Scholar] [CrossRef]
- Isenschmid, D.S.; Levine, B. Principles of Forensic Toxicology; AACC Press: Washington, DC, USA, 2013; pp. 293–316. [Google Scholar]
- Saeed, F.S.; Al Bashit, A.; Viswanathan, V.; Valles, D. An initial machine learning-based victim’s scream detection analysis for burning sites. Appl. Sci. 2021, 11, 8425. [Google Scholar] [CrossRef]
- Davies, V.; Turner, J.; Greenway, M. Toxic inhalational injury. BMJ Case Rep. 2020, 13, e232875. [Google Scholar] [CrossRef]



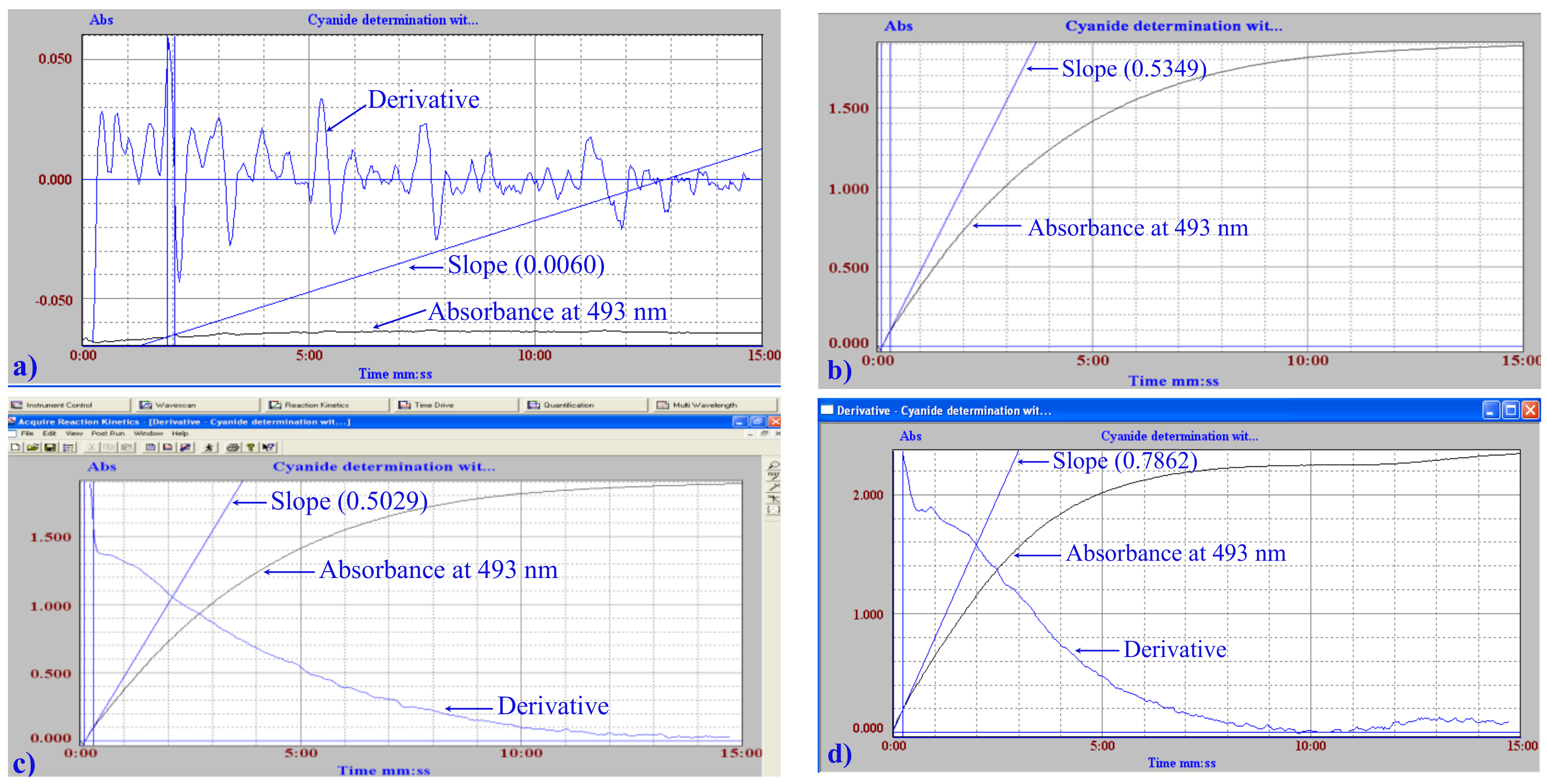
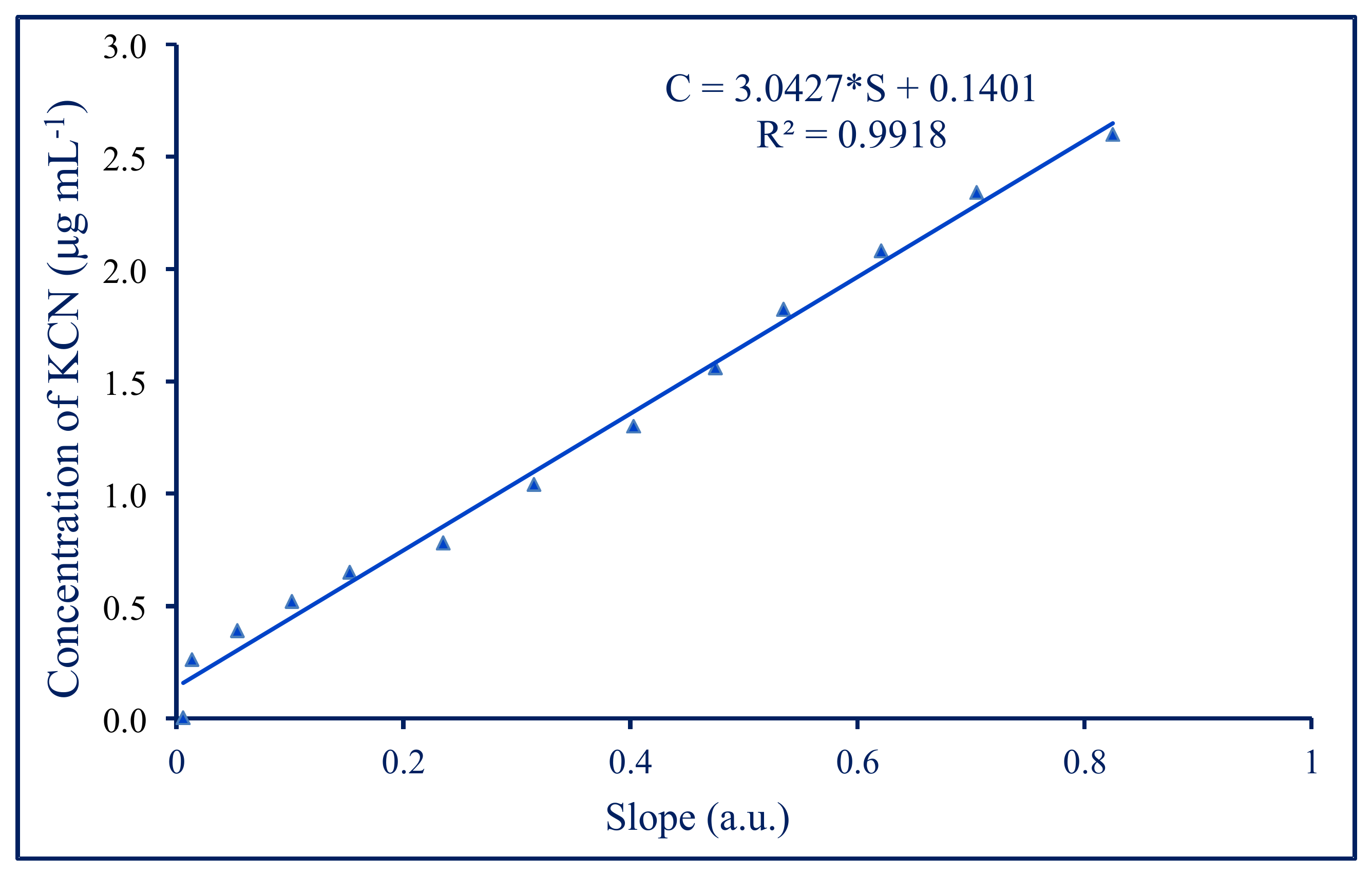
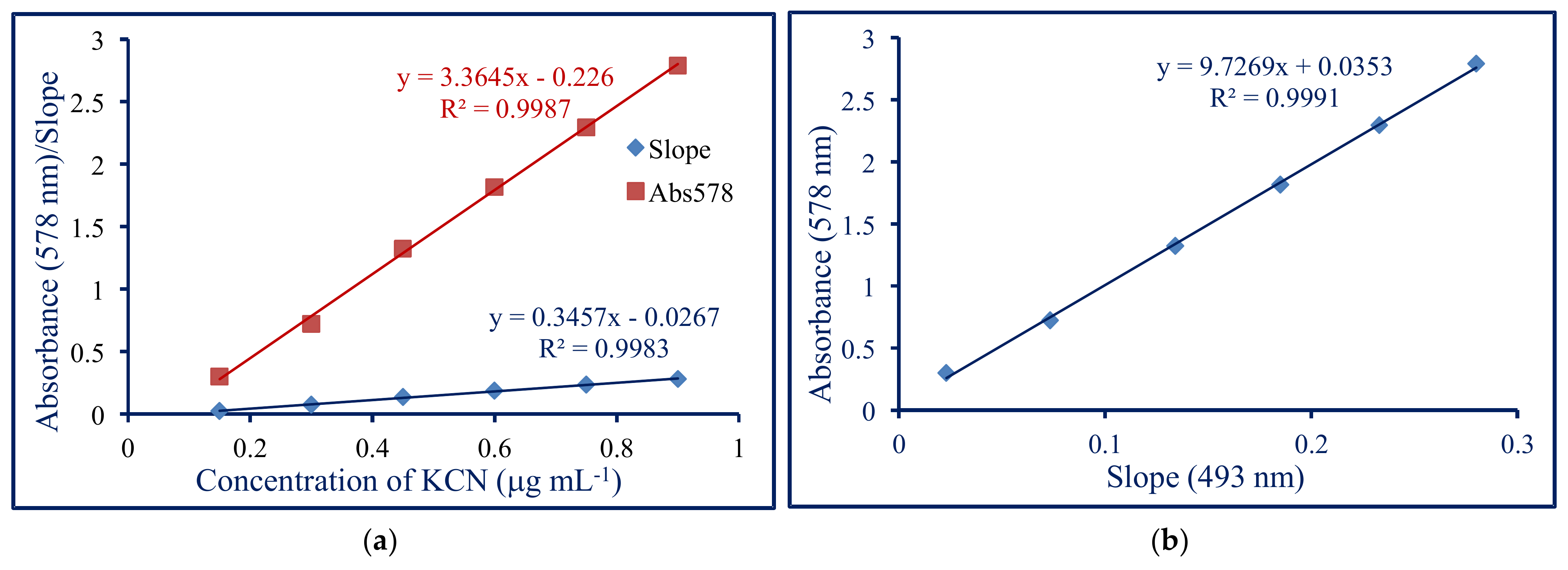
| Sample | KCN, * μg·mL−1 | Absorbance 493 nm | KCN, ** μg·mL−1 |
|---|---|---|---|
| 0 | 0.00 | 0.000 | 0.2676 |
| 1 | 0.26 | 0.090 | 0.4283 |
| 2 | 0.52 | 0.146 | 0.6152 |
| 3 | 0.78 | 0.219 | 0.7385 |
| 4 | 1.04 | 0.351 | 0.9535 |
| 5 | 1.30 | 0.587 | 1.2999 |
| 6 | 1.56 | 0.802 | 1.6233 |
| 7 | 1.82 | 0.992 | 1.8926 |
| 8 | 2.08 | 1.049 | 2.0617 |
| 9 | 2.34 | 1.213 | 2.2717 |
| 10 | 2.60 | 1.432 | 2.6117 |
| Sample 3, D1 (M.L.) | - | 1.275 | 2.3680 |
| Sample 3, D2 (M.L.) | - | 1.389 | 2.5450 |
| Sample | Absorbance 493 nm | KCN, ** μg·mL−1 | KCN μg/0.5 mL of Blood *** | HCN, μg/0.5 mL of Blood **** | HCN, μg/mL of Blood ***** |
|---|---|---|---|---|---|
| Sample 3, D1 (M.L.) | 1.275 | 2.3680 | 3.6836 | 1.5301 | 3.0602 |
| Sample 3, D2 (M.L.) | 1.389 | 2.5450 | 3.9589 | 1.6444 | 3.2890 |
| Average | 3.1746 |
| Volume of KCN Solution (μL) | KCN Concentration (μg mL−1) | Read Time (min) and the Wavelength (nm) | ||||
|---|---|---|---|---|---|---|
| 10; 493 | 20; 493 | 30; 493 | 50; 493 | 15; 485 | ||
| 10 | 0.15 | 0.004 | −0.009 | −0.006 | −0.002 | −0.003 |
| 20 | 0.30 | 0.188 | 0.159 | 0.133 | 0.059 | 0.123 |
| 30 | 0.45 | 0.342 | 0.332 | 0.317 | 0.266 | 0.286 |
| 40 | 0.60 | 0.507 | 0.464 | 0.390 | 0.353 | 0.390 |
| 50 | 0.75 | 0.648 | 0.732 | 0.731 | 0.650 | 0.628 |
| 60 | 0.90 | 0.760 | 0.794 | 0.792 | 0.680 | 0.688 |
| 70 | 1.05 | 0.902 | 0.944 | 0.894 | 0.889 | 0.811 |
| 80 | 1.20 | 1.196 | 1.220 | 1.291 | 1.182 | 1.111 |
| 90 | 1.35 | 1.482 | 1.491 | 1.471 | 1.444 | 1.338 |
| 100 | 1.50 | 1.474 | 1.529 | 1.522 | 1.462 | 1.361 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabian, D.; Bulgaru Iliescu, D.; Diac, M.M.; Badea, M.; Toma, S.I.; Drochioiu, G. Evaluation of Hydrogen Cyanide in the Blood of Fire Victims Based on the Kinetics of the Reaction with Ninhydrin. Appl. Sci. 2022, 12, 2329. https://doi.org/10.3390/app12052329
Tabian D, Bulgaru Iliescu D, Diac MM, Badea M, Toma SI, Drochioiu G. Evaluation of Hydrogen Cyanide in the Blood of Fire Victims Based on the Kinetics of the Reaction with Ninhydrin. Applied Sciences. 2022; 12(5):2329. https://doi.org/10.3390/app12052329
Chicago/Turabian StyleTabian, Daniel, Diana Bulgaru Iliescu, Madalina Maria Diac, Mihaela Badea, Sebastian Ionut Toma, and Gabi Drochioiu. 2022. "Evaluation of Hydrogen Cyanide in the Blood of Fire Victims Based on the Kinetics of the Reaction with Ninhydrin" Applied Sciences 12, no. 5: 2329. https://doi.org/10.3390/app12052329
APA StyleTabian, D., Bulgaru Iliescu, D., Diac, M. M., Badea, M., Toma, S. I., & Drochioiu, G. (2022). Evaluation of Hydrogen Cyanide in the Blood of Fire Victims Based on the Kinetics of the Reaction with Ninhydrin. Applied Sciences, 12(5), 2329. https://doi.org/10.3390/app12052329







