Thermal Conductivity and Rheology of Graphene Oxide Nanofluids and a Modified Predication Model
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Materials
2.2. Nanofluid Preparation
2.3. Stability Characterization
2.4. Characterization and Measurements
3. Results and Discussion
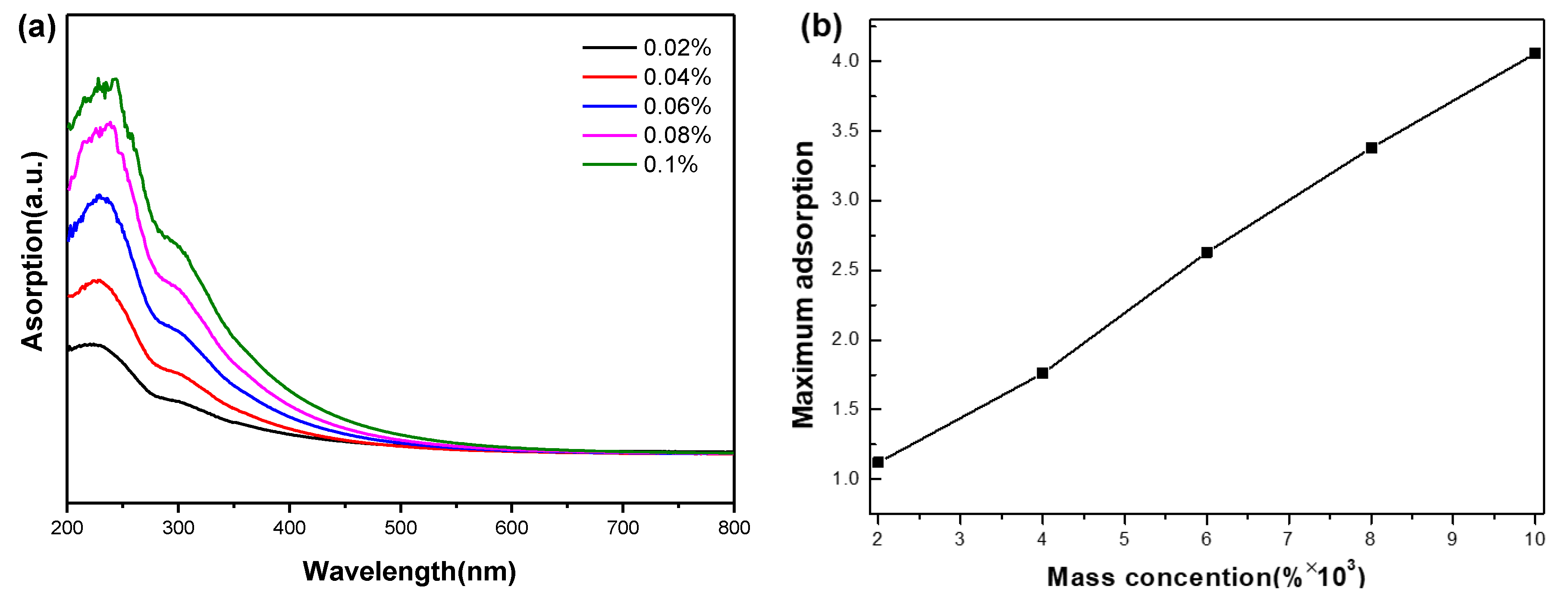
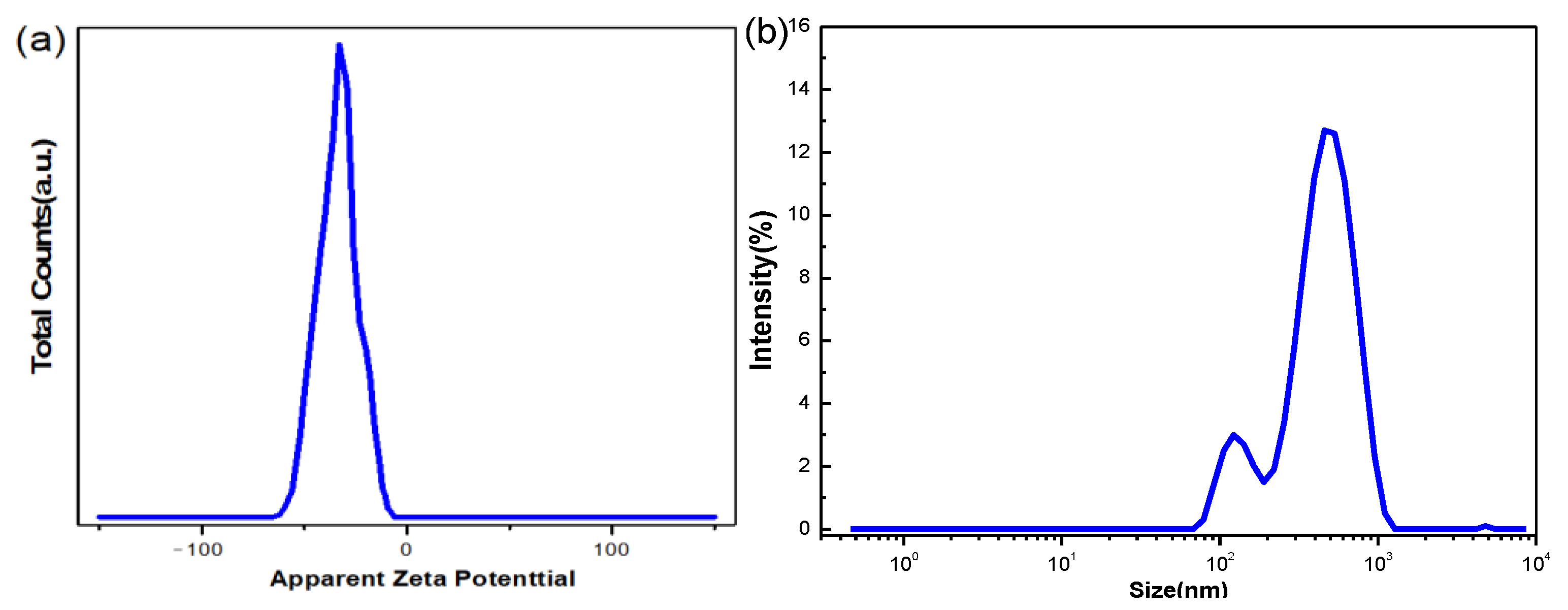
3.1. Stability of Graphene Oxide Nanofluids
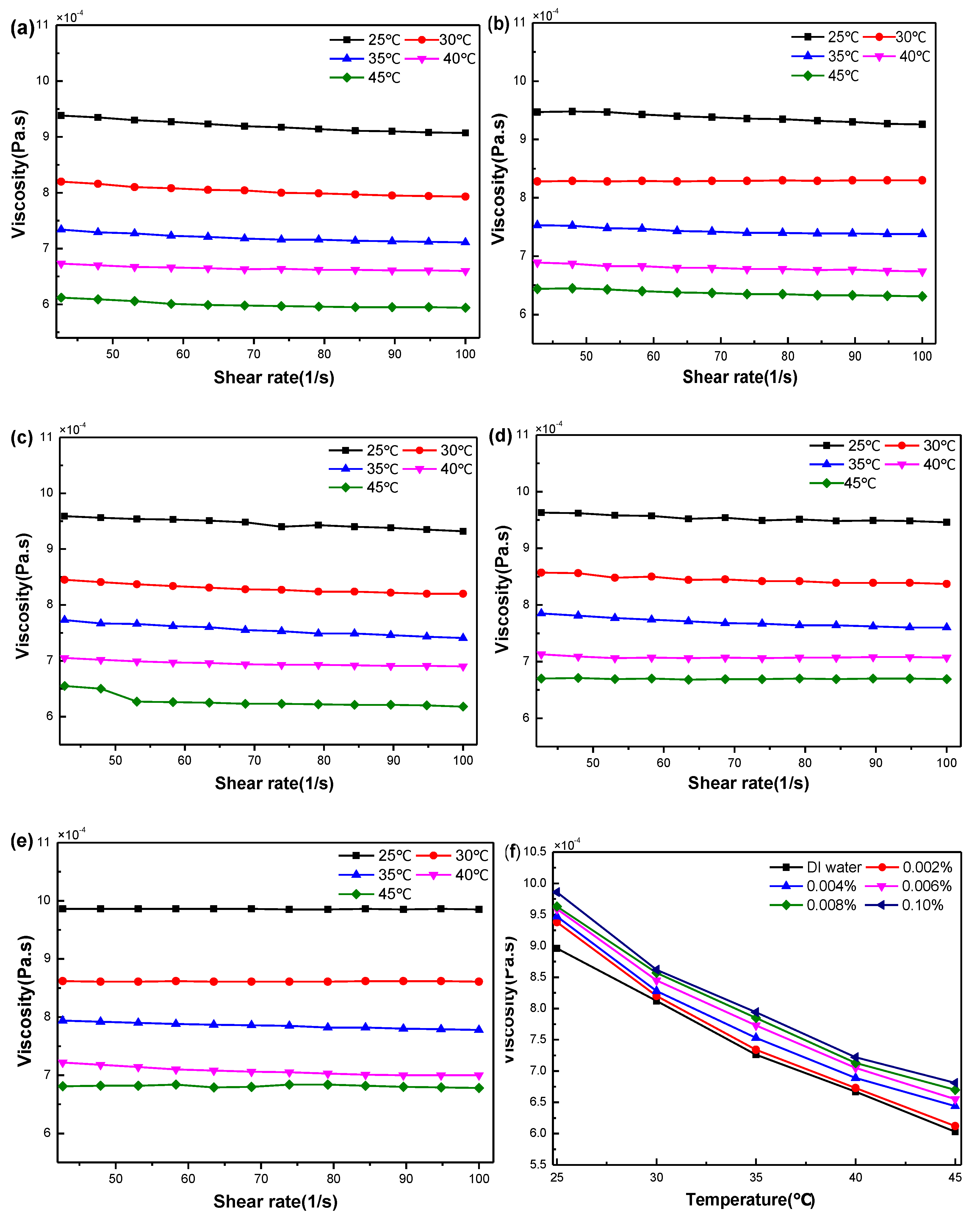
3.2. Rheological Properties of GO Nanofluids
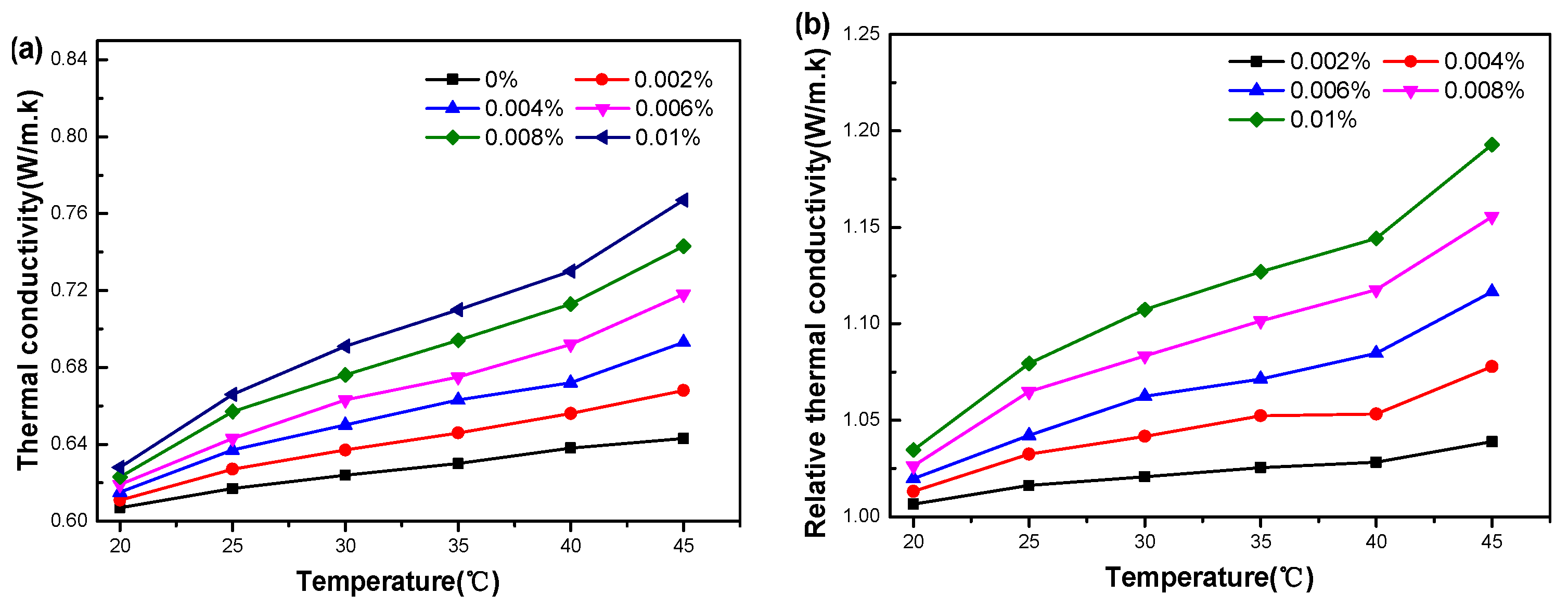
3.3. Thermal Conductivity of Graphene Oxide Nanofluids
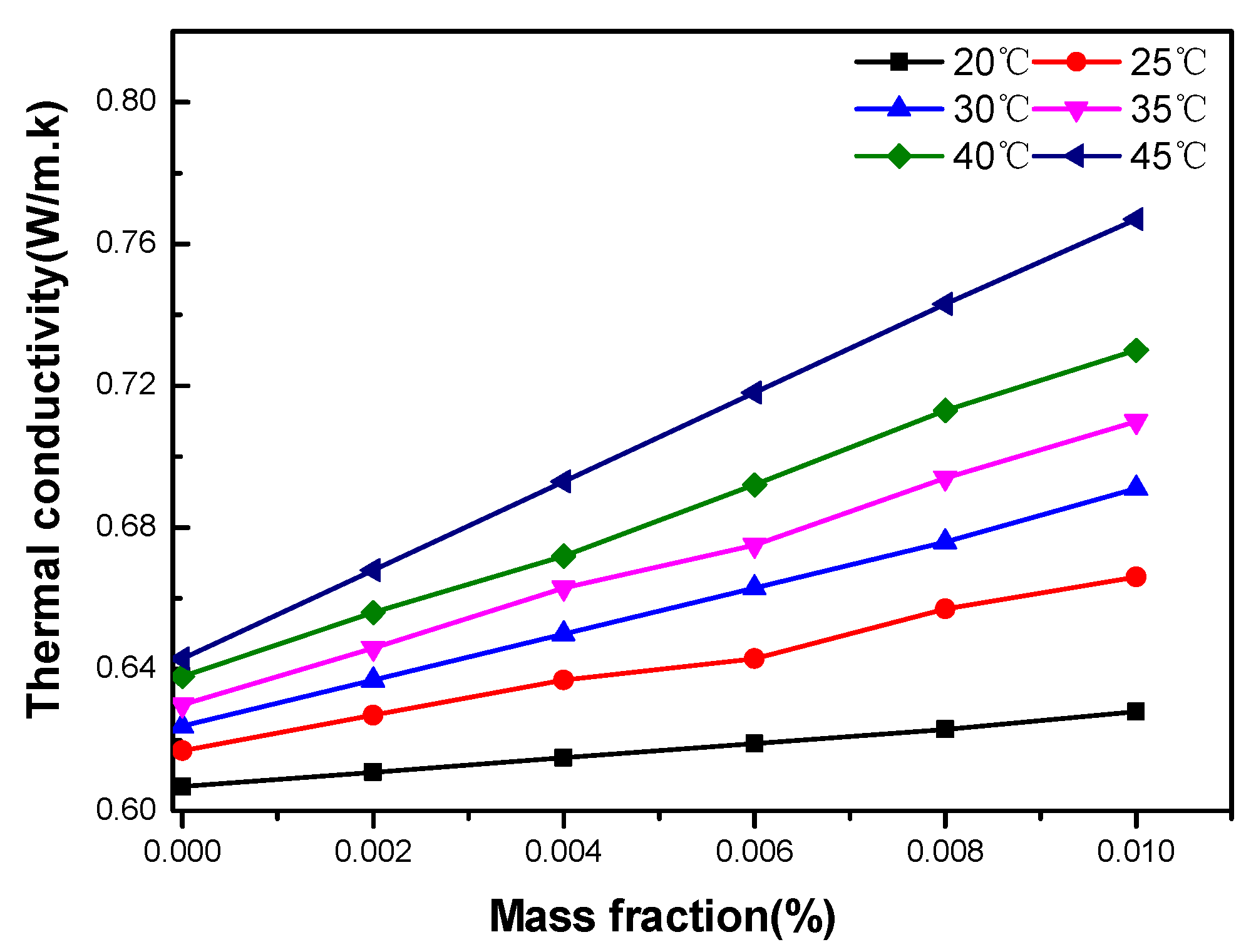
3.3.1. Effect of Temperature
3.3.2. Effect of Particle Concentration
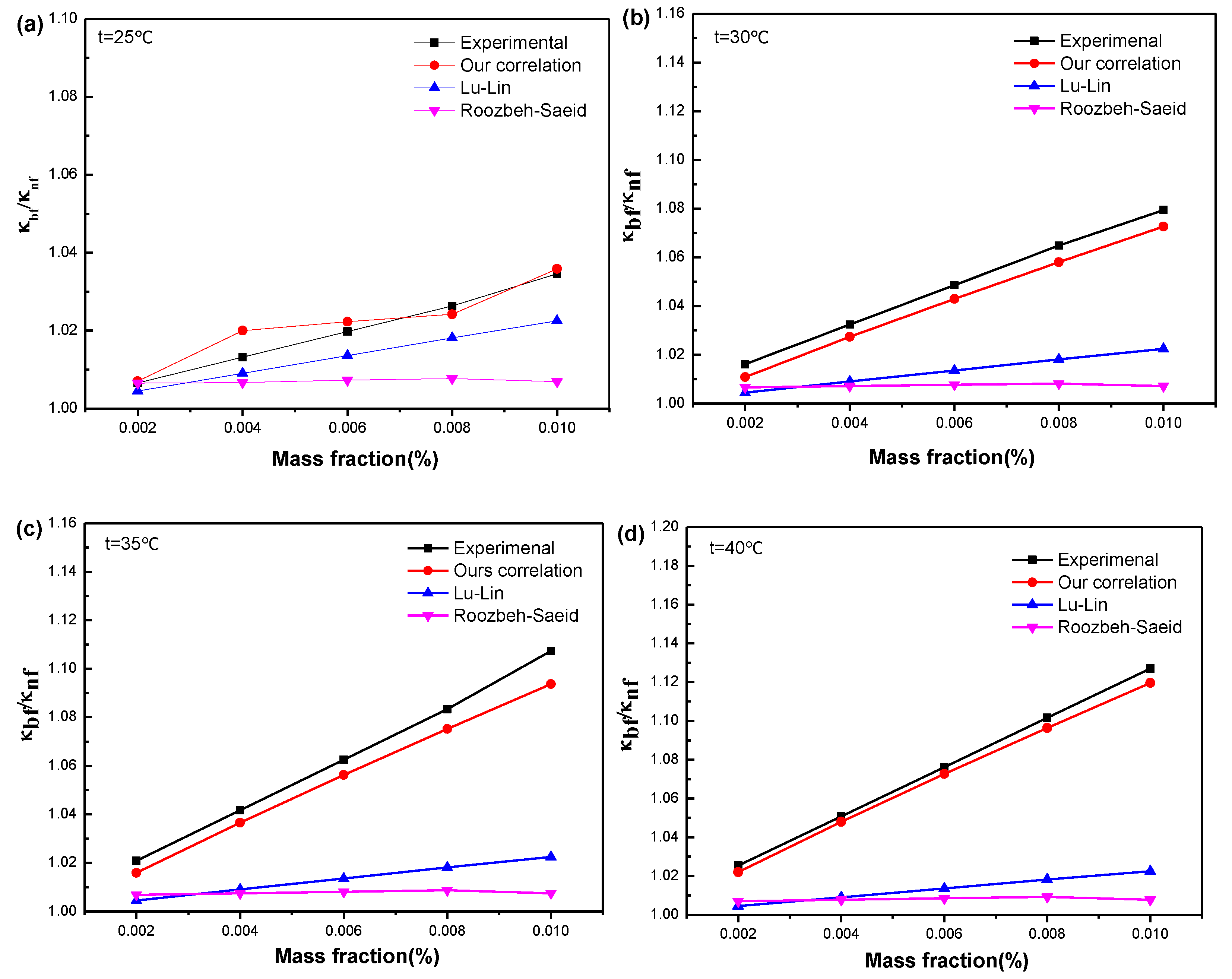
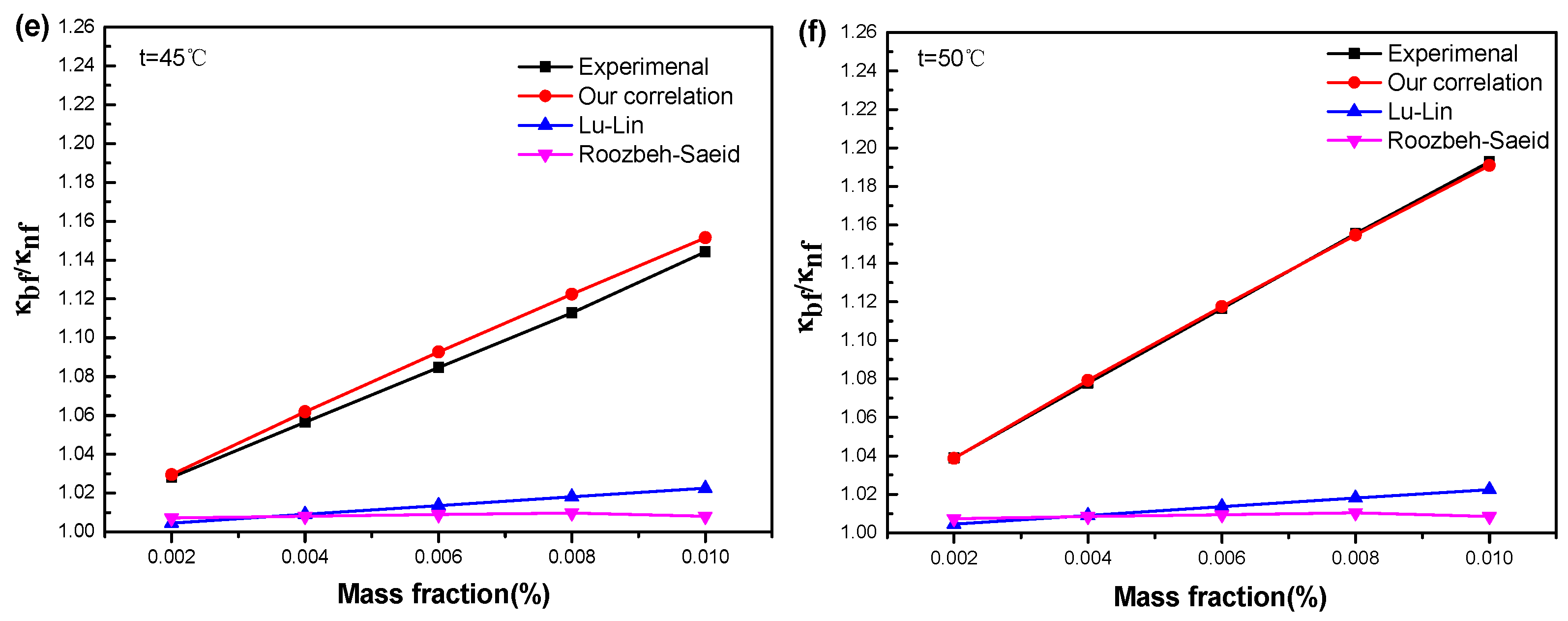
3.3.3. Existing Models for the Effective Thermal Conductivity of Nanofluid
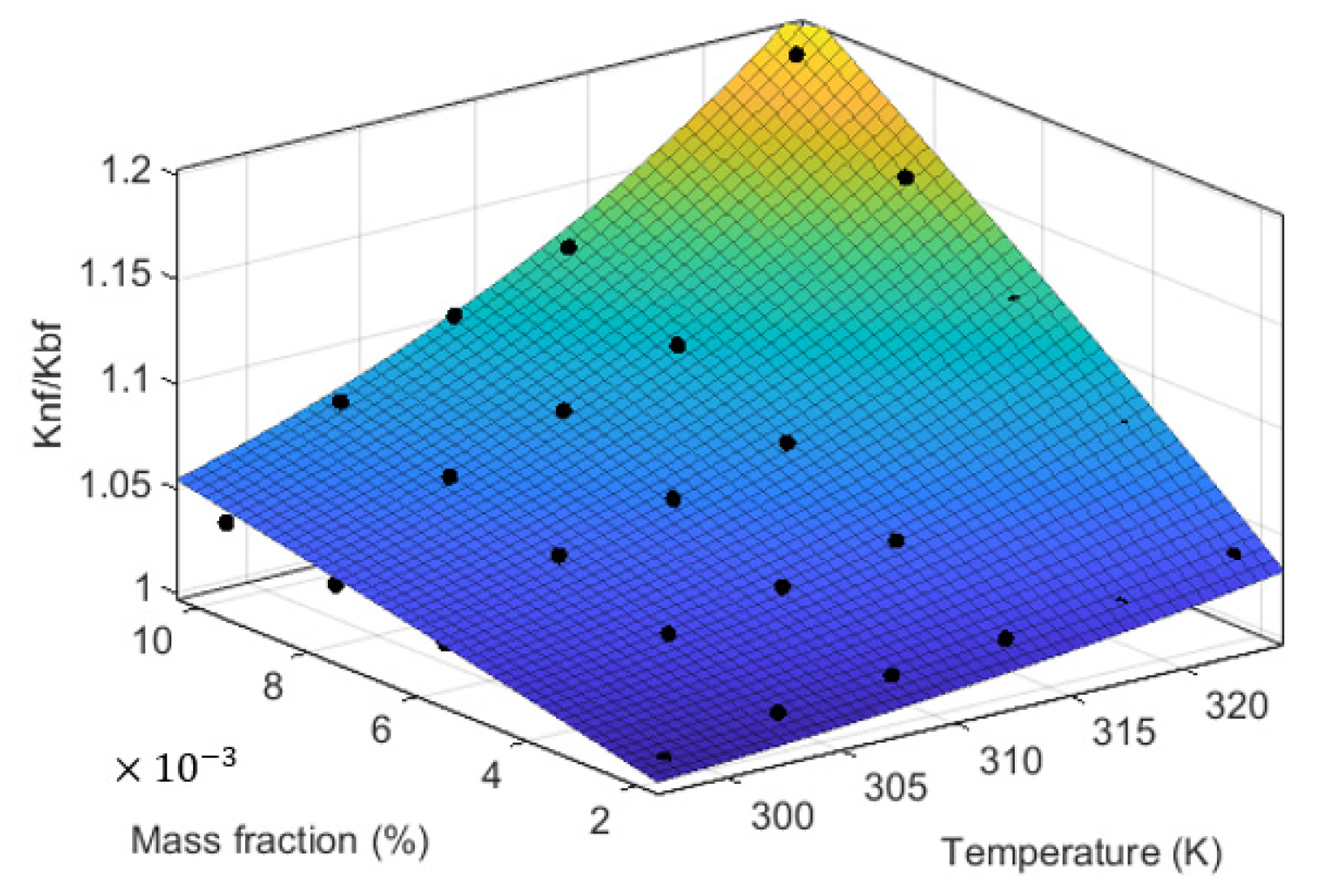
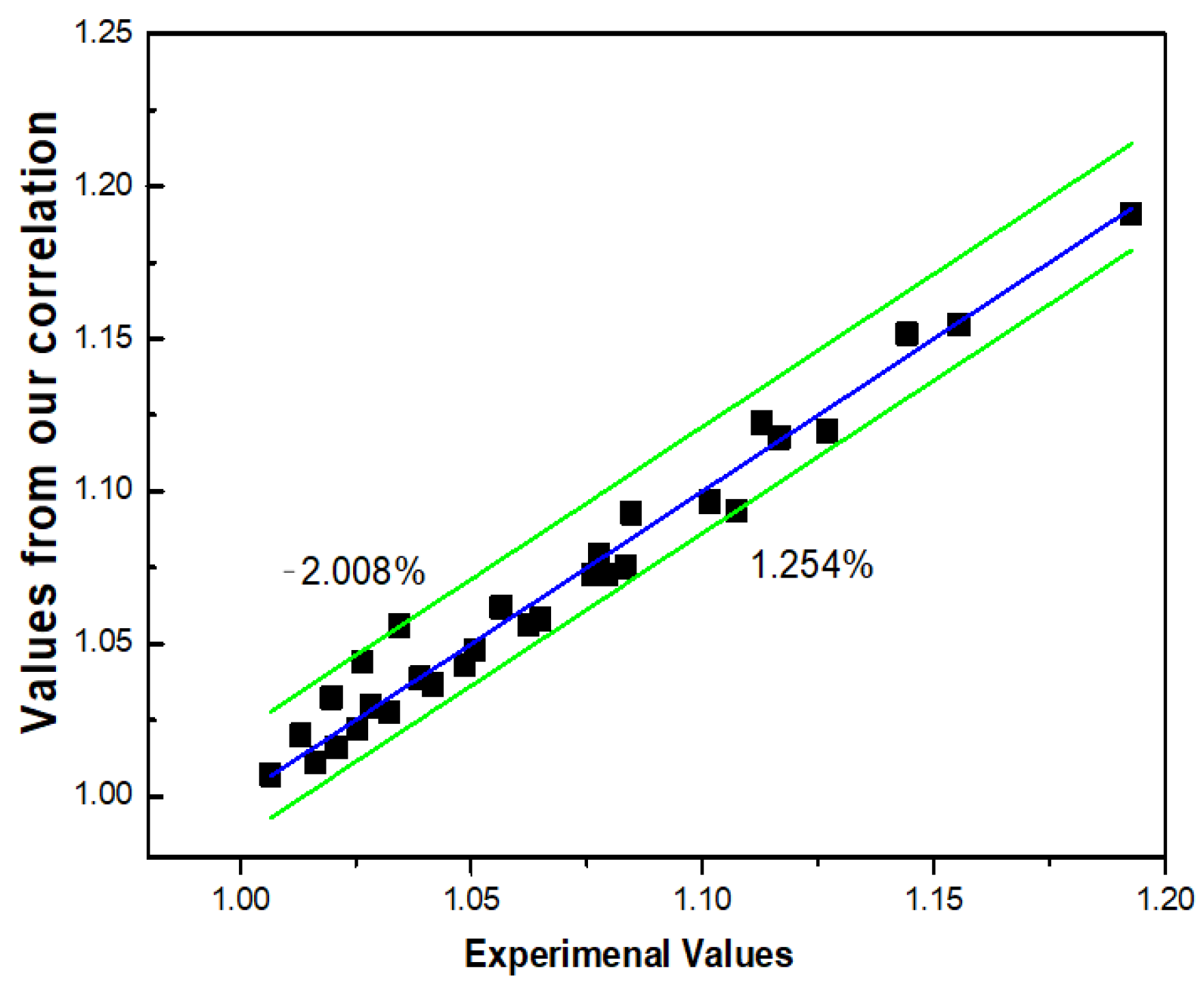
3.3.4. Our Proposed Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonçalves, I.; Souza, R.; Coutinho, G.; Miranda, J.; Moita, A.; Pereira, J.; Moreira, A.; Lima, R. Thermal Conductivity of Nanofluids: A Review on Prediction Models, Controversies and Challenges. Appl. Sci. 2021, 11, 2525. [Google Scholar] [CrossRef]
- Yu, S.-P.; Lue, Y.-F.; Teng, T.-P.; Hsieh, H.-K.; Huang, C.-C. Enhanced Heat Transfer Performance of the Tube Heat Exchangers Using Carbon-Based Nanofluids. Appl. Sci. 2021, 11, 8139. [Google Scholar] [CrossRef]
- Jin, J.Y.; Hatami, M.; Jing, D.W. Experimental investigation and prediction of the thermal conductivity of water-based oxide nanofluids with low volume fractions. J. Therm. Anal. Calorim. 2019, 135, 257–269. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Yu, F. Experimental and theoretical studies of nanofluid thermal conductivity enhancement: A review. Nanoscale Res. Lett. 2011, 6, 229. [Google Scholar] [CrossRef] [Green Version]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Preparation and properties of highly conductive palmitic acid/graphene oxide composites as thermal energy storage materials. Energy 2013, 58, 628–634. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. Enhanced convective heat transfer using graphene dispersed nanofluids. Nanoscale Res. Lett. 2011, 6, 289–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Xie, H. Surfactant-free nanofluids containing double- and single-walled carbon nanotubes functionalized by a wet-mechanochemical reaction. Thermochim. Acta 2010, 497, 67–71. [Google Scholar] [CrossRef]
- Wakif, A.; Zaydan, M.; Alshomrani, A.S.; Muhammad, T.; Sehaqui, R. New insights into the dynamics of alumina-(60% ethylene glycol + 40% water) over an isothermal stretching sheet using a renovated Buongiorno’s approach: A numerical GDQLLM analysis. Int. Commun. Heat Mass Transf. 2022, 133, 105937. [Google Scholar] [CrossRef]
- Nayak, M.K.; Wakif, A.; Animasaun, I.L.; Alaoui, M.S.H. Numerical Differential Quadrature Examination of Steady Mixed Convection Nanofluid Flows Over an Isothermal Thin Needle Conveying Metallic and Metallic Oxide Nanomaterials: A Comparative Investigation. Arab. J. Sci. Eng. 2020, 45, 5331–5346. [Google Scholar] [CrossRef]
- Zaydan, M.; Wakif, A.; Alshomrani, I.L.; Khan, U.; Baleanu, D.; Sehaqui, R. Significances of blowing and suction processes on the occurrence of thermo-magneto-convection phenomenon in a narrow nanofluidic medium: A revised Buongiorno’s nanofluid model. Case Stud. Therm. Eng. 2022, 22, 100726. [Google Scholar] [CrossRef]
- Rasool, G.; Wakif, A. Numerical spectral examination of EMHD mixed convective flow of second-grade nanofluid towards a vertical Riga plate using an advanced version of the revised Buongiorno’s nanofluid model. J. Therm. Anal. Calorim. 2021, 143, 2379–2393. [Google Scholar] [CrossRef]
- Xia, W.F.; Alshomrani, A.S.; Wakif, A.; Shah, N.A.; Yook, S.-J. Gear-generalized differential quadrature analysis of oscillatory convective Taylor-Couette flows of second-grade fluids subject to Lorentz and Darcy-Forchheimer quadratic drag forces. Int. Commun. Heat Mass Transf. 2021, 126, 105395. [Google Scholar] [CrossRef]
- Wakif, A.; Animasaun, I.L.; Khan, U.; Shah, N.A.; Thumma, T. Dynamics of radiative-reactive Walters-b fluid due to mixed convection conveying gyrotactic microorganisms, tiny particles experience haphazard motion, thermo-migration, and Lorentz force. Phys. Scr. 2021, 96, 125239. [Google Scholar] [CrossRef]
- Alghamdi, M.; Wakif, A.; Thumm, T.; Khan, U.; Baleanu, D.; Rasool, G. Significance of variability in magnetic field strength and heat source on the radiative-convective motion of sodium alginate-based nanofluid within a Darcy-Brinkman porous structure bounded vertically by an irregular slender surface. Case Stud. Therm. Eng. 2021, 28, 101428. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Shariaty-Niasar, M.; Rashidi, A.M. Preparation of nanofluids from functionalized Graphene by new alkaline method and study on the thermal conductivity and stability. Int. Commun. Heat Mass Transf. 2013, 42, 89–94. [Google Scholar] [CrossRef]
- Jing, D.W.; Hu, Y.; Liu, M.C.; Wei, J.J.; Guo, L.J. Preparation of highly dispersed nanofluid and CFD study of its utilization in a concentrating PV/T system. Sol. Energy 2015, 112, 30–40. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Q.Y.; Zhang, Y.M.; Gao, W.; Jing, D.W. Insights into the rheological behavior of ethanol-based metal oxide nanofluids. J. Mol. Liq. 2021, 323, 115006. [Google Scholar] [CrossRef]
- Chopkar, M.; Sudarshan, S.; Das, P.; Manna, I. Effect of Particle Size on Thermal Conductivity of Nanofluid. Met. Mater. Trans. 2008, 39, 1535–1542. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Lin, H.-C. Effective conductivity of composites containing aligned spheroidal inclusions of finite conductivity. J. Appl. Phys. 1996, 79, 6761. [Google Scholar] [CrossRef]
- Taherialekouhi, R.; Rasouli, S.; Khosravi, A. An experimental study on stability and thermal conductivity of water-graphene oxide/aluminum oxide nanoparticles as a cooling hybrid nanofluid. Int. J. Heat Mass Transf. 2019, 145, 118751. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, X.; Sha, X.; Jing, D.; Ma, L. Thermal Conductivity and Rheology of Graphene Oxide Nanofluids and a Modified Predication Model. Appl. Sci. 2022, 12, 3567. https://doi.org/10.3390/app12073567
Mei X, Sha X, Jing D, Ma L. Thermal Conductivity and Rheology of Graphene Oxide Nanofluids and a Modified Predication Model. Applied Sciences. 2022; 12(7):3567. https://doi.org/10.3390/app12073567
Chicago/Turabian StyleMei, Xinyu, Xin Sha, Dengwei Jing, and Lijing Ma. 2022. "Thermal Conductivity and Rheology of Graphene Oxide Nanofluids and a Modified Predication Model" Applied Sciences 12, no. 7: 3567. https://doi.org/10.3390/app12073567
APA StyleMei, X., Sha, X., Jing, D., & Ma, L. (2022). Thermal Conductivity and Rheology of Graphene Oxide Nanofluids and a Modified Predication Model. Applied Sciences, 12(7), 3567. https://doi.org/10.3390/app12073567




