Alkali-Activated Materials with Pre-Treated Municipal Solid Waste Incinerator Bottom Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Precursors
2.2. Alkaline Activator and Water Reducing Admixtures
2.3. Aggregates
2.4. Pre-Treatment MIBA
2.5. Mix Design
2.6. Test Methods
3. Results and Discussion
3.1. Characterization of the Precursors
3.2. Characterization of the Aggregates
3.3. MIBA Pre-Treatment
3.4. Fresh-State Properties
3.4.1. Consistence
3.4.2. Density
3.5. Hardened-State Properties
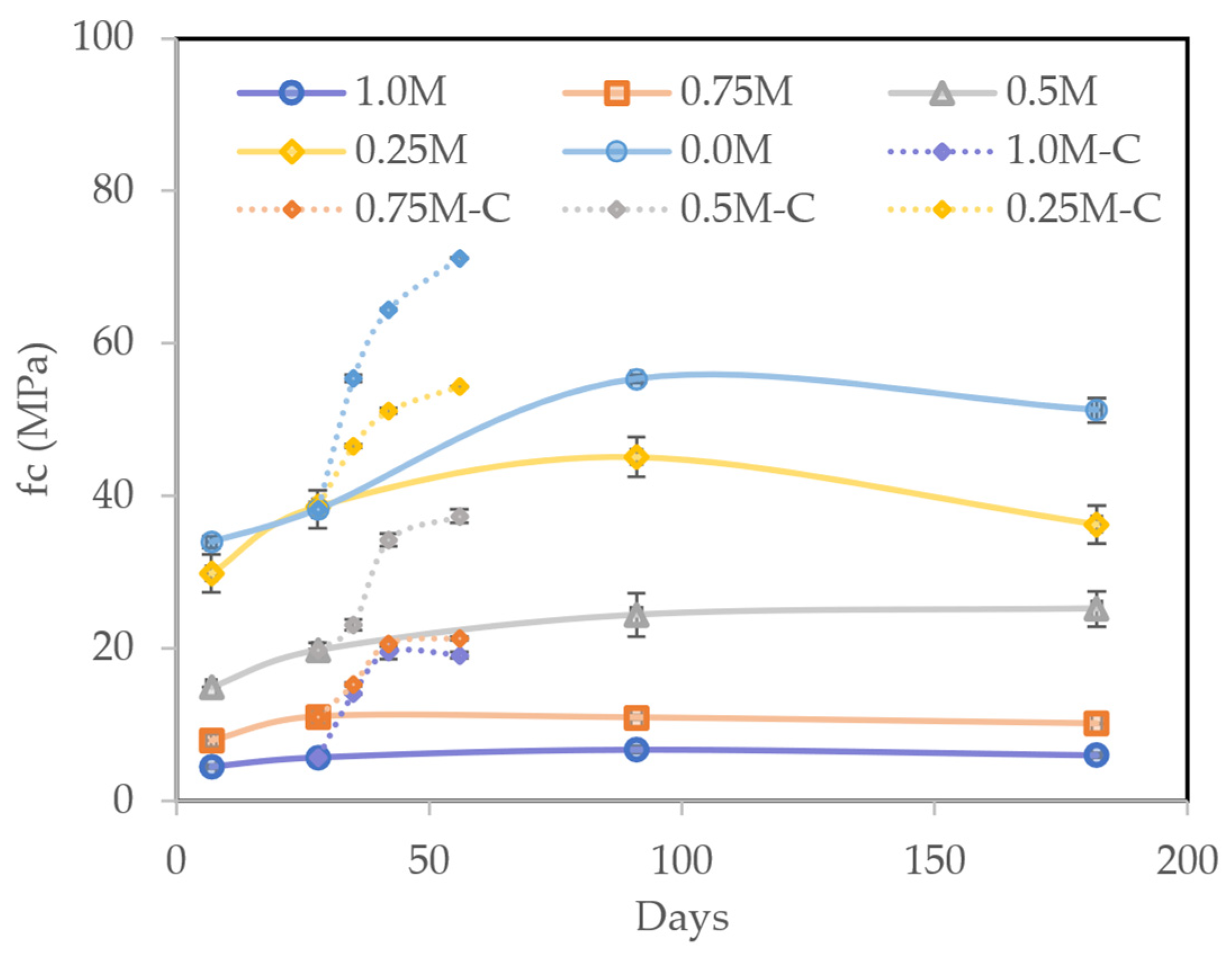
3.5.1. Compressive Strength
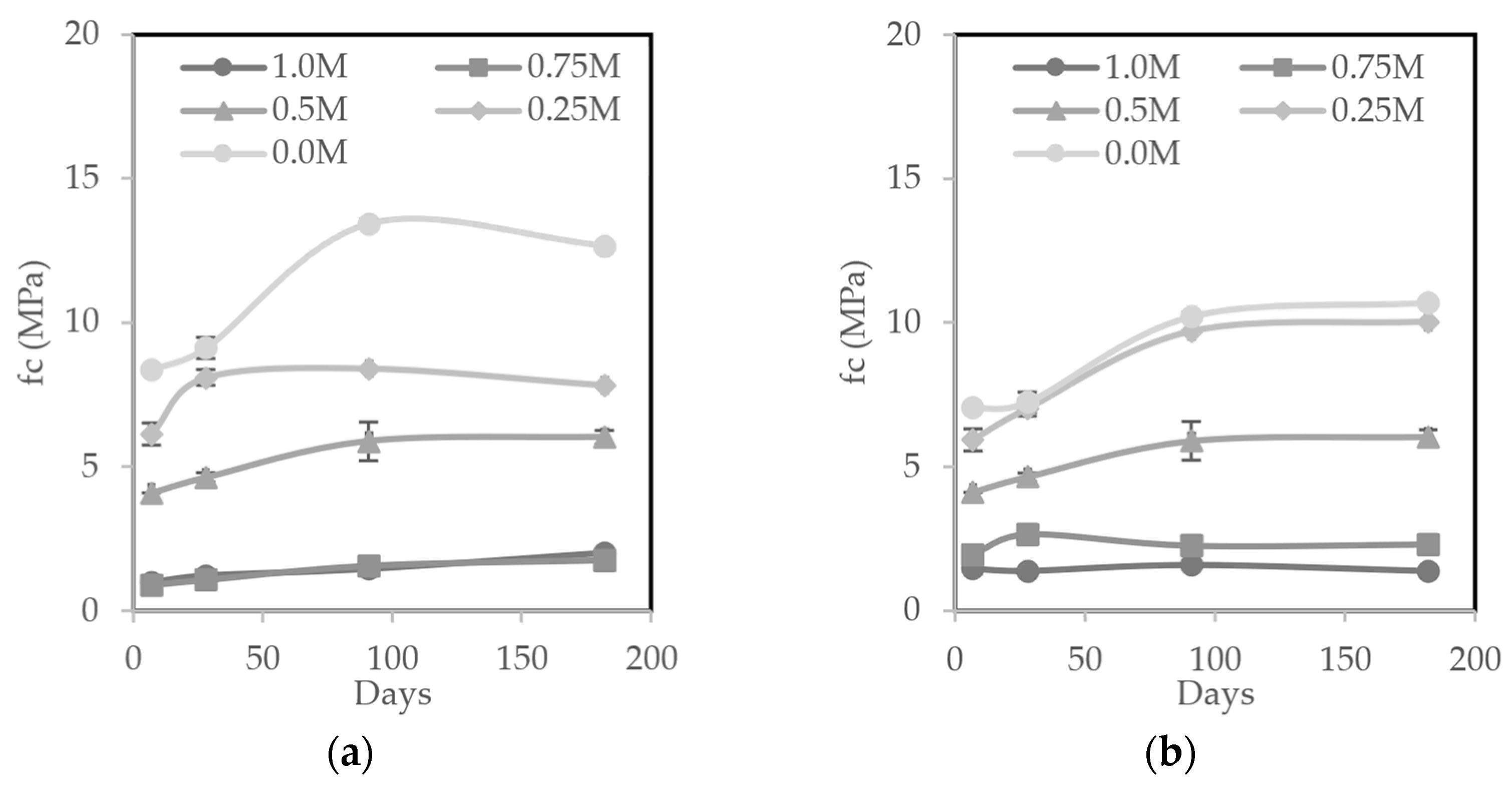
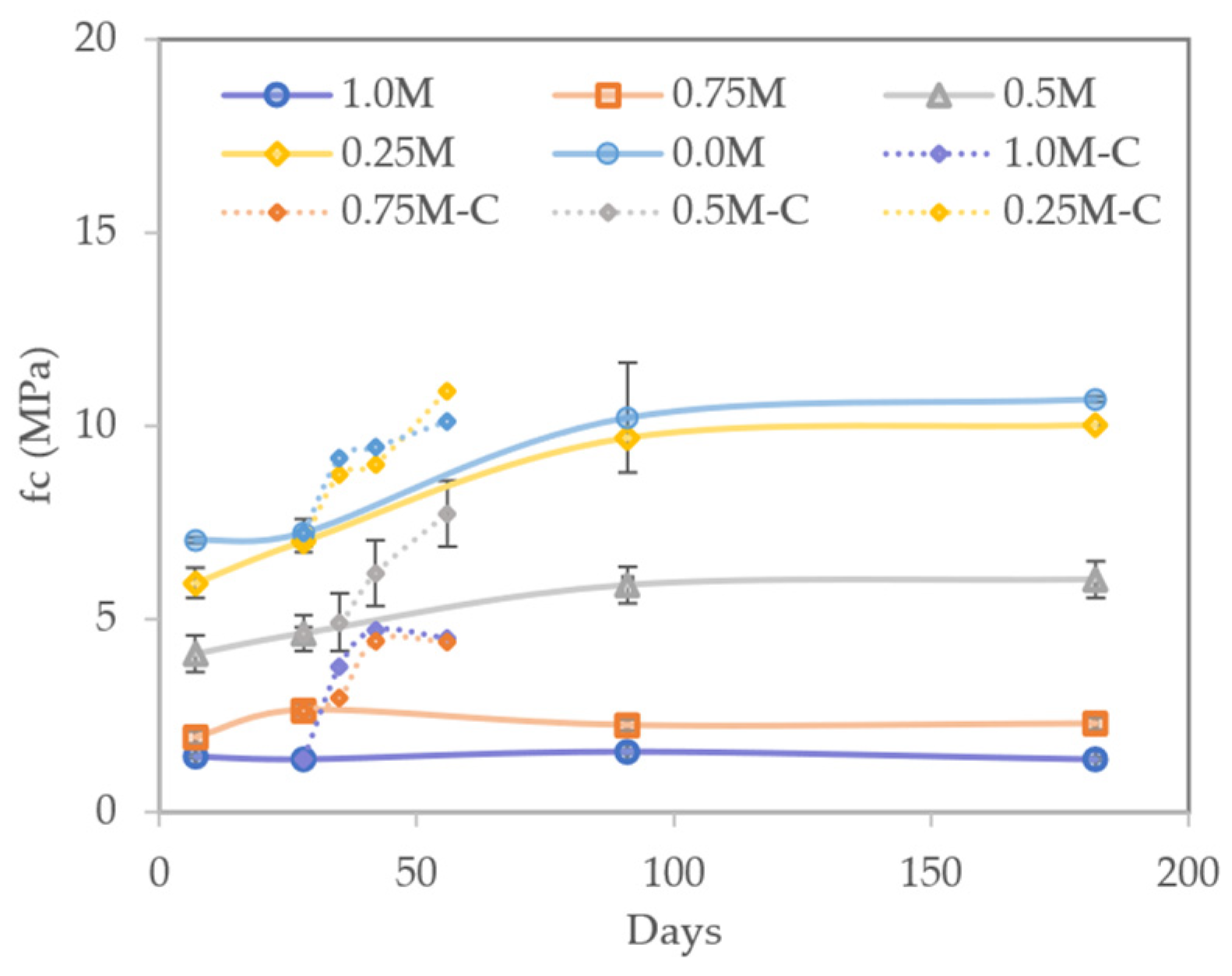
3.5.2. Flexural Strength
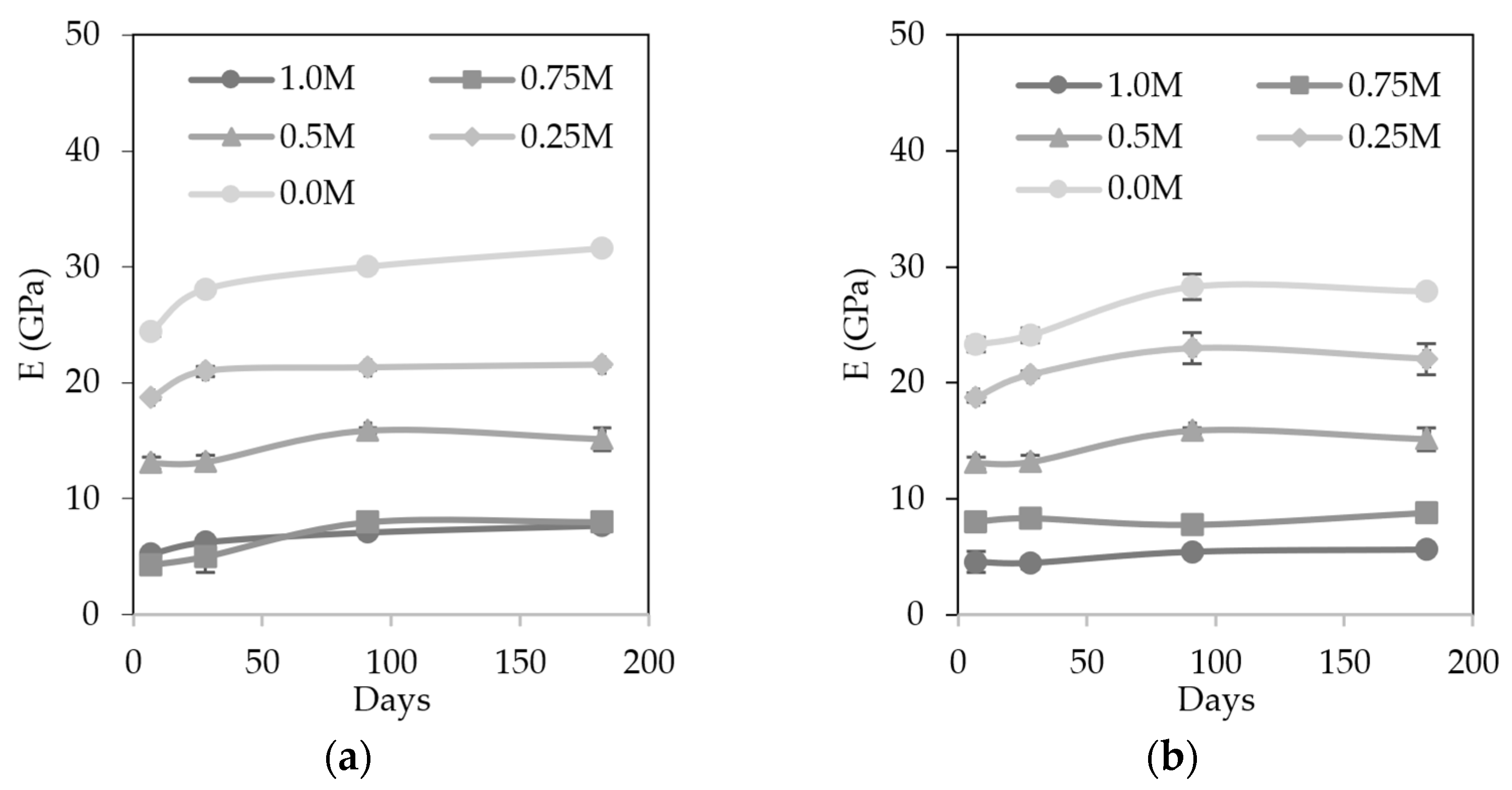
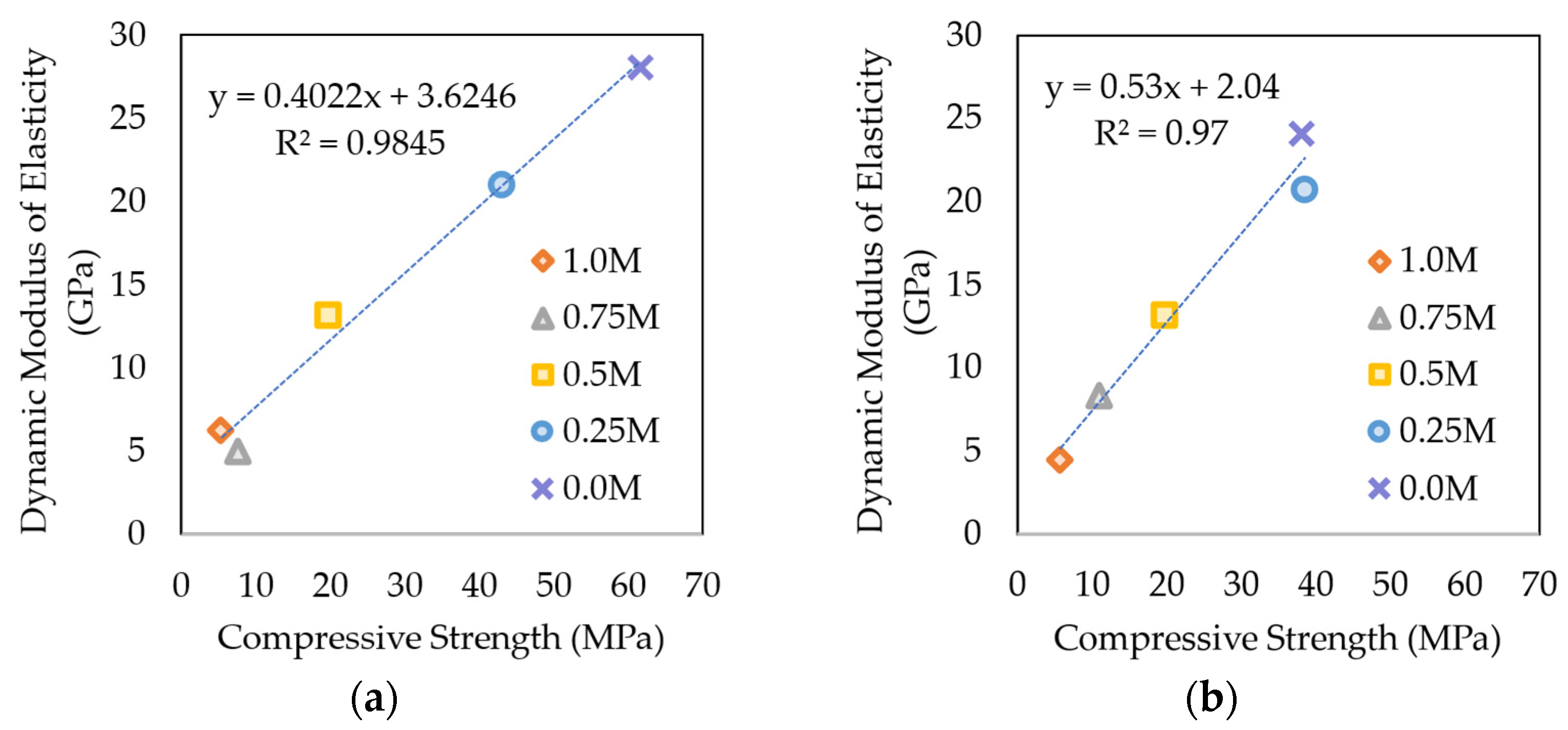
3.5.3. Dynamic Modulus of Elasticity
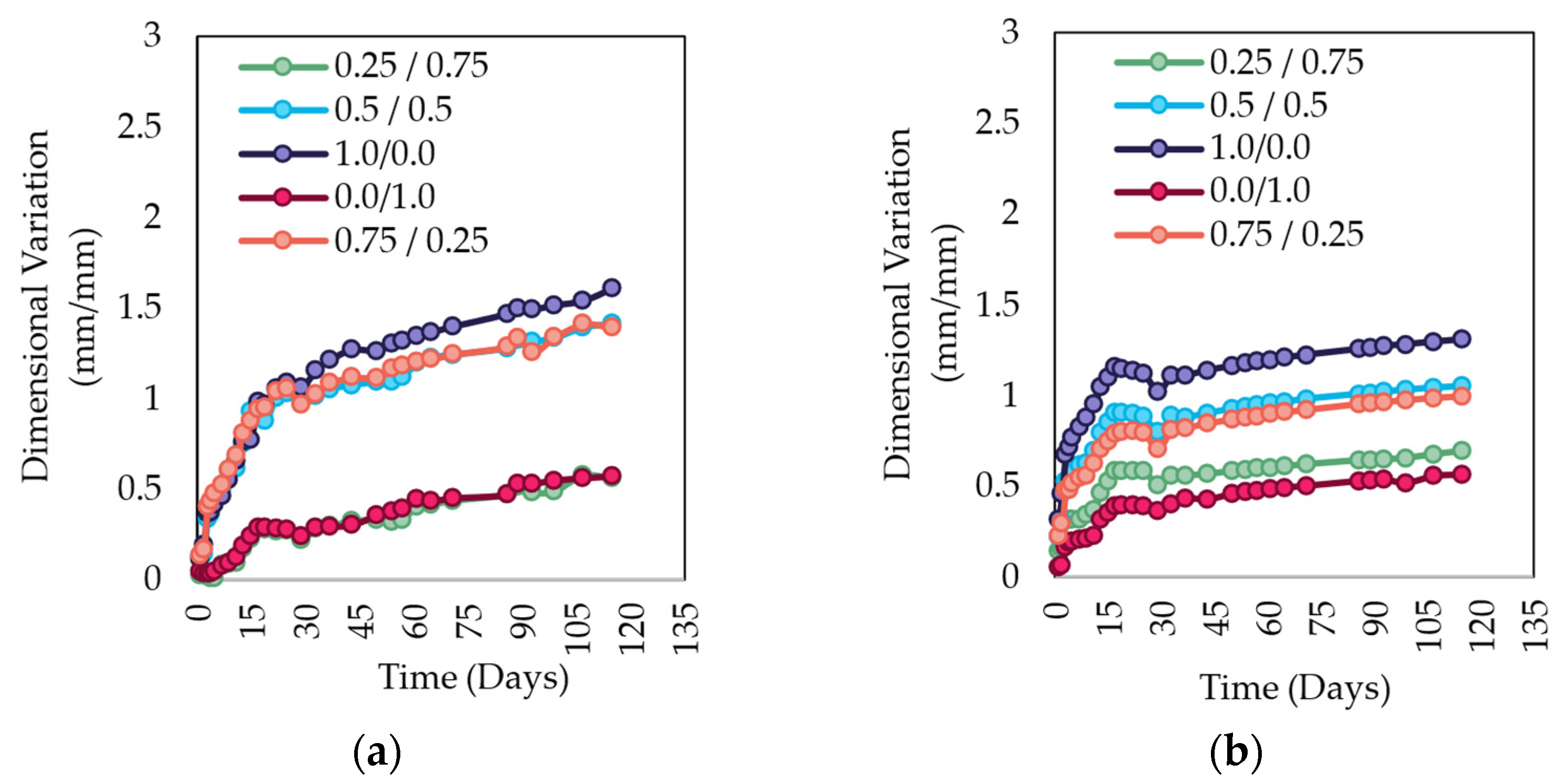
3.5.4. Shrinkage
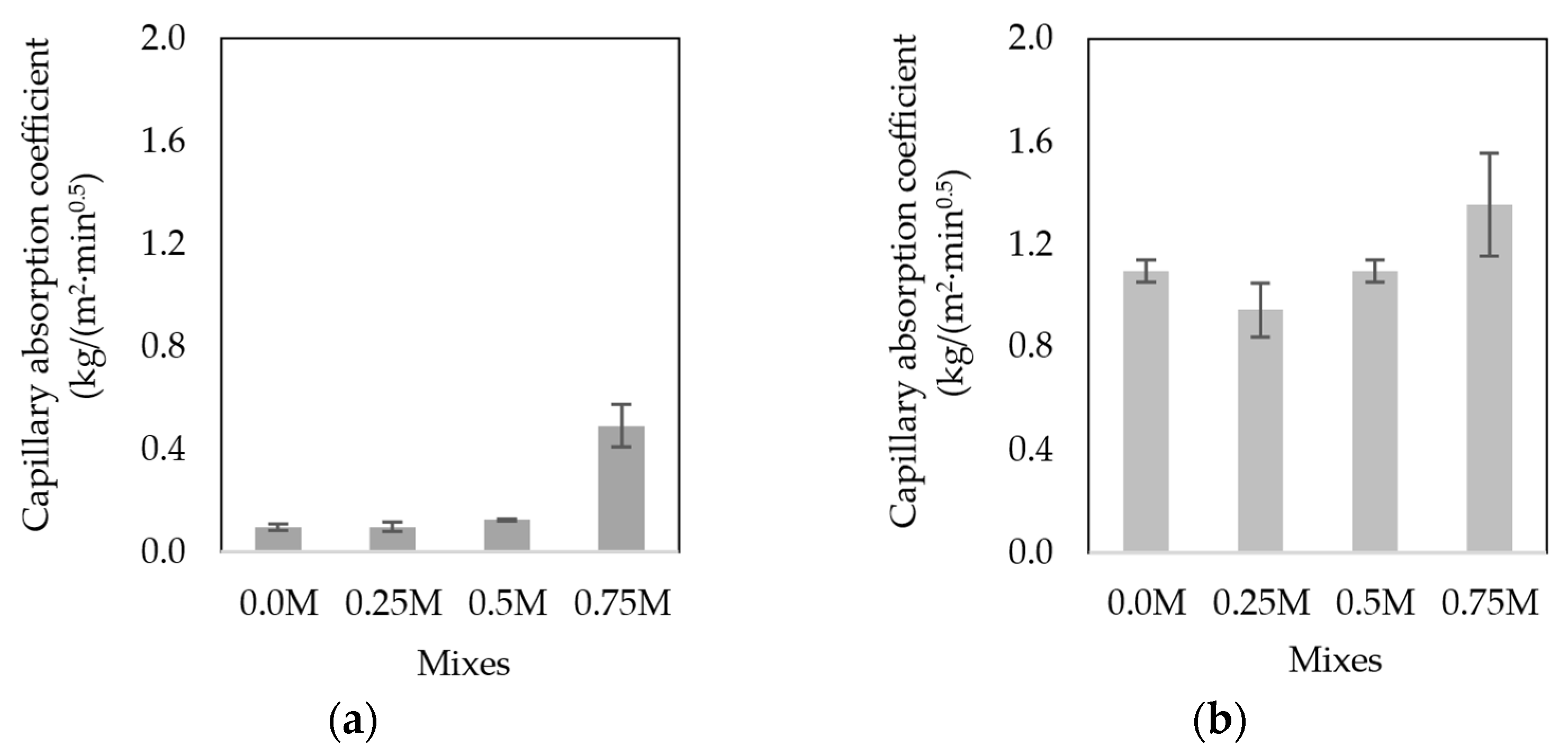
3.5.5. Water Absorption by Capillary Action
3.5.6. Water Absorption by Immersion
4. Conclusions
- The chemical and mineralogical characterization of MIBA showed that it has potential to be activated, but the high aluminum content is problematic in alkali-activated materials due to the formation of hydrogen gas.
- The pre-treatment was effective at reducing the amount of pure aluminum available to react, thereby allowing the production of mortars with adequate dimensional stability;
- FA improved the workability of mixes in the fresh state, regardless of the activator design. Nonetheless, the mixes with the CAA design presented a higher fluidity since they had the lowest sodium silicate content;
- MIBA led to a less dense material due to the foaming reaction between the residual aluminum with the alkaline activator. This caused a higher porosity, lower mechanical strength, and higher water absorption, especially in contents equal to or greater than 75%. However, mixes with a maximum of 50% MIBA content did not show an excessive deterioration in the properties evaluated;
- Carbonation substantially improved the mechanical properties of AAM, especially in those with higher contents of MIBA, as it is a precursor with a high calcium content;
- Shrinkage of the sealed and unsealed specimens is influenced by thermal curing and storage conditions; therefore, the sealed specimens that experienced minimal exchange of water with the environment exhibited higher shrinkage associated with the internal reactions within the AAM matrix;
- All mixes presented sodium migration when performing the capillary test, the magnitude of which was greater in mixes with a greater amount of MIBA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Labor Organization. Compilation of Decisions of the Committee. 2018. Available online: http://ilo.org/dyn/normlex/en/f?p=NORMLEXPUB:70002:0::NO::P70002_HIER_ELMENT_ID,P70002_HIER_LEVEL:3945366,1 (accessed on 16 November 2021).
- Stevano, S.; Ali, R.; Jamieson, M. Essential for what? A global social reproduction view on the re-organisation of work during the COVID-19 pandemic. Can. J. Dev. Stud./Rev. Can. D’études Dév. 2021, 42, 178–199. [Google Scholar] [CrossRef]
- Wong, S.; Mah, A.X.Y.; Nordin, A.H.; Nyakuma, B.B.; Ngadi, N.; Mat, R.; Amin, N.A.S.; Ho, W.S.; Lee, T.H. Emerging trends in municipal solid waste incineration ashes research: A bibliometric analysis from 1994 to 2018. Environ. Sci. Pollut. Res. 2020, 27, 7757–7784. [Google Scholar] [CrossRef] [PubMed]
- Explained Eurostat. Municipal Waste Statistic. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=File:Municipal_waste_landfilled,_incinerated,_recycled_and_composted,_EU-28,_1995-017.png (accessed on 7 July 2021).
- Explained Eurostat. Working from Home across EU Regions in 2020. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20210923-1 (accessed on 25 September 2021).
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C.W. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [CrossRef] [PubMed]
- Tang, P. Municipal Solid Waste Incineration (MSWI) Bottom Ash—From Waste to Value: Characterization, Treatments, and Application. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2017. [Google Scholar]
- Simões, B.; da Silva, P.; Silva, R.; Avila, Y.; Forero, J. Ternary mixes of self-compacting concrete with fly ash and municipal solid waste incinerator bottom ash. Appl. Sci. 2021, 11, 107. [Google Scholar] [CrossRef]
- Clavier, K.A.; Paris, J.M.; Ferraro, C.C.; Townsend, T.G. Opportunities and challenges associated with using municipal waste incineration ash as a raw ingredient in cement production—A review. Resour. Conserv. Recycl. 2020, 160, 104888. [Google Scholar] [CrossRef]
- Birgisdottir, H.; Bhander, G.; Hauschild, M.Z.; Christensen, T.H. Life cycle assessment of disposal of residues from municipal solid waste incineration: Recycling of bottom ash in road construction or landfilling in Denmark evaluated in the ROAD-RES model. Waste Manag. 2007, 27, S75–S84. [Google Scholar] [CrossRef]
- Keppert, M.; Pavlík, Z.; Tydlitat, V.; Volfová, P.; Švarcová, S.; Šyc, M.; Černý, R. Properties of municipal solid waste incineration ashes with respect to their separation temperature. Waste Manag. Res. 2012, 30, 1041–1048. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Alkali-activated materials and geopolymer: A review of common precursors and activators addressing circular economy. Circ. Econ. Sustain. 2022, 2, 165–196. [Google Scholar] [CrossRef]
- Gökçe, H.S.; Tuyan, M.; Nehdi, M.L. Alkali-activated and geopolymer materials developed using innovative manufacturing techniques: A critical review. Constr. Build. Mater. 2021, 303, 124483. [Google Scholar] [CrossRef]
- Puertas, F.; Amat, T.; Fernández-Jiménez, A.; Vázquez, T. Mechanical and durable behaviour of alkaline cement mortars reinforced with polypropylene fibres. Cem. Concr. Res. 2003, 33, 2031–2036. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Casanova, S.; Silva, R.V.; de Brito, J.; Pereira, M.F.C. Mortars with alkali-activated municipal solid waste incinerator bottom ash and fine recycled aggregates. J. Clean. Prod. 2021, 289, 125707. [Google Scholar] [CrossRef]
- Carvalho, R.; Silva, R.V.; de Brito, J.; Pereira, M.F.C. Alkali activation of bottom ash from municipal solid waste incineration: Optimization of NaOH- and Na2SiO3-based activators. J. Clean. Prod. 2021, 291, 125930. [Google Scholar] [CrossRef]
- Alderete, N.M.; Joseph, A.M.; Van den Heede, P.; Matthys, S.; De Belie, N. Effective and sustainable use of municipal solid waste incineration bottom ash in concrete regarding strength and durability. Resour. Conserv. Recycl. 2021, 167, 105356. [Google Scholar] [CrossRef]
- Wang, H.-W.; Chung, H.-W.; Teng, H.-T.; Cao, G. Generation of hydrogen from aluminum and water–effect of metal oxide nanocrystals and water quality. Int. J. Hydrogen Energy 2011, 36, 15136–15144. [Google Scholar] [CrossRef]
- Luan, C.; Shi, X.; Zhang, K.; Utashev, N.; Yang, F.; Dai, J.; Wang, Q. A mix design method of fly ash geopolymer concrete based on factors analysis. Constr. Build. Mater. 2021, 272, 121612. [Google Scholar] [CrossRef]
- Cho, Y.K.; Yoo, S.W.; Jung, S.H.; Lee, K.M.; Kwon, S.J. Effect of Na2O content, SiO2/Na2O molar ratio, and curing conditions on the compressive strength of FA-based geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- Kuenzel, C.; Vandeperre, L.J.; Donatello, S.; Boccaccini, A.R.; Cheeseman, C. Ambient temperature drying shrinkage and cracking in metakaolin-based geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef] [Green Version]
- Rocha, T.D.; Dias, D.P.; Franca, F.C.C.; Guerra, R.R.D.; Marques, L. Metakaolin-based geopolymer mortars with different alkaline activators (Na+ and K+). Constr. Build. Mater. 2018, 178, 453–461. [Google Scholar] [CrossRef]
- Mary Joseph, A.; Snellings, R.; Nielsen, P.; Matthys, S.; De Belie, N. Pre-treatment and utilisation of municipal solid waste incineration bottom ashes towards a circular economy. Constr. Build. Mater. 2020, 260, 120485. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Sun, Y.; Lu, Z.; Zhang, X.; Zuo, L.; Feng, Y.; Qian, R.; Qi, Y.; Ji, Y.; et al. Influence of NaOH content on the alkali conversion mechanism in MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 248, 118582. [Google Scholar] [CrossRef]
- Maldonado-Alameda, A.; Mañosa, J.; Giro-Paloma, J.; Formosa, J.; Chimenos, J.M. Alkali-activated binders using bottom ash from waste-to-energy plants and aluminium recycling waste. Appl. Sci. 2021, 11, 3840. [Google Scholar] [CrossRef]
- Cristelo, N.; Segadães, L.; Coelho, J.; Chaves, B.; Sousa, N.R.; de Lurdes Lopes, M. Recycling municipal solid waste incineration slag and fly ash as precursors in low-range alkaline cements. Waste Manag. 2020, 104, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Chen, L.; Lu, Z.; Sun, Y.; Zhang, X.; Feng, Y.; Ji, Y.; Xu, Z. Use of pretreatment to prevent expansion and foaming in high-performance MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 245, 118471. [Google Scholar] [CrossRef]
- Wang, C.; He, H.; Wang, Y.; Xue, W. Effects of fluorogypsum and flue-gas desulfurization gypsum on the hydration and hardened properties of alkali slag cement. Crystals 2021, 11, 1475. [Google Scholar] [CrossRef]
- Yurt, Ü.; Bekar, F. Comparative study of hazelnut-shell biomass ash and metakaolin to improve the performance of alkali-activated concrete: A sustainable greener alternative. Constr. Build. Mater. 2022, 320, 126230. [Google Scholar] [CrossRef]
- Lima, S.; Gomes, T.; Moraes, J. Novel one-part alkali-activated binder produced with coffee husk ash. Mater. Lett. 2022, 313, 131733. [Google Scholar] [CrossRef]
- European Council. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Communities 1998, 330, 32–54. [Google Scholar]
- Topçu, İ.; Ateşin, Ö. Effect of high dosage lignosulphonate and naphthalene sulphonate based plasticizer usage on micro concrete properties. Constr. Build. Mater. 2016, 120, 189–197. [Google Scholar] [CrossRef]
- EN 12620; Aggregates for Concrete. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2002.
- Rosenband, V.; Gany, A. Application of activated aluminum powder for generation of hydrogen from water. Int. J. Hydrogen Energy 2010, 35, 10898–10904. [Google Scholar] [CrossRef]
- EN-196-1; Methods of Testing Cement—Part 1: Determination of Strength. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2005; 36p.
- ASTM D4972; Standard Test Methods for pH of Soils. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- EN-933-1; Tests for Geometrical Properties of Aggregates—Part 1: Determination of Particle Size Distribution. Sieving Method. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2012; 22p.
- EN-1097-6; Tests for Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2013; 54p.
- EN-1015-3; Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (By Flow Table). Comité Européen de Normalisation (CEN): Brussels, Belgium, 1999; 10p.
- EN-1015-6; Methods of Test for Mortar for Masonry—Part 6: Determination of Bulk Density of Fresh Mortar. Comité Européen de Normalisation (CEN): Brussels, Belgium, 1999; 8p.
- EN 1015-11; Determination of Flexural and Compressive Strength of Hardened Mortar. Comité Européen de Normalisation (CEN): Brussels, Belgium, 1999.
- EN-1015-10; Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened Mortar. Comité Européen de Normalisation (CEN): Brussels, Belgium, 1999; 10p.
- EN 14146; Natural Stone Test Methods. Determination of the Dynamic Elastic Modulus of Elasticity (By Measuring the Fundamental Resonance Frequency). Comité Européen de Normalisation (CEN): Brussels, Belgium, 2004; 18p.
- EN-1015-13; Methods of Test for Mortar for Masonry—Part 13: Determination of Dimensional Stability of Hardened Mortars. Comité Européen de Normalisation (CEN): Brussels, Belgium, 1993; 20p.
- EN-1015-18; Methods of Test for Mortar for Masonry—Part 18: Determination of Water Absorption Coefficient Due to Capillary action of Hardened Mortar. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2002; 12p.
- LNEC-E394; Concrete: Determination of Water Absorption by Immersion—Testing at Atmospheric Pressure (in Portuguese). National Laboratory in Civil Engineering (LNEC—Laboratório Nacional de Engenharia Civil): Lisbon, Portugal, 1993; 2p.
- EN-13295; Products and Systems for the Protection and Repair of Concrete Structures. Test Methods. Determination of Resistance To Carbonation. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2004; 18p.
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- Lynn, C.J.; Dhir, R.K.; Ghataora, G.S. Municipal incinerated bottom ash use as a cement component in concrete. Mag. Concr. Res. 2017, 69, 512–525. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Saffarzadeh, A.; Shimaoka, T.; Zhao, C.; Peng, X.; Gao, J. Geoenvironmental weathering/deterioration of landfilled MSWI-BA glass. J. Hazard. Mater. 2014, 278, 610–619. [Google Scholar] [CrossRef]
- Bayuseno, A.P.; Schmahl, W.W. Understanding the chemical and mineralogical properties of the inorganic portion of MSWI bottom ash. Waste Manag. 2010, 30, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.M.; Zeedan, S.R. The effect of activator concentration on the residual strength of alkali-activated fly ash pastes subjected to thermal load. Constr. Build. Mater. 2011, 25, 3098–3107. [Google Scholar] [CrossRef]
- Engineering ToolBox. Hydrogen—Density and Specific Weight. 2018. Available online: https://www.engineeringtoolbox.com/hydrogen-H2-density-specific-weight-temperature-pressure-_2044.html (accessed on 17 July 2019).
- Das, S.K.; Mustakim, S.M.; Adesina, A.; Mishra, J.; Alomayri, T.S.; Assaedi, H.S.; Kaze, C.R. Fresh, strength and microstructure properties of geopolymer concrete incorporating lime and silica fume as replacement of fly ash. J. Build. Eng. 2020, 32, 101780. [Google Scholar] [CrossRef]
- Kuri, J.; Khan, M.; Sarker, P. Fresh and hardened properties of geopolymer binder using ground high magnesium ferronickel slag with fly ash. Constr. Build. Mater. 2021, 272, 121877. [Google Scholar] [CrossRef]
- Provis, J.L.; Duxson, P.; van Deventer, J.S. The role of particle technology in developing sustainable construction materials. Adv. Powder Technol. 2010, 21, 2–7. [Google Scholar] [CrossRef]
- Kujawa, W.; Olewnik-Kruszkowska, E.; Nowaczyk, J. Concrete strengthening by introducing polymer-based additives into the cement matrix—A mini review. Materials 2021, 14, 6071. [Google Scholar] [CrossRef]
- Burgos-Montes, O.; Palacios, M.; Rivilla, P.; Puertas, F. Compatibility between superplasticizer admixtures and cements with mineral additions. Constr. Build. Mater. 2012, 31, 300–309. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Ruiz-Molina, S.; Pérez-Villarejo, L.; Castro, E.; Sánchez-Soto, P.J. Dust filter of secondary aluminium industry as raw material of geopolymer foams. J. Build. Eng. 2020, 32, 101656. [Google Scholar] [CrossRef]
- Provis, J.L.; Fernández-Jiménez, A.; Kamseu, E.; Leonelli, C.; Palomo, A. Binder chemistry—Low-calcium alkali-activated materials. In Alkali Activated Materials; Provis, J.L., van Deventer, J.S.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 93–123. [Google Scholar]
- Bernal, S.A.; Provis, J.L.; Fernández-Jiménez, A.; Krivenko, P.V.; Kavalerova, E.; Palacios, M.; Shi, C. Binder chemistry–high-calcium alkali-activated materials. In Alkali Activated Materials; Provis, J.L., van Deventer, J.S.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 59–91. [Google Scholar]
- Fernandez-Jimenez, A.; Palomo, A.; Lopez-Hombrados, C. Engineering properties of alkali-activated fly ash concrete. ACI Mater. J. 2006, 103, 106. [Google Scholar]
- Duxson, P.; Mallicoat, S.; Lukey, G.; Kriven, W.; van Deventer, J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Khalid, H.; Lee, N.; Choudhry, I.; Wang, Z.; Lee, H.K. Evolution of zeolite crystals in geopolymer-supported zeolites: Effects of composition of starting materials. Mater. Lett. 2019, 239, 33–36. [Google Scholar] [CrossRef]
- Moudar, J.; El Fami, N.; Diouri, A.; Taibi, M. Identification and characterization of faujasite zeolite phase in alkali activated class F fly ash. Mater. Today Proc. 2022. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Rao, R.; Wang, C.; Fang, C. Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos. Part B Eng. 2019, 164, 179–190. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of fresh and hardened fly ash/slag based geopolymer concrete: A review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Jin, C. Use of slaked lime and Portland cement to improve the resistance of MSWI bottom ash-GBFS geopolymer concrete against carbonation. Constr. Build. Mater. 2018, 166, 290–300. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z. Influence of fly ash on the pore structure and shrinkage characteristics of metakaolin-based geopolymer pastes and mortars. Constr. Build. Mater. 2017, 153, 284–293. [Google Scholar] [CrossRef]
- Archez, J.; Farges, R.; Gharzouni, A.; Rossignol, S. Influence of the geopolymer formulation on the endogeneous shrinkage. Constr. Build. Mater. 2021, 298, 123813. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Geopolymer Institute: Saint-Quentin, France, 2008. [Google Scholar]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- San Nicolas, R.; Provis, J. Interfacial transition zone in alkali-activated slag concrete. In Proceedings of the 12th International Conference on Recent Advances in Concrete Technology and Sustainability Issues, Prague, Czech Republic, 30 October 2012. [Google Scholar]
- Bernal, S.A.; San Nicolas, R.; Provis, J.L.; De Gutiérrez, R.M.; van Deventer, J.S. Natural carbonation of aged alkali-activated slag concretes. Mater. Struct. 2014, 47, 693–707. [Google Scholar] [CrossRef]
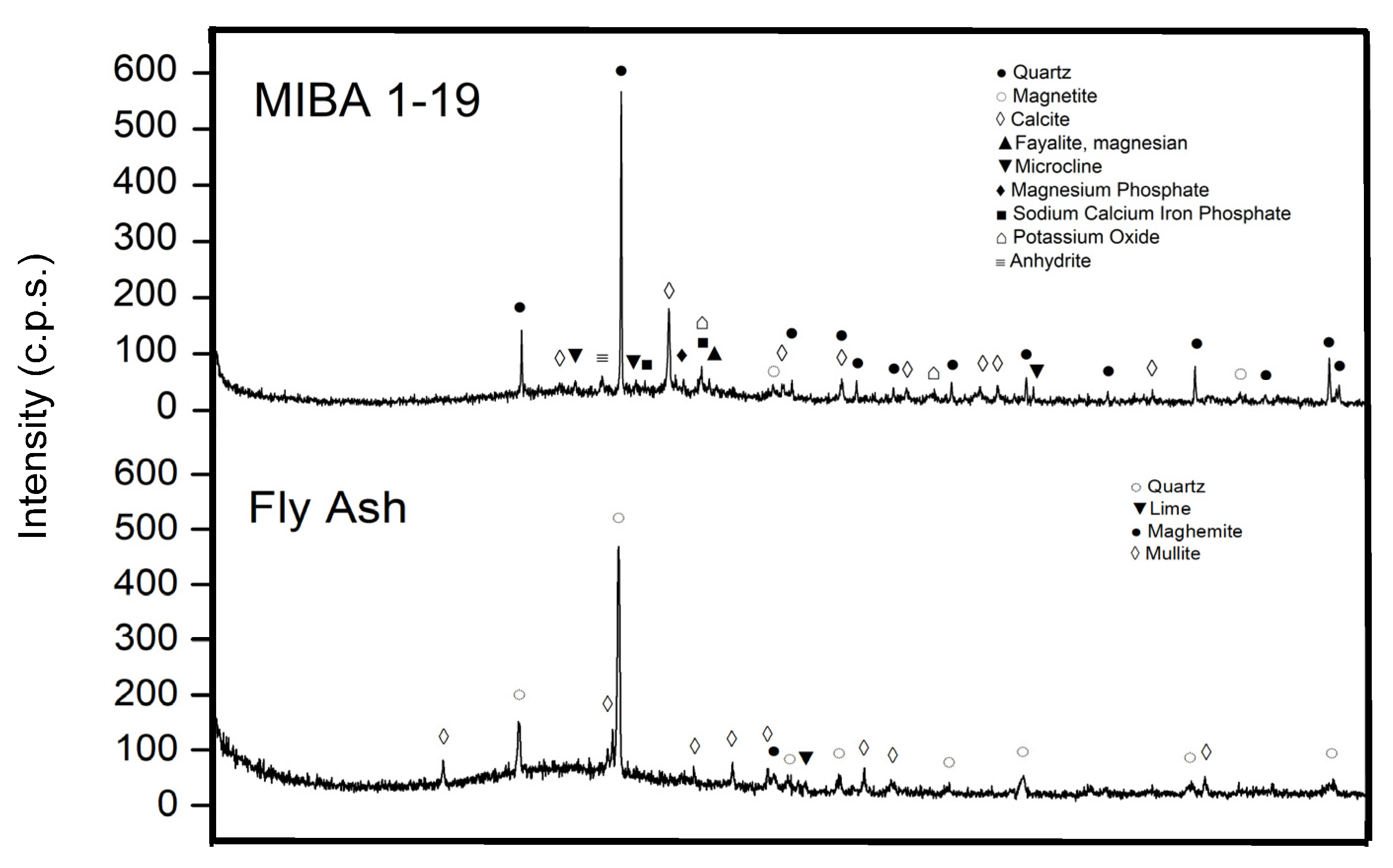


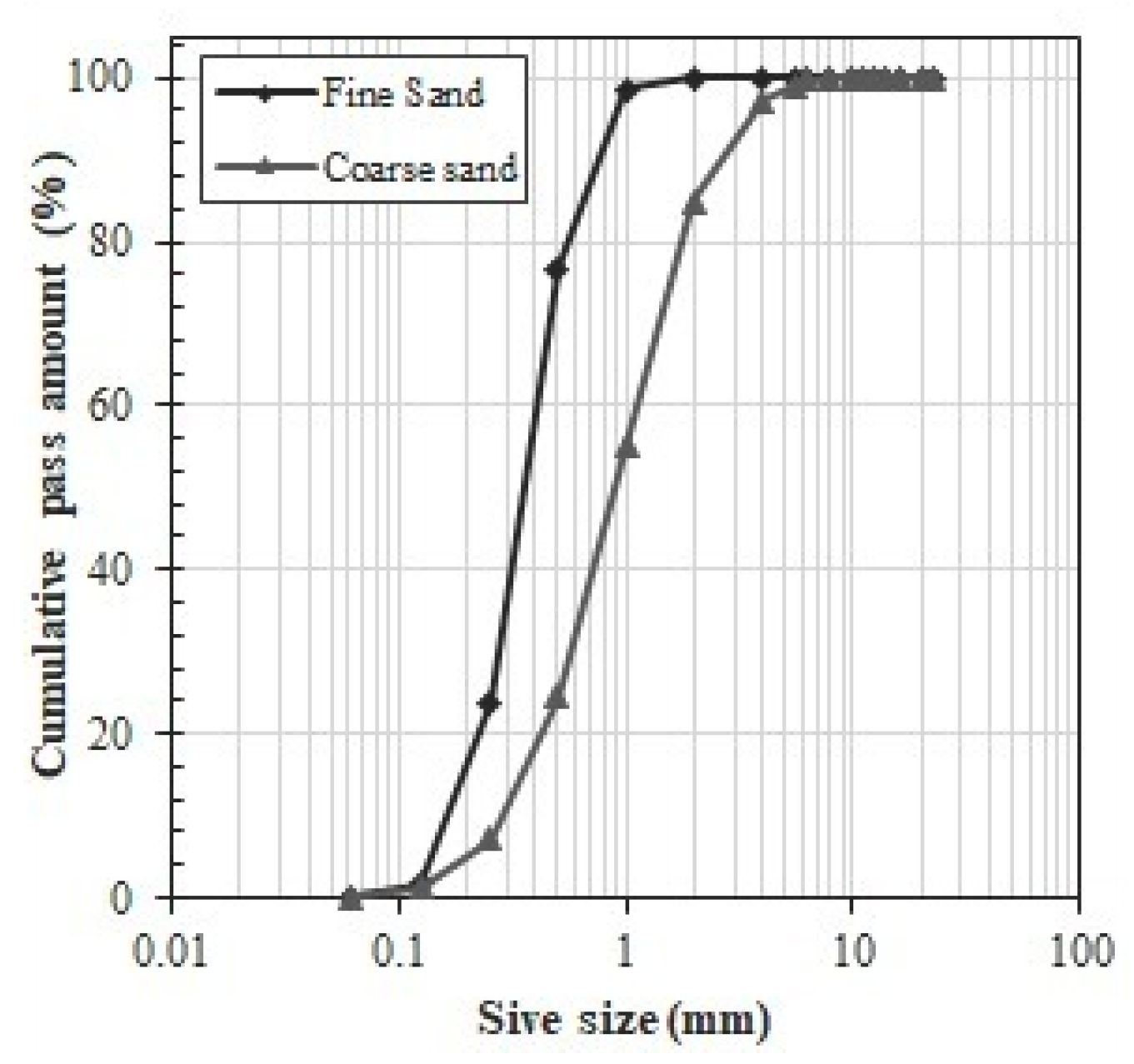


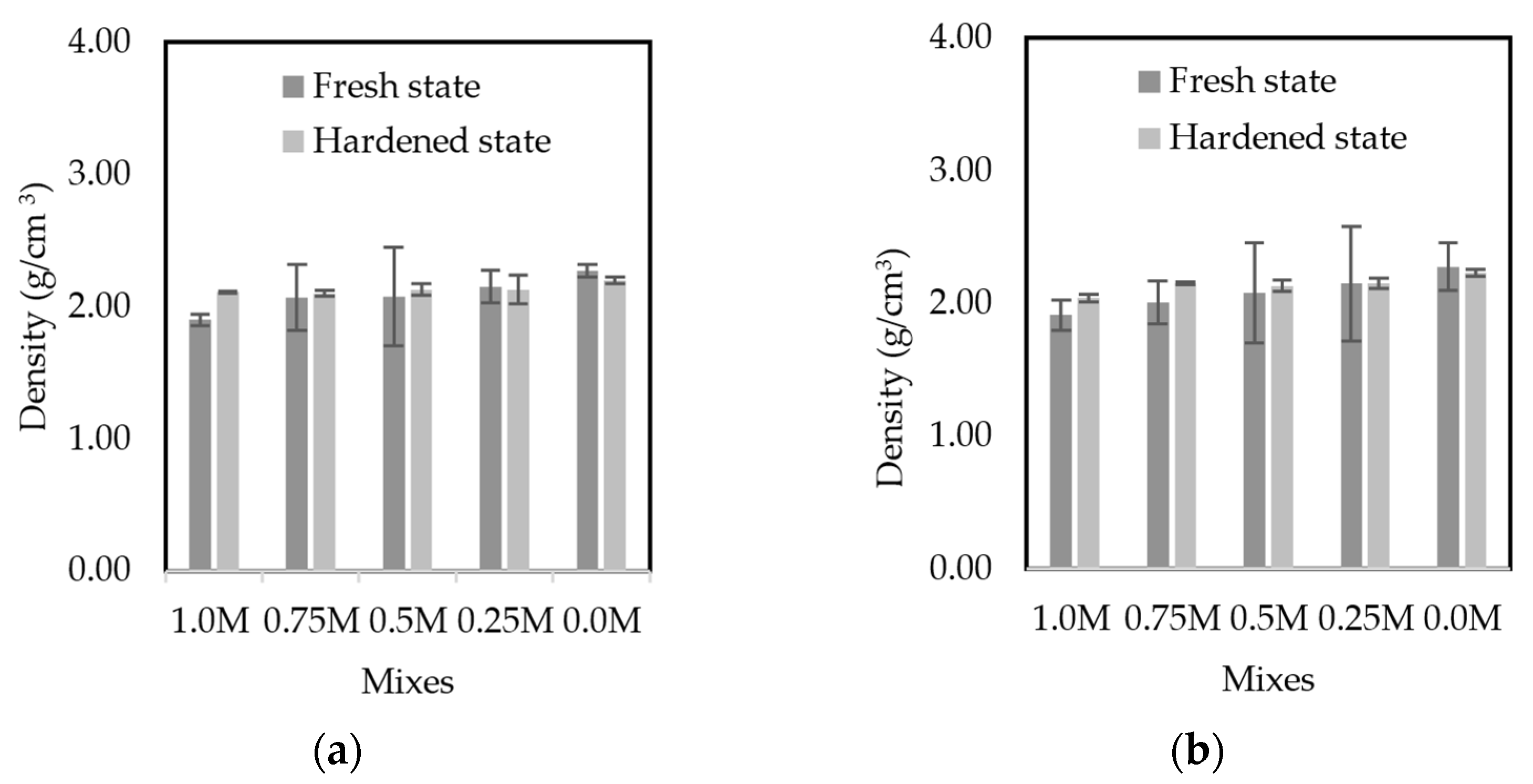
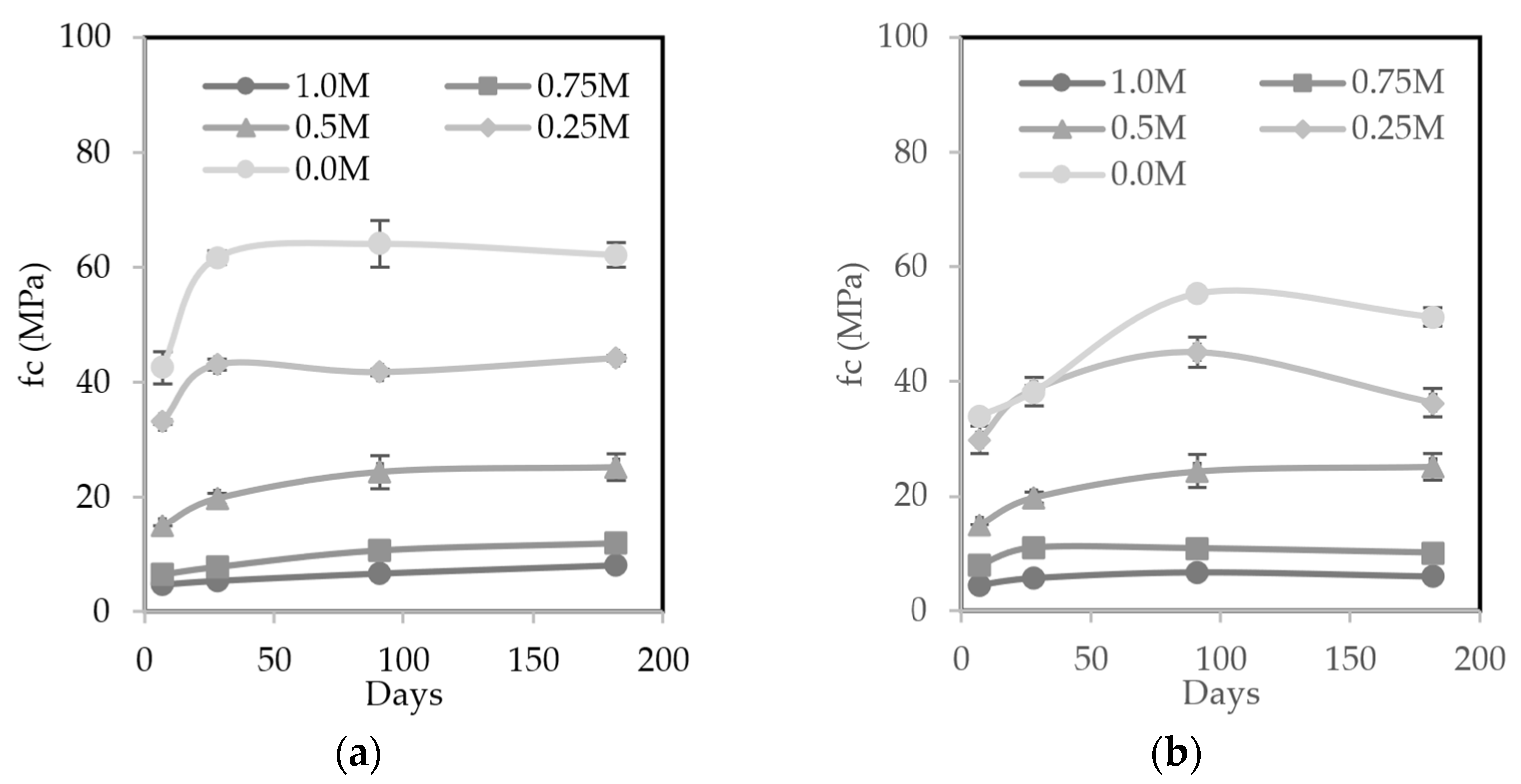
| MIX | MIBA | FA | WRA | Water | NaOH | Na2SiO3 | Fine Sand | Coarse Sand |
|---|---|---|---|---|---|---|---|---|
| 1.0 M | 514.31 | 0.00 | 5.14 | 210.11 | 55.44 | 0.00 | 452.01 | 1050.66 |
| 0.75 M | 398.56 | 127.21 | 5.26 | 175.69 | 61.79 | 49.06 | 447.20 | 1039.49 |
| 0.5 M | 262.68 | 251.53 | 5.14 | 130.45 | 66.15 | 114.43 | 442.12 | 1027.67 |
| 0.25 M | 129.64 | 372.40 | 5.02 | 74.80 | 68.51 | 195.19 | 436.38 | 1014.34 |
| 0.0 M | 0.00 | 491.34 | 0.00 | 9.47 | 69.22 | 291.55 | 431.82 | 1003.74 |
| MIX | MIBA | FA | SP | Water | NaOH | Na2SiO3 | Fine Sand | Coarse Sand |
|---|---|---|---|---|---|---|---|---|
| 1.0 M | 524.87 | 0.00 | 5.25 | 130.32 | 66.08 | 114.32 | 441.70 | 1026.70 |
| 0.75 M | 393.84 | 125.70 | 5.20 | 130.39 | 66.11 | 114.37 | 441.91 | 1027.18 |
| 0.5 M | 262.68 | 251.53 | 5.14 | 130.45 | 66.15 | 114.43 | 442.12 | 1027.67 |
| 0.25 M | 131.40 | 377.47 | 5.09 | 130.51 | 66.18 | 114.48 | 442.33 | 1028.16 |
| 0.0 M | 0.00 | 505.64 | 0.00 | 131.12 | 66.49 | 115.01 | 444.39 | 1032.95 |
| Materials | FA (%) | MIBA (%) |
|---|---|---|
| Al2O3 | 25.55 | 8.85 |
| CaO | 2.28 | 18.33 |
| Fe2O3 | 6.92 | 6.69 |
| K2O | 2.75 | 1.59 |
| MgO | 1.83 | 4.02 |
| Na2O | 1.30 | 6.55 |
| SiO2 | 56.44 | 48.92 |
| SO3 | 0.80 | 1.36 |
| Cl− | 0.00 | 0.00 |
| Cr2O3 | 0.49 | 0.06 |
| TiO2 | 1.14 | 0.48 |
| ZnO | 0.02 | 0.35 |
| P2O5 | 0.44 | 2.52 |
| V2O5 | 0.05 | - |
| CuO | 0.00 | 0.16 |
| MnO2 | - | 0.12 |
| Mix | Water Absorption by Immersion (%) | Standard Deviation |
|---|---|---|
| 0.0 M | 13.4 | 0.14 |
| 0.25 M | 13.1 | 0.09 |
| 0.5 M | 14.8 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, Y.; Silva, R.V.; de Brito, J. Alkali-Activated Materials with Pre-Treated Municipal Solid Waste Incinerator Bottom Ash. Appl. Sci. 2022, 12, 3535. https://doi.org/10.3390/app12073535
Avila Y, Silva RV, de Brito J. Alkali-Activated Materials with Pre-Treated Municipal Solid Waste Incinerator Bottom Ash. Applied Sciences. 2022; 12(7):3535. https://doi.org/10.3390/app12073535
Chicago/Turabian StyleAvila, Yoleimy, Rui Vasco Silva, and Jorge de Brito. 2022. "Alkali-Activated Materials with Pre-Treated Municipal Solid Waste Incinerator Bottom Ash" Applied Sciences 12, no. 7: 3535. https://doi.org/10.3390/app12073535
APA StyleAvila, Y., Silva, R. V., & de Brito, J. (2022). Alkali-Activated Materials with Pre-Treated Municipal Solid Waste Incinerator Bottom Ash. Applied Sciences, 12(7), 3535. https://doi.org/10.3390/app12073535








