Abstract
Background: In recent years, the incidence of neoplastic diseases in children has increased significantly. Immunodepression triggers undesirable effects in the oral cavity such as mucositis, opportunistic infections, oral bleeding, xerostomia, dysgeusia, decay, dental malformations and pain. Objective: We aim to assess, through salivary tests, the quality of saliva in pediatric patients affected by oncohematological diseases and treated with chemo/radiotherapy compared to non-treated subjects. Methods: A total of 20 subjects aged between 5 and 18 years, with oncological pathologies undergoing radio and/or chemotherapy, were evaluated. The control group consisted of 20 healthy children of the same age. The two groups of subjects were asked to undergo salivary tests. Descriptive statistics were computed for each item. Fisher’s exact test was conducted to compare case and control groups. Results: Subjects treated with chemo/radiotherapy had a lower pH and therefore a higher salivary acidity that predisposes to caries and the oral hygiene of children with oncohematological diseases was more deficient than that of the control group. Conclusions: The early detection of these indicators, and the prevention carried out to limit their severity, is an important aspect of the comprehensive care of oncohematological subjects. Pediatric dentists can play a crucial a role enabling good quality of life during cancer therapy.
1. Introduction
Malignant tumors affecting the pediatric population account for 1–2% of all neoplasms and are one of the most frequent causes of death in this age group. In recent years, the incidence of neoplastic diseases in children has increased significantly. This is due to increased exposure to carcinogenic environmental noxae and the diagnostic possibilities now available [1,2,3,4]. In contrast to adult tumors, which are mainly carcinomas, tumors of embryonic and connective tissue origin predominate in children, namely leukemias, central nervous system tumors, lymphomas, neuroblastomas, bone and soft-tissue sarcomas. Acute lymphoblastic leukemia, among childhood leukemias, represents most cases, with the remaining 25% being various types of acute myeloblastic leukemia. The treatment of these diseases involves the use of cytotoxic drugs, such as alkylating agents, cytotoxic antibiotics, antimetabolites and agents that can be used in combination and inhibit microtubule formation [5,6]. Immunodepression, caused by antineoplastic treatments and local tissue changes resulting from possible radiotherapy, trigger undesirable effects in the oral cavity such as: mucositis, opportunistic infections, oral bleeding, xerostomia, dysgeusia, decay, dental malformations and pain. The oral cavity is damaged because its tissues are affected by the cytotoxic impact of these drugs: antineoplastic agents, which are not selective, act on both tumor cells and healthy cells, thus inhibiting their replication [7].
Mucosal cells in the oral cavity are particularly susceptible to the action of these agents, resulting in atrophy and loss of mucosal barrier integrity [8]. Mucositis is one of the most disabling and frequent complications. The earliest clinical sign is the appearance of an erythematous area, often in the non-keratinized mucosa. The clinical course is variable and in the most severe cases leads to the appearance of ulcerated and hemorrhagic lesions, responsible for intense pain, incompatible with the intake of solid food and liquids, making it necessary to resort to parenteral nutrition and pain-relieving therapies [9]. Another recurring complication occurring in the oral cavity is xerostomia associated with dysgeusia. Poor saliva production leads to a decreased buffering effect against the acidic pH, as well as to poor cleansing of the oral cavity and to a weakening of the mucous membranes. These factors, combined with changes in the oral microbial flora, are responsible for an increased risk of cavities [10]. The early detection of these symptoms and the prevention carried out to limit their severity are important aspects of the treatment process, enabling an improvement in quality of life during cancer therapy.
We aim to assess, through salivary tests, the quality of saliva in pediatric patients affected by oncohematological diseases and treated with chemo/radiotherapy. A control group of healthy children was selected for comparison.
2. Materials and Methods
The study was carried out in cooperation with the Pediatric Hematology Department of Translational and Precision Medicine, and the Department of Oral and Maxillo-Facial Sciences, Sapienza University of Rome. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Department of Oral and Maxillo-Facial Sciences, Sapienza University of Rome (protocol code 11/24062021). Informed consent was obtained from all subjects involved in the study.
The subjects for the study group were recruited over 30 working days. Twenty subjects aged between 5 and 18 years—10 males and 10 females, with oncological pathologies (acute and chronic myeloid leukemias, acute lymphoid leukemias, Hodgkin’s and non-Hodgkin’s lymphomas) undergoing radio and/or chemotherapy, were evaluated. All patients were enrolled between 0 and 8 months from disease onset (mean 5 months). They received similar chemotherapy drugs (vincristine, etoposide, cyclophosphamide, methotrexate, cytarabine, anthracyclines) even if they were treated with different protocols according to the underlying disease.
The control group was randomly selected from the patients undergoing a first visit in the unit of the Department of Translational and Precision Medicine, and the Department of Oral and Maxillo-Facial Sciences, Sapienza University of Rome. Among the children booked for a first visit in the Department in a 14-day period, a sample of 20 was randomly chosen from the list according to an algorithm generated though the SAS Software (Analytics Software & Solutions-SAS Inc., 100 SAS Campus Drive, Cary, NC 27513-2414, USA, 2020). The sample of 20 children was made up of 10 males and 10 females, of the same age. Subjects with a previous history of oncohematological and oncological disease were excluded.
Salivary test kits were needed for the purpose of evaluating the stimulated and non-stimulated saliva of these patients. Their use is aimed at analyzing the flow, viscosity and consistency of non-induced saliva; studying the pH of the patient’s resting saliva; analyzing the amount of induced saliva that the patient can produce, and the buffering capacity. Salivary tests were carried out through the Salivary Check Buffer kits by GC (GC EUROPE N.V. Researchpark Haasrode-Leuven 1240, Interleuvenlaan 33, B-3001 Leuven, Belgium). Patients were instructed not to consume food or drink, brush teeth or use a mouthwash for at least one hour prior to the test. Table 1 describes and explains the different tests carried out according to the instructions of the previously mentioned Salivary Check Buffer kits by GC.

Table 1.
Description of the different tests carried out according to the instructions of the Salivary Check Buffer kits by GC.
Statistical Analysis
The data gathered were recorded with a specially designed computer program and collected in a Microsoft Excel spreadsheet. Descriptive statistics were computed for each item. Fisher’s exact test was conducted to compare case and control groups [11,12]. Analysis of the data was performed using SPSS 28.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
Visual examinations of the salivary secretion at rest between the two groups are reported in Figure 1. The control group resulted in having slightly more subjects with slow saliva flow, when compared to the test group. This relationship was not significant.
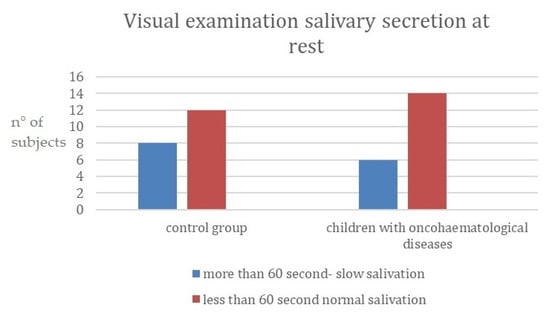
Figure 1.
Visual examination of salivary secretion at rest. (y-axis: n° of subjects = number of subjects for each category).
Figure 2, below, reports data collected on the viscosity of saliva at rest; children with oncohematological diseases had having higher-viscosity saliva compared to the control group. This relationship was significant (see Table 2 further in the Results section).
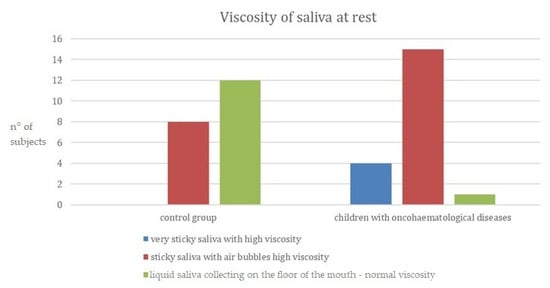
Figure 2.
Viscosity of saliva at rest. (y-axis: n° of subjects = number of subjects for each category).

Table 2.
Fisher’s exact test for the salivary test executed on controls and oncohematological group [11,12].
It can be observed that in the control group, 60% of the patients have liquid saliva with a normal viscosity. By contrast, in the group of children with oncohematological diseases, 75% of the patients have sticky saliva with air bubbles corresponding to a high viscosity. In contrast to the control group, a large proportion of the children also had very sticky saliva with high viscosity.
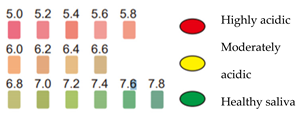
The acidity (i.e., the pH of the resting saliva) was then examined. Results are shown in Figure 3; children with oncohematological diseases had more acidic saliva compared to the control group. This relationship was significant (see Table 2 further in the Results section).
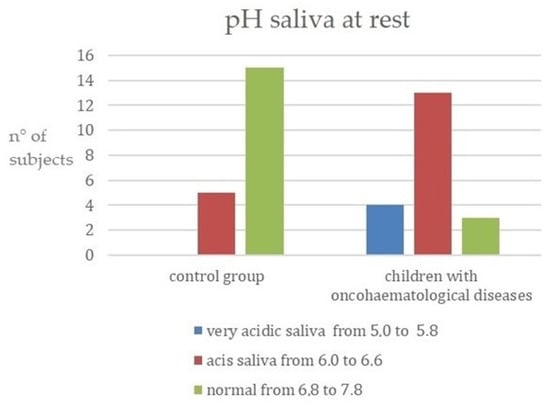
Figure 3.
pH of saliva at rest. (y-axis: n° of subjects = number of subjects for each category).
By stimulating saliva using a paraffin tablet, the amount produced by the two groups was examined. A total of 30% of children with oncohematological diseases had low capacity to produce saliva (3.5–5 mL). In both cases, however, the majority had very low capacity (less than 3.5 mL). From the previous data collected through the salivary tests it can be stated that the ability of saliva to neutralize acids is low in both groups of children. However, in particular, in the group of children affected by oncohematological diseases, 20% have a rather low capacity, between 0 and 5 (Figure 4).
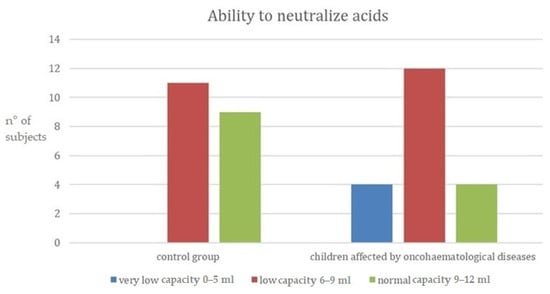
Figure 4.
Ability of saliva to neutralize acids. (y-axis: n° of subjects = number of subjects for each category).
The ability to neutralize acids within two groups are highlighted in Figure 4.
As summarized in Table 2, the comparison between the study and control group proved to be significant for viscosity, pH of saliva at rest, and ability to neutralize acids, showing that these parameters can be influenced by oncohematological conditions. On the other hand, the p-value for the salivary secretion test was not significant and the difference between the two groups was probably not related to the disease.
4. Discussion
After analyzing the results of the salivary tests, various comparisons were made with studies in the literature. Only a few studies carried out salivary tests on oncohematological patients.
Despite the sample being relatively small, this was enough to complete a thorough examination, collect data for our objectives and confirm some results from the previous literature.
In a survey conducted in 2010 by Ou-Young et al., whose aim was to assess the caries activity of children with oncohematological diseases, a group of apparently healthy children and a group of sick children were sampled and compared. Using salivary tests, stimulated saliva samples were collected, and their buffering capacity assessed. The results of these tests show that the group of children with oncohematological diseases had a lower salivary buffer capacity than the control group [13], confirming the findings of the present study, where, in the group of children affected by oncohematological diseases, 20% had very low capacity, between 0 and 5 (Figure 4).
In another study carried out in 2017 by Mazaheri et al., apparently healthy children and children with oncohematological diseases of the same age and belonging to the same socioeconomic status were compared. Sampling was undertaken to determine the salivary pH of the participants. It was observed that between the two groups, children with oncohematological diseases had a higher susceptibility to caries and a lower salivary pH [14]. The same results were found by Hedge et al. [15] and in this work, comparing apparently healthy children and children affected by oncohematological diseases. The data in the present work showed that in the control group, 75% had a saliva pH of 6.8 to 7.8 (normal saliva). In contrast to the latter, only a few children in the other group had non-acidic saliva, with the majority having an acidic salivary pH between 6.0 and 6.6. A total of 20% had very acidic saliva with a pH ranging from 5.0 to 5.8 (Figure 3). Moreover, using salivary tests, it was possible to assess that 60% of the sick children had a low buffering capacity in their saliva and 20% had a rather low capacity, compared to the control group, where 55% of the children showed a low capacity to neutralize acids and 45% a normal capacity. Furthermore, by analyzing the pH, it was possible to observe a higher acidity of the saliva at rest in the group of children suffering from oncohematological pathologies, who have a greater predisposition to dental caries. It can therefore be deduced that the studies reported in the literature are confirmed by the development of this study, since the low production of saliva leads to a decrease in the buffering effect, poor cleansing of the oral cavity, and a weakening of the mucous membranes. These factors, combined with a change in the oral microbial flora, are responsible for an increased risk of cavities.
In a study carried out in 2012 by Morais et al., the oral symptoms that most frequently occur in the mouths of children with leukemia during and after antineoplastic therapies were identified. Mucositis, gingivitis, periodontitis and candidiasis were diagnosed most frequently through careful clinical examinations [16]. In another survey conducted in 2010 by Ponce-Torres et al., the prevalence of oral manifestations observed in these children was gingivitis, decay, mucositis, cheilitis, periodontitis, recurrent herpes, primary herpetic gingivostomatitis, xerostomia, mucosal pallor, ecchymoses and induced ulcers [17]. In a study by Kapoor et al. [18], mean unstimulated salivary flow rate and mean salivary pH scores were found to be lower in the group of children with leukemia and were higher in the group of healthy children. Several authors have reported similar findings [15,19,20,21]. In the present study, visual examination of salivary secretion at rest between the two groups showed that most of the children had normal salivation that accumulated on the floor of the oral cavity in less than 60 s, in both groups, and just a few of them had reduced saliva flow. This might be influenced by the small sample size, but it should also be taken into consideration that saliva flow rate may be affected by the severity of the disease.
The findings of this study must be seen in the light of some limitations due to sample size. Regarding the sample size, when the power of a test is low, there are two possibilities: increasing the sample size or turning to another test. Since increasing the sample size is one of our goals in the near future, we must take into consideration that we are dealing with oncological patients, and therefore it would be unethical and not advisable to reach such a big sample size in a relatively short period of time. Regarding that, our study focused on the qualitative and quantitative aspects of saliva in the oncohematological patient.
Undoubtedly, considering this study as a preliminary report, the results obtained could have been influenced by different phases of the disease and by the time the medication was taken. A more precise evaluation can be obtained in future studies comparing groups of healthy and sick children of the same age. A group of sick children should be at the same stage of the disease (since its onset) and should take the same medications for the same duration.
For both groups, oral health status, presence of caries and gingival status evaluation were part of the first visit; nevertheless, data or statistics on caries or gingival indexes were not carried out, as the work was mainly focused on the evaluation of the quality of the saliva of the oncohematological patient sample. Therefore, it would be advisable if future studies would undertake a clinical oral assessment of the patient including a complete overview with indexes and data regarding the presence of dental caries, dentine erosion, and oral health status.
5. Conclusions
Oral hygiene and preventive protocols implemented in children with oncohematological diseases can provide greater comfort to the oncohematological patient and help prevent complications that may affect the child’s quality of life.
It can be deduced that the latter have a lower pH and therefore a higher salivary acidity that predisposes to caries, and that the oral hygiene of children with oncohematological diseases is more deficient than that of the control group. It is, therefore, of fundamental importance to emphasize oral hygiene training programs for both children and their parents and to have them undergo regular dental examinations. Therefore, hygienists and pediatric dentists must be part of the pediatric oncology team in order to apply therapeutic interventions to lessen the impact of oral manifestations and improve the quality of life of these young patients and their families.
Author Contributions
Conceptualization, M.S. and I.V.; methodology, I.V., N.M. and M.S.; statistical analysis, G.D.C. and M.S.; investigation, K.G., L.S. and I.V.; data curation G.Z. and I.V.; writing—original draft preparation, M.S. and G.D.C.; writing—review and editing G.D.C., G.Z. and M.S.; supervision, A.P. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by Ethics Committee of Department of Oral and maxillo-facial science Sapienza University of Rome (protocol code 11/24062021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bowen, D.M.; Pieren, M.S.J.A. Darby and Walsh Dental Hygiene: Theory and Practices, 5th ed.; Saunders: Philadelphia, PA, USA, 2019. [Google Scholar]
- De Felice, F.; Di Carlo, G.; Saccucci, M.; Tombolini, V.; Polimeni, A. Dental Cone Beam Computed Tomography in Children: Clinical Effectiveness and Cancer Risk due to Radiation Exposure. Oncology 2019, 96, 173–178. [Google Scholar] [CrossRef]
- Staderini, E.; De Luca, M.; Candida, E.; Rizzo, M.I.; Rajabtork Zadeh, O.; Bucci, D.; Zama, M.; Lajolo, C.; Cordaro, M.; Gallenzi, P. Lay People Esthetic Evaluation of Primary Surgical Repair on Three-Dimensional Images of Cleft Lip and Palate Patients. Medicina 2019, 55, 576. [Google Scholar] [CrossRef] [PubMed]
- Patini, R.; Staderini, E.; Camodeca, A.; Guglielmi, F.; Gallenzi, P. Case Reports in Pediatric Dentistry Journals: A Systematic Review about Their Effect on Impact Factor and Future Investigations. Dent. J. 2019, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Deininger, M.W.; Rosti, G.; Hochhaus, A.; Soverini, S.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Guilhot, F.; et al. European Leukemia Net Recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013, 122, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.J.; Montserrat, E.; Rai, K.R.; et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008, 111, 5446–5456. [Google Scholar] [CrossRef] [PubMed]
- Velten, D.B.; Zandonade, E.; Monteiro de Barros Miotto, M.H. Prevalence of oral manifestations in children and adolescents with cancer submitted to chemotherapy. BMC Oral Health 2017, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Pathobiology of oral mucositis: Novel insights and opportunities. J. Support. Oncol. 2007, 5 (Suppl. S4), 3–11. [Google Scholar] [PubMed]
- Luzzi, V.; Ierardo, G.; Corridore, D.; Di Carlo, G.; Di Giorgio, G.; Leonardi, E.; Campus, G.G.; Vozza, I.; Polimeni, A.; Bossù, M. Evaluation of the orthodontic treatment need in a paediatric sample from Southern Italy and its importance among paediatricians for improving oral health in pediatric dentistry. J. Clin. Exp. Dent. 2017, 9, e995–e1001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fisher, R.A. On the interpretation of χ2 from contingency tables, and the calculation of P. J. R. Stat. Soc. 1922, 85, 87–94. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical Methods for Research Workers; Oliver and Boyd: Edinburgh, UK, 1954; ISBN 0-05-002170-2. [Google Scholar]
- Ou-Yang, L.-W.; Chang, P.-C.; Tsai, A.I.; Jaing, T.-H.; Lin, S.-Y. Salivary microbial counts and buffer capacity in children with acute lymphoblastic leukemia. Pediatr. Dent. 2010, 32, 218–222. [Google Scholar]
- Mazaheri, R.; Jabbarifar, E.; Ghasemi, E.; Akkafzadeh, E.; Poursaeid, E. Oral health status, salivary pH status, and Streptococcus mutans counts in dental plaques and saliva of children with acute lymphoblastic leukemia. Dent. Res. J. 2017, 14, 188–194. [Google Scholar]
- Hegde, A.M.; Joshi, S.; Rai, K.; Shetty, S. Evaluation of oral hygiene status, salivary characteristics and dental caries experience in acute lymphoblastic leukemic (ALL) children. J. Clin. Pediatr. Dent. 2011, 35, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.F.D.; Lira, J.A.D.S.; Macedo, R.A.D.P.; Santos, K.S.D.; Elias, C.T.V.; Morais, M.D.L.S.D.A. Oral manifestations resulting from chemotherapy in children with acute lymphoblastic leukemia. Braz. J. Otorhinolaryngol. 2014, 80, 78–85. [Google Scholar] [PubMed]
- Ponce-Torres, E.; Ruíz-Rodríguez, M.D.S.; Alejo-González, F.; Hernández-Sierra, J.F.; Pozos-Guillén, A.D. Oral manifestations in pediatric patients receiving chemotherapy for acute lymphoblastic leukemia. J. Clin. Pediatr. Dent. 2010, 34, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, G.; Goswami, M.; Sharma, S.; Mehta, A.; Dhillon, J.K. Assessment of oral health status of children with Leukemia: A cross-sectional study. Spec. Care Dentist. 2019, 39, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Pels, E.; Mielnik-Błaszczak, M. Oral hygiene in children suffering from acute lymphoblastic leukemia living in rural and urban regions. Ann. Agric. Environ. Med. 2012, 19, 529–533. [Google Scholar] [PubMed]
- Azher, U.; Shiggaon, N. Oral health status of children with acute lymphoblastic leukemia undergoing chemotherapy. Indian J. Dent. Res. 2014, 24, 523. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Utreja, A.; Bello Correa, F.O.; Al-Askar, M.; Hudieb, M.; Qayyum, F.; Al-Rasheed, A.; Almas, K.; Al-Hezaimi, K. Oral health status in children with acute lymphoblastic leukemia. Crit. Rev. Oncol. Hematol. 2012, 83, 303–309. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).