Abstract
Background. Preoperative planning and 3D printing can be used to treat pelvic bone fractures using pre-contoured surgical plates, in particular complex, comminuted fractures involving the acetabulum and quadrilateral plate. The aim of the study was to develop a Fast-Track-Protocol (fast track methodology) for creating 3D anatomical models, that could be used to shape surgical plates, using open-source software and budget 3D printers. Such a ‘low-budget’ approach would allow a hospital-based multidisciplinary team to carry out pre-surgical planning and treat complex pelvic fractures using 3D technology. Methods. The study included 5 patients with comminuted pelvic fractures. For each patient, CT (computed tomography) data were converted into two 3D models of the pelvis-injured side and mirrored model of the contralateral, uninjured hemipelvis. These models were 3D printed and used as templates to shape surgical plates. Results. A Fast-Track-Protocol was established and used to successfully treat 5 patients with complex, comminuted fractures of the pelvis. Conclusion. Using the Fast-Track-Protocol it was possible to prepare 3D printed models and patient-specific pre-contoured plates within 2 days of hospital admittance. Such an approach resulted in better surgical technique and shorter operative times, while incurring relatively low costs.
1. Introduction
Since its invention in the 1980s 3D printing has developed rapidly and come to play an important role in various fields of manufacturing including the automotive, medical devices, and aerospace industries [1,2]. Not long after this technology became available, its potential use in medicine, in particular reconstructive surgery, became obvious [3,4,5,6]. Today 3D printing has numerous applications and in 2020 the International Medical Device Regulators Forum (IMDRF) published its strategic plan for 2021–2025 [7], which described it as a rapidly developing technology in the medical sector. At present many different 3D printing technologies are available, the most common are Vat Photopolymerization (Stereolithography (SLA), Digital Light Processing (DPL)), Material Jetting (PolyJet, NanoParticle Jetting), Powder Bed Fusion technology (Electron Beam Melting (EBM), Selective Laser Sintering (SLS), Selective Heat Sintering (SHS), Direct Metal Laser Sintering (DMLS)) and Material Extrusion (MEX), commonly referred to as Fused Deposition Modeling (FDM) or Fused Filament Fabrication (FFF). Of these technologies, FDM/FFF is currently the most commonly used for rapid prototyping and development of different products [2]. Additionally, this technology is most frequently used to create anatomical models that are used in medicine. This is mainly because such 3D printers have relatively low purchase costs, with a large number of manufacturers and numerous 3D printers readily available on the market. In addition, they are reasonably easy to operate and use low-cost materials [2,8,9,10,11,12], of which Acrylonitrile Butadiene Styrene (ABS) and Polylactic Acid (PLA) are the most commonly used. The latter is particularly useful, with regard to anatomical models used in medicine, due to its biodegradability, adequate mechanical properties for such applications, ease of printing (well-defined print parameters), and low purchase costs [2,8,9,10,11,12]. 3D printed 1:1 scale anatomical models can be used to visualize and classify complex pelvic fractures as well as optimally plan surgical access for their reconstruction. They can also serve as templates for selecting and pre-contouring surgical plates together with choosing suitable screw sizes. Furthermore, such models can also be used to plan pelvic tumor resection and custom implant design [13]. Studies have shown that such an approach shortens operative times and consequently the duration of general anesthesia; reduces patient blood loss, as well as intraoperative exposure to ionizing radiation [14]. An additional benefit is better patient communication. Of all pelvic injuries, fractures involving the acetabulum are the most challenging [15,16,17]. Currently, the number of low-energy osteoporotic fractures involving the acetabulum is increasing, with an estimated annual incidence of 224 per 100,000, in patients over 60 years of age [18]. Open reconstruction together with fracture stabilization, is essential for optimal long-term results and prevention of degenerative changes. In the literature [19], two main approaches to preparing and using 3D printed models as templates for pre-contouring fixation plates, have been described [19] (Figure 1). The two approaches can outlined as shown in Table 1.
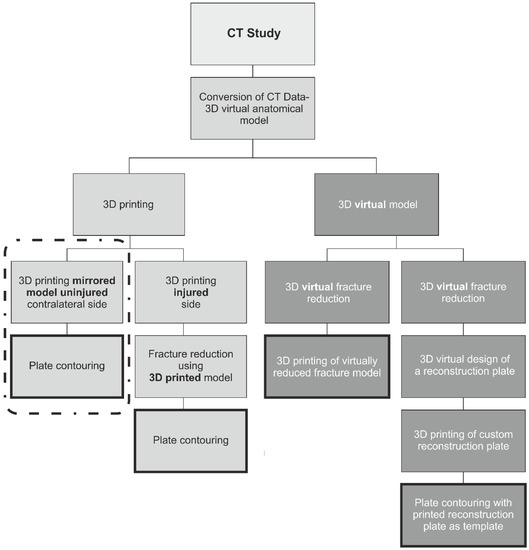
Figure 1.
Diagram showing current approaches to preparing and using 3D printed models as templates for pre-contouring fixation plates.

Table 1.
Two approaches to preparing 3D printed models as templates.
Both of these approaches can be complex and time-consuming processes. Both require knowledge of radiological anatomy and the use of different types of software that can be difficult to master. Furthermore, some basic knowledge of the many 3D printing methods available today is necessary, to choose the optimal technology for a given task. Unfortunately, most software packages used to convert CT data into virtual models as well the 3D printing technologies required to build them are prohibitively expensive. Furthermore, the technique of virtual fracture reduction that consists of accurately repositioning virtual bone fragments is unpredictable.
To greatly simplify and shorten the entire process of going from CT scan to 3D model and finally preparing surgical plates, it was necessary to develop a straightforward and rapid methodology. Such an approach would allow a hospital-based multidisciplinary team to scan, convert and print 3D models using free, open-source software and an inex-pensive 3D printing technology.
The aim of this paper was to develop the Fast-Track-Protocol that describes each stage of a low-cost process, using open-source software and a budget MEX (i.e., FDM/FFF) printer, to create PLA anatomical models and treat patients with acetabular and/or quadrilateral plate fractures.
2. Materials and Methods
A total of 5 patients, aged 50–65 years, who sustained comminuted fractures involving the acetabulum and quadrilateral plate were included in the study. CT scans were performed on admission to the hospital, using one of the following scanners: Siemens Sensation Cardiac 64 or GE Medical Systems Optima CT540, according to the CT study protocol shown in Table 1. CT data were saved as DICOM files and next converted into 3D models (Figure 2) using the free, open-source software 3D Slicer 4.11 [20]. For each patient, two models of the pelvic bones were created: the injured side and a mirrored model of the undamaged, contralateral side (Figure 3). A more detailed description of the segmentation process is provided in the Supplementary Materials.
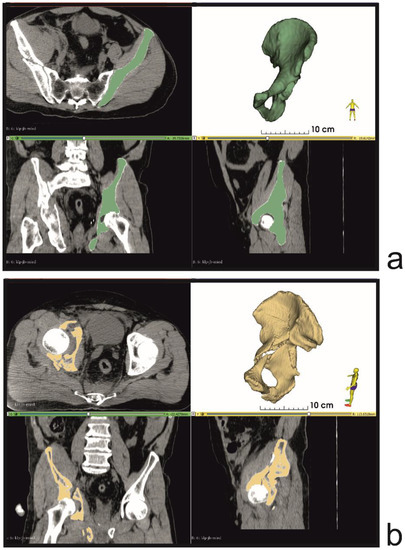
Figure 2.
Bone tissue segmentation and virtual 3D models (a) uninjured contralateral side (b) injured side of the pelvis.
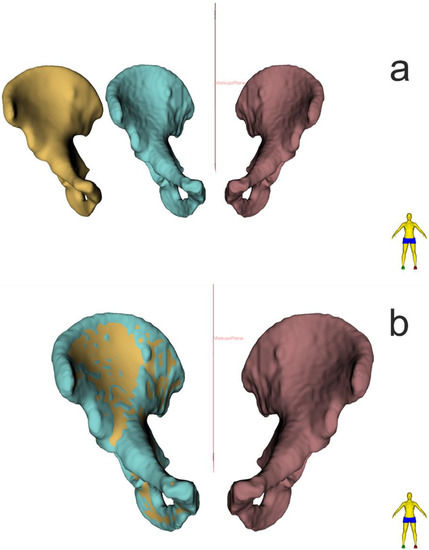
Figure 3.
(a) Optimization and smoothing of model surface ‘SURFACE TOOLBOX’ module, function ‘Smoothing’ (b) 3D comparison analysis of 3D model before and after smoothing (3D Slicer).
The resulting 3D models were exported as STL (.stl) files and later imported into CURA 3.6.0, 3D printing software (CURA 3.6.0, Ultimaker B.V, Utrecht, The Netherlands), which was used to configure the printing process. Models were next printed on an Ultimaker U3 3D printer (Ultimaker B.V, Utrecht, The Netherlands) from PLA, using the print parameters shown in Table 2. Following the printing process, models were cleaned and dried. In the final stage, a set of 3.5 mm Low Profile Reconstruction Plates (Low Profile Pelvic System, DePuy Synthes, Raynham, MA, USA) was prepared. This was achieved by first shaping the bending template, which is part of the Low Profile Pelvic System, to conform to the contours of the mirrored, uninjured contralateral hemipelvis model, followed by 3D bending of the reconstruction plates according to manufacturer instructions (Figure 4 and Figure 5). To reduce the risk of contamination, during the pre-contouring process the anatomical models, bending template, and reconstruction plates were placed and handled within a laminar flow chamber -Telstar Bio II Advance (Azbil Telstar, Elacourt, France) (Figure 4 and Figure 5). The pre-contoured plates were sterilized in the hospital’s central sterile services department according to local procedures (steam sterilization).

Table 2.
CT scan parameters.
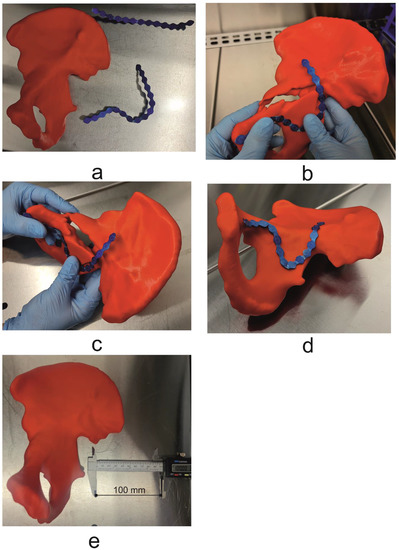
Figure 4.
(a) Mirrored uninjured hemipelvis with bending template, (b,c) process of shaping and fitting bending template to the patient’s unique anatomy, (d) final shaped bending template, (e) 3D model with scale bar (100 mm).
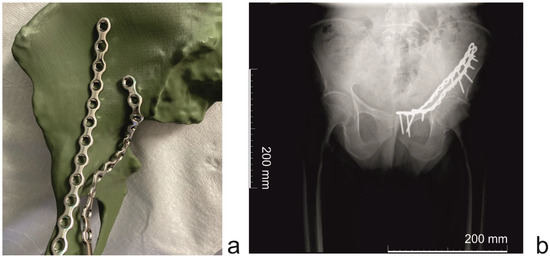
Figure 5.
(Patient 3) (a) Mirrored uninjured hemipelvis with reconstruction plates (b) (Patient 4) Post-operative X-ray image (scale bar = 200 mm).
3. Results
CT studies were performed in accordance with the parameters outlined in Table 1-the total examination time was: 30 min ± 5 min. The average segmentation time, together with virtual planning and optimization of the 3D model, was 180 min ± 11 min. The models were then printed according to the parameters outlined in Table 2. The average printing time was 600 min ± 60 min (10 h ± 1 h)-The printing was carried out overnight to make the best use of the available time. Removal of support materials required 180 min. ± 10 min and the pre-contouring stage was 30 min ± 5 min. Model cleaning and sterilization lasted 120 min. Sterile plates were delivered to the operating room on the morning of the operation. Total time from CT scan to sterile pre-contoured plate (details provided in Table 3),

Table 3.
3D printing parameters.
- Day 1:
- ○
- Patient CT study
- ○
- Segmentation and virtual planning;
- ○
- 3D printing;
- Day 2:
- ○
- Support material removal;
- ○
- Surgical plate pre-contouring;
- ○
- Sterilization of pre-contoured plates;
On the basis of, the above specifications of the Fast-Track-Protocol were defined (Supplementary Materials).
4. Discussion
In total 3D models were prepared for 5 patients, who had sustained acetabular fractures, within 48 h of hospital admission. The average time required to go from CT scan to ready pre-contoured surgical plate was 1140 min (19.0 h), with a maximum time of 1220 min (20.33 h) and a minimum time of 1050 min (17.5 h).
Fractures of the acetabulum and the quadrilateral plate (quadrilateral surface) require precise anatomical repositioning and stable fixation of displaced bone fragments. It is generally recognized that clinically acceptable bone fragment displacement, in the form of fracture gaps or step-offs, should not exceed 2 mm. On the other hand, gaps of around 10 mm or more, almost universally portend a poor prognosis with accelerated posttraumatic arthrosis [1,21]. The use of anatomical models and virtual planning at the pre-surgical stage allows surgeons to precisely plan the order of bone fragment repositioning and prepare patient-specific pre-contoured surgical plates, based on the patient’s unique anatomy. The use of ready pre-formed surgical plates primarily shortens operative time as well as reduces intra-operative blood loss, shortens general anesthesia, and lowers surgery costs [14,22]. Additionally, if two surgical plates must be used, pre-contouring allows for excellent planning of the placement of these plates, without conflict due to any overlap. Despite the many advantages of using 3D printing for medical purposes, it has yet to become the gold standard in the treatment of complex trauma. This is most probably due to several factors that make the process of converting CT data into physical templates appear to be very daunting. First of all, to create a detailed 3D model that accurately depicts patient anatomy, it is necessary to scan a patient using a multi-slice CT scanner, to acquire detailed imaging data. At present such scanners are widely available, therefore the only requirement is to use a CT study protocol that will result in the necessary imaging data. The acquired CT data must consist of isometric voxels that will allow for accurate segmentation in all three planes. Additionally, it is crucial to perform the scan with a gantry tilt of 0-degrees as any tilt (±) in the z-axis can result in a deformed and inaccurate 3D model, following segmentation. The next stage requires the use of software that can convert the CT data into a detailed 3D model, which can be converted to a format compatible with 3D printers. Usually, such software is prohibitively expensive and rather complex with a long learning curve, requiring some level of expertise. Finally, a 3D printer is required to create the physical models. Essentially 3D printers can be divided into two groups-high-end industrial printers and budget, desktop printers. Industrial 3D printers are expensive and usually require specialized personnel and infrastructure to operate them. At the same time, budget printers have not always been capable of printing accurate three-dimensional models. However, a large number of manufacturers active in this industry sector and recent advances, have resulted in low-cost 3D printers that can produce relatively high-quality models.
The Fast-Track-Protocol assumes that medical staff, in the form of a multidisciplinary team, will take a Do It Yourself (DIY) approach and create 3D objects using open source, software for converting CT data and then build them using a low-cost FFF/FDM 3D printer, within 2 working days. This will not delay any surgical treatment, as on average patients are operated on 3–5 days from the moment of injury (optimal time is up to 7 days following trauma). Data from the literature [19] show two basic approaches to preparing and using 3D printed templates for pre-contouring fixation plates (Figure 3, Figure 4 and Figure 5). The first approach focuses on 3D printing only, whilst the second uses virtual planning and fragment reduction, followed by 3D printing. The 3D printing only method greatly reduces the number of procedures required; as a result, it is a much faster process. However, such an approach assumes that the human bony pelvis is symmetric [23] and that the mirrored uninjured contralateral hemipelvis can serve as a template for pre-contouring reconstruction plates [14,24]. On the other hand, virtual fracture reduction that consists of repositioning virtual bone fragments is inaccurate and unpredictable. The authors chose the 3D printing only method as it is the shortest process and requires the least amount of work. Whereas the CT study protocol we propose can be used on any modern CT scanner and results in detailed imaging.
The use of open-source software was one of the crucial aspects of this study. In many papers describing the use of anatomical models for reconstructive surgery, commercial software packages are typically used to convert CT data [24,25,26]. Such software is very effective and reliable, in particular, if it has been validated and certified for medical use. However, this usually means that such certified software packages are expensive and would require a substantial financial investment that may be difficult to justify in a hospital environment. For this reason, the authors decided to use open-source software, for which there are online tutorials and documentation, as well as an active community of users. Consequently, 3D Slicer (www.slicer.org accessed on 10 March 2021) was chosen as it is relatively easy to learn, whilst having many advanced functions. Nevertheless, users will have to invest some time in order to learn to use the software. In particular, if they have had little or no experience reconstructing CT data (Table 4). Although 3D Slicer has not been certified for medical use, it has a long history of use in research with a very strong focus on clinical and biomedical applications [27,28,29,30,31,32,33]. Furthermore, a multidisciplinary approach must be used when converting CT data with constant input from the attending surgeon who will ultimately decide how to treat the patient and, ideally, with the support of an experienced radiologist. The final stage of the process involves the use of a low-budget desktop FFF/FMD 3D printer. There are a large number of such printers on the market, however, we suggest using a slightly more expensive dual extruder 3D printer that uses dissolvable support material. Such machines can build significantly more precise models and are the best comprise between quality and cost. They are relatively easy to operate and do not require specialist knowledge. Nevertheless, 3D printers can at times still be unpredictable and will usually require regular maintenance (Table 4). This would be the only potential major cost for a hospital/surgery department wanting to use this workflow. However, even this can be avoided, if necessary, by outsourcing the printing process, without incurring the cost of data conversion, virtual planning, etc. The authors involved in the development of the Fast-Track-Protocol were an experienced team consisting of several orthopedic surgeons, an engineer, and a radiologist. Such an approach provided an excellent opportunity for an interdisciplinary solution to a complex problem and could provide other teams the chance to benefit from 3D technologies (Table 5) and to optimize different surgical procedures using imaging, reverse engineering, and surgical pre-planning.

Table 4.
Time required to carry out each step in the Fast Track protocol (mins—minutes, h—hours, sd—standard deviation).

Table 5.
Advantages and disadvantages of the Fast Track Protocol.
We believe that such a combination of diagnostic imaging, tissue segmentation, 3D image reconstruction, 3D printed models, and pre-contoured plates will enable junior surgeons to gain a better understanding of trans-articular hip joint fractures and the surgical techniques involved in their treatment, resulting in a shorter learning curve. An additional benefit is better patient communication.
5. Conclusions
The Fast-Track-Protocol provides a simple and easy-to-understand workflow, which does not require in-depth knowledge of 3D technologies, to create 3D printed anatomical models of the pelvis and prepare patient-specific pre-contoured surgical plates within 2 days of hospital admission.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app12073492/s1, Table S1. Fast Track protocol, Figure S1. Select the ‘CROP VOLUME’ module. Select the area/volume for segmentation (pelvic bones), Figure S2. Select the “SEGMENT EDITOR” module. Create two segments, the first segment can be named, e.g., MASK 01 and the second segment, e.g., Pelvis L/R or uninjured, Figure S3. Segmentation correction process (removal of artifacts) using the ‘SCISSORS’ function, Figure S4. Segmentation correction process (removal of artifacts) using the ‘ERASE’ function, Figure S5. Segmentation correction process (removal of artifacts) using the ‘PAINT’ function, Figure S6A–C. Removal of the femur using the ‘ERASE’ function and ‘ISLAND’ function, Figure S7A,B. Creating a 3D model for printing. Select the ‘WRAP SOLIDFY’ function, Figure S8. Select the ‘MARKUP’ module, Figure S9. Select the ‘DYNAMIC MODEL’ module, Figure S10. Select the ‘SURFACE TOOLBOX’ module, Figure S11. Export the model.
Author Contributions
Conceptualization, K.A., M.E. and P.K.; methodology, visualization and supervision, K.A., M.E. and P.K.; writing—original draft preparation, K.A., M.E., P.K. and B.R.; administration: M.D.; software, M.E. and P.K.; investigation, K.A., M.E., B.R., J.P. and P.K. other, M.D. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodríguez, F.C.; Mañanes, R.P.; Cárceles, F.N.; Gil Martínez, P. 3D printing utility for surgical treatment of acetabular fractures. Rev. Esp. Cir. Ortop. Traumatol. (Engl. Ed.) 2018, 62, 231–239. [Google Scholar] [CrossRef]
- Khorasani, M.; Ghasemi, A.; Rolfe, B.; Gibson, I. Additive manufacturing a powerful tool for the aerospace industry. Rapid Prototyp. J. 2022, 28, 87–100. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Qiao, G.; Liu, H.; Ji, A.; Ye, F. Computer-assisted virtual surgical procedure for acetabular fractures based on real CT data. Injury 2011, 42, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Merema, B.J.; Kraeima, J.; ten Duis, K.; Wendt, K.W.; Warta, R.; Vos, E.; Schepers, R.H.; Witjes, M.J.H.; Ijpma, F.F.A. The design, production and clinical application of 3D patient-specific implants with drilling guides for acetabular surgery. Injury 2017, 48, 2540–2547. [Google Scholar] [CrossRef]
- Boudissa, M.; Oliveri, H.; Chabanas, M.; Tonetti, J. Computer-assisted surgery in acetabular fractures: Virtual reduction of acetabular fracture using the first patient-specific biomechanical model simulator. Orthop. Traumatol. Surg. Res. 2018, 104, 359–362. [Google Scholar] [CrossRef]
- Tostain, O.; Debuyzer, E.; Benad, K.; Putman, S.; Pierache, A.; Girard, J.; Pasquier, G. Ten-year outcomes of cementless anatomical femoral implants after 3D computed tomography planning. Follow-up note. Orthop. Traumatol. Surg. Res. 2019, 105, 937–942. [Google Scholar] [CrossRef]
- IMDRP Management Committee. IMDRF Strategic Plan 2021–2025; International Medical Device Regulators Forum, 25 September 2020. Available online: https://www.hsa.gov.sg/medical-devices/international-collaboration-medical-devices/imdrf (accessed on 16 January 2022).
- Rashed, K.; Kafi, A.; Simons, R.; Bateman, S. Fused filament fabrication of nylon 6/66 copolymer: Parametric study comparing full factorial and Taguchi design of experiments. Rapid Prototyp. J. 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Nahar, C.; Gurrala, P.K. Transient thermal finite-element analysis of fused filament fabrication process. Rapid Prototyp. J. 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Herzog, T.; Schnell, G.; Tille, C.; Seitz, H. Investigation of suitable material and adhesion promoter combinations for fused filament fabrication on flexible silicone build plates. Rapid Prototyp. J. 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Chand, R.; Sharma, V.S.; Trehan, R.; Gupta, M.K. A physical investigation of dimensional and mechanical characteristics of 3D printed nut and bolt for industrial applications. Rapid Prototyp. J. 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ponnusamy, S.; Nallamilli, M.S.R. The influence of process parameters on the impact resistance of 3D printed PLA specimens under water-absorption and heat-treated conditions. Rapid Prototyp. J. 2021, 27, 1108–1123. [Google Scholar] [CrossRef]
- Durastanti, G.; Belvedere, C.; Ruggeri, M.; Donati, D.M.; Spazzoli, B.; Leardini, A. A Pelvic Reconstruction Procedure for Custom-Made Prosthesis Design of Bone Tumor Surgical Treatments. Appl. Sci. 2022, 12, 1654. [Google Scholar] [CrossRef]
- Chana-Rodríguez, F.; Mañanes, R.P.; Rojo-Manaute, J.; Gil, P.; Martínez-Gómiz, J.M.; Vaquero-Martín, J. 3D surgical printing and pre contoured plates for acetabular fractures. Injury 2016, 47, 2507–2511. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Xing, W.; Wu, Z.; Huang, H.; Huang, W. A combination of three-dimensional printing and computer-assisted virtual surgical procedure for preoperative planning of acetabular fracture reduction. Injury 2016, 47, 2223–2227. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Newman, S.; Lin, Y.; Chen, X.; Xu, L.; Wang, Q. Application of an innovative computerized virtual planning system in acetabular fracture surgery: A feasibility study. Injury 2016, 47, 1698–1701. [Google Scholar] [CrossRef]
- Citak, M.; Gardner, M.J.; Kendoff, D.; Tarte, S.; Krettek, C.; Nolte, L.-P.; Hüfner, T. Virtual 3D planning of acetabular fracture reduction. J. Orthop. Res. 2008, 26, 547–552. [Google Scholar] [CrossRef]
- Oberkircher, L.; Ruchholtz, S.; Rommens, P.M.; Hofmann, A.; Bücking, B.; Krüger, A. Osteoporotic Pelvic Fractures. Dtsch. Ärztebl. Int. 2018, 115, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Marinescu, R.; Popescu, D.; Laptoiu, D. A Review on 3D-Printed Templates for Precontouring Fixation Plates in Orthopedic Surgery. J. Clin. Med. 2020, 9, 2908. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Pérez, C.; Rodríguez-Lozano, G.; Rojo-Manaute, J.; Vaquero-Martín, J.; Chana-Rodríguez, F. 3D surgical printing for preoperative planning of trabecular augments in acetabular fracture sequel. Injury 2018, 49, S36–S43. [Google Scholar] [CrossRef]
- Li, L.; Gao, J. Comparison of three-dimensional printing and conventional imaging in surgical treatment of Tile C pelvic fractures: A long-term follow-up study. Int. J. Clin. Exp. Med. 2017, 10, 12433–12439. [Google Scholar]
- Ead, M.S.; Duke, K.K.; Jaremko, J.L.; Westover, L. Investigation of pelvic symmetry using CAD software. Med Biol. Eng. Comput. 2019, 58, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, F.; Yao, S.; Xiong, Z.; Sun, T.; Zhu, F.; Telemacque, D.; Drepaul, D.; Ren, Z.; Guo, X. Application of computer-assisted virtual surgical procedures and three-dimensional printing of patient-specific pre-contoured plates in bicolumnar acetabular fracture fixation. Orthop. Traumatol. Surg. Res. 2019, 105, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Pierannunzii, L.; Fischer, F.; Tagliabue, L.; Calori, G.M.; D’Imporzano, M. Acetabular both-column fractures: Essentials of operative management. Injury 2010, 41, 1145–1149. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Zhang, G.; Lin, H.; Yu, Z.; Wu, C.; Li, X.; Lin, Y.; Huang, W. Accurate fixation of plates and screws for the treatment of acetabular fractures using 3D-printed guiding templates: An experimental study. Injury 2017, 48, 1147–1154. [Google Scholar] [CrossRef]
- Damon, A.; Clifton, W.; Valero-Moreno, F.; Quinones-Hinojosa, A. Cost-Effective Method for 3-Dimensional Printing Dynamic Multiobject and Patient-Specific Brain Tumor Models: Technical Note. World Neurosurg. 2020, 140, 173–179. [Google Scholar] [CrossRef]
- Virzì, A.; Muller, C.O.; Marret, J.-B.; Mille, E.; Berteloot, L.; Grévent, D.; Boddaert, N.; Gori, P.; Sarnacki, S.; Bloch, I. Comprehensive Review of 3D Segmentation Software Tools for MRI usable for pelvic surgery planning. J. Digit. Imaging 2020, 33, 99–110. [Google Scholar] [CrossRef]
- Czeibert, K.; Sommese, A.; Petneházy, Ö.; Csörgő, T.; Kubinyi, E. Digital endocasting in comparatirve canine brain morphology. Front. Vet. Sci. 2020, 7, 749. [Google Scholar] [CrossRef]
- Kikinis, R.; Pieper, S. 3D Slicer as a tool for interactive brain tumor segmentation. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 6982–6984. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, J.; Paniagua, B.; Oliveira Ruellas, A.C.; Fillion-Robin, J.C.; Prietro, J.C.; Gonçalves, J.R.; Hoctor, J.; Yatabe, M.; Styner, M.; Li, T.; et al. 3D Slicer craniomaxillofacial moduler support patient-specific decision-making for personalized healthcare in dental research. In Multimodal Learning for Clinical Decision Support and Clinical Image-Based Procedures; Springer: Cham, Switzerland, 2020; pp. 44–53. [Google Scholar] [CrossRef]
- Shetty, H.; Shetty, S.; Kakade, A.; Shetty, A.; Karobari, M.I.; Pawar, A.M.; Marya, A.; Heboyan, A.; Venugopal, A.; Nguyen, T.H.; et al. Three-dimensional semi-automated volumetric assessment of the pulp space of teeth following regenerative dental procedures. Sci. Rep. 2021, 11, 21914. [Google Scholar] [CrossRef]
- Velazquez, E.R.; Parmar, C.; Jermoumi, M.; Mak, R.H.; Van Baardwijk, A.; Fennessy, F.M.; Lewis, J.H.; De Ruysscher, D.; Kikinis, R.; Lambin, P.; et al. Volumetric CT-based segmentation of NSCLC using 3D-Slicer. Sci. Rep. 2013, 3, 3529. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).