Abstract
Extremely low-frequency and low-intensity electromagnetic fields show positive effects on the treatment of several osteoarticular diseases, such as osteoarthritis, and are currently applied in the clinical setting with promising results on tissue regeneration. However, the biological mechanisms underlying the beneficial effects triggered by this type of physical stimulation still need to be deciphered. We tested the hypothesis that ultra-low complex electromagnetic fields stimulation using an innovative medical device could enhance chondrogenesis in human adipose-derived stem cells (ADSCs), and analyzed its biological effects. Chondrogenic lineage markers, like ACAN, SOX9, RUNX2, COL2A1, and COL10A1, were evaluated after 21 days of treatment. Thus far, we have provided preliminary evidence that a dedicated pattern of ultra-weak complex electromagnetic sequences emitted by a cutting-edge technology can promote cartilage regeneration, inducing the chondrogenic differentiation and maturity of ADSCs.
1. Introduction
Articular hyaline cartilage is composed of chondrocytes included within an extracellular matrix (ECM) set as a framework of macromolecules like collagens, glycosaminoglycans (GAGs), proteoglycans, glycoproteins and water. It shows a typical stratified organization of fibrils, mainly collagen type II, which supply tensile strengthening to a highly hydrated proteoglycans gel, resulting in a structure able to support the compressive load and protect the bone surface. During life, articular cartilage is subjected to a physiological inner remodeling through neo-synthesis and replacing ECM components with chondrocytes. Nevertheless, chondral tissue possesses low self-repair potential [1]. Moreover, aging reduces the capability of chondrocytes to maintain such turnover rate and to re-establish functional tissue, increasing the risk of progressive degeneration and damage to the joints’ cartilage surface. Age-related and traumatic lesions of articular cartilage, if left untreated, lead to joint pain and impairment, which is clinically defined as osteoarthritis (OA).
Cartilage depletion represents a truly disabling condition, and over 200 million people worldwide suffer from OA [2]. The surgical treatment of these pathological manifestations presents limitations, as mature cell- and tissue-based transplants, such as autologous ex vivo cultured chondrocytes implantation or osteochondral grafts, often fail to restore hyaline cartilage structure and functionality [3,4]. The latter is due to chondrocyte poor in vitro proliferation and de-differentiation susceptibility. In this scenario, adult stem cells rapidly emerged as a valuable cell source for articular cartilage tissue engineering [5].
In particular, Mesenchymal Stem (and/or Stromal) Cells (MSCs), which are adult mesoderm-derived undifferentiated cells, showed the capability to self-renew together with a multilineage differentiation potential (e.g., chondrogenic, osteogenic, and adipogenic) and have been already employed including cartilage regenerative medicine for a variety of clinical applications [6]. Among MSCs populations, Adipose-Derived Stem Cells (ADSCs) have shown promising potential for chondrogenesis and the advantage of being easily obtainable from liposuction waste [7,8,9,10]. Moreover, most in vivo animal studies have reported good results using pre-differentiated or undifferentiated, autologous or allogeneic ASCs to regenerate cartilage in osteochondral defects or surgically induced osteoarthritis.
The application of electromagnetic fields (EMFs) has already shown a positive impact in vitro on enhancing chondrogenesis [11,12,13]. In particular, recent studies highlighted the high biological activity of some extremely low-frequency magnetoelectric fields (ELF-EMFs) [14]. It has been shown that low-frequency pulsed electromagnetic fields improve patients’ recovery both in the short (90 days) and in the long term (3 years), as demonstrated in the results of two level-I clinical studies. The long-term benefit results from biophysical chondroprotection of articular cartilage and prevention of the fibrotic stimuli exerted by pro-inflammatory cytokines on wounded tissue. Regenerative medicine also relies on biophysical stimulation, exerting a protective effect on the repair of tissue from catabolic effects of the inflammatory reaction elicited by the surgical implantation procedure. The microenvironment regulating stem cell differentiation can be cell–matrix adhesions or cell–cell interactions. The microenvironment is certainly helpful for maintaining MSC survival, commitment, and differentiation. It has already been reported that a hyaluronan-enriched microenvironment can both initiate and promote the chondrogenic differentiation of human adipose-derived stem cells (ADSCs) and that single-pulse electromagnetic field (SPEMF) stimulation may promote chondrogenic differentiation and cartilaginous matrix formation thus being applied for articular cartilage tissue engineering. Nevertheless, understanding the biological mechanisms underlying the therapeutic effects of low-intensity electromagnetic field treatment is lacking.
To test the hypothesis, we have investigated the effects of Limfa® Therapy on an in vitro model of ADSC cells to study their differentiation capability towards cartilage lineage. Limfa is based on a non-invasive technology centered on the application of ultra-weak magnetoelectric fields, emerged as one of the most innovative and promising medical devices in electromagnetic therapy. The experimental study had the aim to decipher the molecular mechanism underlying the Limfa functions. This research aims to biologically characterize the specific action mechanism of “connective tissue regeneration” Limfa® Sequence on adipose stem cells chondrogenesis, to better enlighten and validate its efficacy and therapeutic effect.
2. Materials and Methods
2.1. ADSCs Cell Culture
Commercial human ADSCs (ATCC PCS-500-011) from different batches were cultured in Mesenchymal Stem Cell Basal Medium (ATCC PCS-500-030) supplemented with Mesenchymal Stem Cell Growth Kit for Adipose-Derived MSCs (ATCC PCS-500-040) following the manufacturer instructions. Given that all experiments have been performed on three independent cell batches, it is possible to assume that different cell types have been used. After cell expansion, 1 × 105 cells were seeded in p60 culture dishes and maintained either in Basal medium (Basal) or in Chondrogenic Differentiation Medium (Diff) (Chondrocyte Differentiation Tool, ATCC) for 21 days at 37 °C, 5% CO2.
2.2. Limfa® Therapy Treatment
The Limfa® Therapy system (Eywa Srl, Rimini, Italy) (CE c.n. DD 60155923) was used to create extremely low-frequency (2–80 Hz), low-intensity (1–100 μT) complex variable electromagnetic fields, delivered as a predesigned module of patented wave sequences.
Specifically, the Limfa® Therapy apparatus is equipped with an electromedical computer allowing the emission of low multi-frequency signals, available as of specific pre-set treatment programs, free of 99% electromagnetic noise and thermal effect once they reach the targeted tissues. Several sequences of complex variable ultra-weak EMFs have been developed, tested and patented (Limfa® Sequences), showing a biologically active and extremely positive effect. Compared to traditional magnetotherapy, where ELF-EMFs are also exploited, their signals are a combination of one or two pulsed waveforms with the same geometry; Limfa® Therapy operates through several wave geometry shapes and frequencies, integrated into defined sequences that have a tissue-specific and -targeted therapeutic action.
To apply the magnetoelectric field emitted by the Limfa® Therapy transductor to the cell culture vessels, we used an Okolab Mini Stage-Top H301 Incubator (Okolab Srl, Naples, Italy,) allowing optimal cell exposure to the wave sequences pattern while still maintaining standard in vitro culture conditions. The Limfa® Therapy patented pre-loaded program—“connective tissues regeneration”—was applied for the entire standardized duration of 50 min. Eleven treatment sessions were performed every other day for a total duration of 21 days from cell seeding to mimic the standard schedules used in the clinical setting (+Limfa). Cells were examined to monitor cell morphology every day with microscopy evaluation. In contrast, half of the cell cultures did not undergo electromagnetic therapy and were evaluated as the negative control (−Limfa).
2.3. Morphological Cellular Evaluation
Images were acquired using an EVOS microscope (ThermoFisher, Waltham, MA, USA) using 40× magnification. The evaluation was performed by two independent operators, JI and GB. A scoring system was applied as follows: N, non-differentiated; +, weak differentiation; ++, moderate differentiation; +++, strong differentiation.
2.4. Cell Viability Assay
Cell viability was measured through the Trypan Blue exclusion test. Cells were harvested from plates after treatment with Limfa® Therapy in the complete medium; the treatment was performed following the protocol described in the previous paragraph.
2.5. Real-Time PCR (RT-qPCR)
Real-time PCR was used to evaluate the expression of the main chondrogenesis markers, including ACAN, SOX9, RUNX2, COL2A1, and COL10A1 [6]. At the end of the 21-day culture, RNA extraction of the cellular samples was performed using TRIzol Reagent (ThermoFisher Scientific), following the manufacturer protocol. RNA was quantified using a NanoDrop2000 spectrophotometer (Thermo Fisher Scientific), and 1 μg RNA was retrotranscribed using the SuperScript IV Reverse transcription kit (Invitrogen, Waltham, MA, USA) following the manufacturer’s instructions. RT-qPCR was carried out using 1 μL of the obtained cDNA and 100 μM of gene-specific reverse and forward primers (Eurofins Genomics, listed in Table 1. PCR was carried out using SYBR green chemistry (Applied Biosystems, Waltham, MA, USA). Amplification was performed using a 7500 real-time PCR system and software (Applied Biosystems, Waltham, MA, USA). Samples were held at 50 °C for 2 min and 95 °C for 10 min, then amplified at 95 °C for 15 s and 60 °C for 1 min for 40 cycles. The specificity of the PCR amplification was checked with a continuous heat dissociation curve (measured between 60–95°C) performed subsequently to the final PCR cycle. Gene expression levels were standardized using GAPDH as an internal control. Quantification analysis was performed using the comparative ΔΔCt method [15], and gene expression was expressed as a fold change relative to the control’s untreated Basal medium samples. All experiments were conducted in triplicate on three different cell batches.

Table 1.
Primer sequences used in RT-qPCR experiments. GAPDH was used as an endogenous normalizer in relative expression quantification.
2.6. Western Blot
Western blot was performed to evaluate collagen type-II and collagen type-I protein levels, considering their key role in defining chondrogenesis progression. Western blot was carried out on whole-cell lysates using sequence-specific antibodies directed against collagen I and collagen II (ab138492 and ab188570, respectively, Abcam, 1:1000 in blocking solution), as in Duranti et al., 2021 [16]. All experiments were performed on three different cell batches. Briefly, cells were gently collected by mild scraping and resuspended in ice-cold PBS. Protein extraction was performed using the lysis buffer with the following composition: NP40 (150 mM), NaCl (150 mM), Tris-HCl pH 8 (50 mM), EDTA pH 8 (5 mM), NaF (10 mM), Na4P2O7 (10 mM), Na3VO4 (0.4 mM), and protease inhibitor cocktail (Complete Mini-Roche, Mannheim, Germany).
2.7. Protein Quantification
Data were analyzed with ImageJ and graphs were plotted with OriginPro 8. When quantifying protein variations, the signal was normalized to the signal of the corresponding protein in the total lysate.
2.8. Statistical Analysis
Statistical and graphical data analysis was carried out using Origin V.8 (OriginLab Corporation, Northampton, MA, USA) and GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA). Results were expressed as mean ± SEM. Since data were normally distributed, statistical comparisons between multiple groups were performed using a one-way ANOVA test. For all tests, differences with p < 0.05 were considered statistically significant.
3. Results
3.1. Effects of Limfa® Therapy on Cell Morphology
The morphology of ADSCs was examined every day during the 21 days of treatment. From the images obtained by inversion microscopy, we can observe that cells in the control appear different compared to those after Limfa treatment when considered in Basal conditions with a stretched shape and longer protrusions. In this regard, cells in the Basal condition tend to resemble the ones in the control condition of the differentiation set. It is clear how ADSC differentiation in chondrogenic conditioned medium (Diff) led to the deposition of abundant extracellular matrix, generating interconnected structures where single-cell shape appears to be undetectable. On the contrary, cells cultured in Basal medium (Basal) maintained a fibroblast-like single-cell appearance throughout the entire period of culture (Figure 1A). In Figure 1B, we have reported images taken after 10 days of treatment, showing a differentiated morphology of the cells. Figure 1C shows a graph reporting cell viability after 21 days of treatment, with a percentage of survival of 100%.
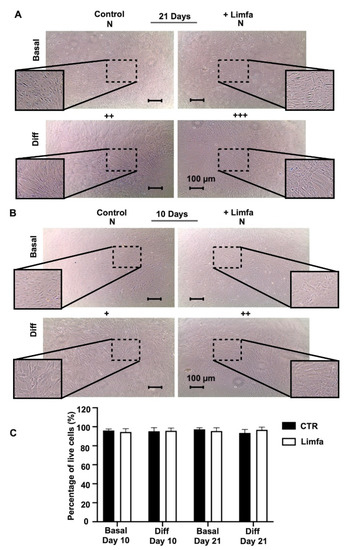
Figure 1.
Morphological examination of ADSCs 2D cultures after 10 and 21 days of culture. Cells have been examined under different conditions. Basal, which corresponds to cells cultured in basal adipose stem cells medium; Basal + Limfa, which corresponds to cells cultured in basal adipose stem cells medium and treated with Limfa® Therapy; Diff, which corresponds to cells cultured in chondrogenic differentiation medium; Diff + Limfa, which corresponds to cells cultured in chondrogenic differentiation medium and treatment with Limfa® Therapy. Scoring system: +, weak differentiation; ++, moderate differentiation; +++, strong differentiation. (A) cell images after 21 days of Limfa treatment (B) cell images after 10 days of Limfa treatment (C) Cytotoxicity assay performed after 10 and 21 days of treatment. The scale bar corresponds to 100 µm.
3.2. Effects of Limfa® Therapy on Chondrogenic Molecular Marker Expression
RT-qPCR analysis was then applied to determine the effects of chondrogenic differentiation, as well as Limfa® Therapy treatment, on the following markers of chondrogenesis: SOX9, RUNX2, COL2A1, COL10A1 and ACAN. COL2A1 showed a significant upregulation in its expression rate following the treatment schedule with Limfa® Therapy under defined medium cultures compared to untreated controls.
The RUNX2 marker exhibited a significant increase in Limfa® Therapy-treated cultures, both under Basal and differentiation medium conditions.
ACAN showed significant expression differences between untreated cells maintained in Basal compared to conditioned medium, but no difference was observed due to the Limfa® Therapy treatment per se.
Interestingly, the COL10A1 marker displayed a significant decrease in cells under both Basal and differentiation conditions when treated with Limfa® Therapy, compared to the untreated group. SOX9 also significantly decreased when comparing Basal vs. Diff + Limfa groups.
SOX9 expression only showed a significant reduction in cells treated with Limfa® Therapy cultured in conditioned media (Diff + Limfa) compared to untreated Basal conditions (Figure 2).
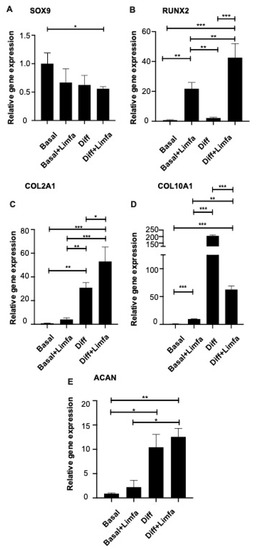
Figure 2.
Expression analysis of the main chondrogenic differentiation markers in different culture and treatment conditions. Cells were examined under different conditions. Basal, which corresponds to cells cultured in basal adipose stem cells medium; Basal + Limfa, which corresponds to cells cultured in basal adipose stem cells medium and treated with Limfa® Therapy; Diff, which corresponds to cells cultured in chondrogenic differentiation medium; Diff + Limfa, which corresponds to cells cultured in chondrogenic differentiation medium and treatment with Limfa® Therapy. SOX9 (A), RUNX2 (B), COL2A1 (C), COL10A1 (D) and ACAN (E) expression levels were assessed by RT-qPCR. Experiments are means of three different repeats. Results are expressed as relative gene expression (2-ΔCt) normalized on Basal culture conditions values. Error standard is reported. * = p < 0.05; ** = p < 0.025; *** = p < 0.01 (One-Way ANOVA).
Overall, RT-qPCR expression analysis of the main chondrogenesis markers revealed that, for most of the transcripts taken into account, the treatment with Limfa® Therapy (Diff + Limfa) induced an increase in the relative gene expression levels when compared to untreated ones.
3.3. Effect of Limfa® Therapy on Collagen Type I and II Protein Expression
The above-mentioned results prompted us to analyze the effects of Limfa® Therapy treatment on the expression of different collagens, which represent the gold standard. Collagen type I protein expression appears to be negatively affected by the treatment with Limfa Therapy, especially under differentiation culture conditions. Consistently, cartilage-specific collagen type II appears to be increased by treatment with Limfa® Therapy (Diff + Limfa) (Figure 3).
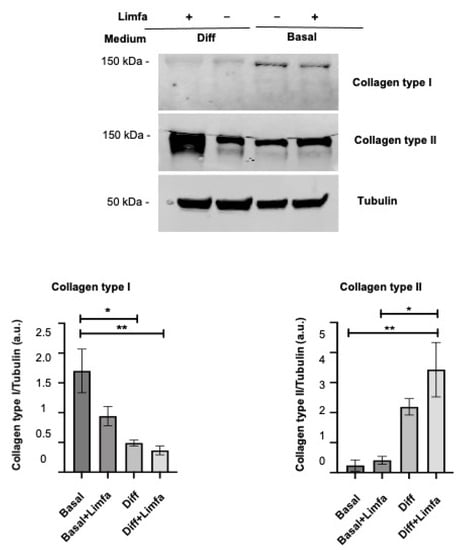
Figure 3.
Western Blot analysis of Collagen I and Collagen II expression in different cultures and treatment conditions. Results are expressed as arbitrary units (a.u.) and normalized on Tubulin input. Basal—Basal medium; Diff—differentiation medium; Limfa—Limfa® Therapy treatment. Experiments are means of three different repeats. Error standard is reported. * = p < 0.05; ** = p < 0.025 (One-Way ANOVA).
4. Discussion
Even though some studies have already examined the effects of ultra-weak electromagnetic fields on mature human chondrocytes, their interaction with chondrogenic lineage commitment of MSCs sources such as ADSCs, has not been completely elucidated. This study aimed to investigate the biological effects of ultra-low complex electromagnetic fields delivered by an innovative medical device, Limfa® Therapy, on an in vitro cartilage regeneration model.
Our results indicate that ADSCs treatment with Limfa® Therapy is able to induce the modulation of some of the main genetic chondrogenesis markers. Evidence has emerged that SOX9 did not show significant differences when compared to untreated and Basal medium conditions. This is in line with the early-stage nature of this chondrogenesis marker. Such a transcription factor is described as an early gene driving the initial switch to the chondrogenic commitment of undifferentiated progenitor cells [17].
The genetic markers related to extracellular matrix composition showed modifications induced by Limfa® Therapy which are highly representative of ADSCs chondrogenic lineage commitment. Cartilage-specific collagen type-II expression showed the most significant increase in cells treated with Limfa® Therapy and maintained in a Differentiation medium (Diff + Limfa) when compared to both its untreated relative control (Diff) and Basal conditions. Limfa® Therapy was also able to significantly decrease collagen type 10 expression, both in Basal and conditioned medium cultures. As previously described [18], COL10A1 is known to be a specific marker for late chondrocyte hypertrophy, found in network-like rather than in fibril-like collagen structures, suggesting that Limfa® Therapy preferentially promotes hyaline cartilage formation instead of bone tissue. This represents an innovative finding considering that many studies show how cartilage derived in vitro from MSCs commonly shows hypertrophic rather than hyaline features, making it unsuitable for functional cartilage tissue regeneration purposes [19].
Finally, collagen type-II protein expression evaluated by Western blot confirms the hypothesis that Limfa® Therapy is able to potentiate the induction of chondrogenesis, exploiting the soluble factors contained in pro-chondrogenic medium and therefore enhancing the deposition of cartilage-specific ECM. Moreover, we have envisaged a significant downregulation of collagen type-I protein expression after performing Limfa® Therapy treatment on cells maintained with differentiation medium. Such findings strengthen the data obtained so far by our group, as collagen type I represents one of the main osteogenic markers, which is repressed during chondrogenic lineage commitment [20].
Overall, our results suggest that Limfa® Therapy efficacy might be ascribable to its ability to promote adipose mesenchymal stem cells’ chondrogenic lineage commitment and tissue repair [10,11]. Moreover, concerning extracellular matrix deposition, Limfa® Therapy application effectively promotes ADSC differentiation when coupled with biochemical stimuli contained in a pro-chondrogenic medium.
Such results open a new path as it is known that the literature lacks studies regarding the use of ADSCs in humans for orthopedic pathologies [13]. Nevertheless, preliminary outcomes are very encouraging, with a low rate of complications. Different delivery systems for these stem cells have been tested so far. ADSCs can be administered either with a simple injection or during a surgical procedure. Together, the evidence from the few available clinical studies shows promising outcomes in the treatment of select musculoskeletal pathologies [21]. The limitation to most of this published literature is the inclusion of other therapeutic biologics. In this scenario, our findings urge an additional validation using ADSCs derived from lipoaspirate samples to assess the effects of Limfa® Therapy on endogenous stem cells. On the one hand, further investigation will validate the utility of Limfa® Therapy in the clinical treatment of osteoarthritis. On the other hand, it will highlight the potential improvement of the technology represented by adding an autologous ADSCs intra-articular injection to boost cell regeneration capacity.
5. Conclusions
Overall, our study places itself along an entirely new line emerging from the possibility of using electromagnetic fields coupled with ultrasound for biomedical applications, as our group has recently demonstrated [22]. It would be of great interest to observe the treatment effects of this process on human bone marrow stromal stem cells. Even though it was not possible to include a comprehensive series of human cases at this stage, we are willing to pursue this as our next step. Overall, our preliminary findings have shown that the effects of Limfa® Therapy can induce ADSC differentiation in vitro.
Author Contributions
Conceptualization, project administration, C.D., R.D., P.D.B. and A.A.; methodology, investigation, formal analysis, writing—original draft preparation, C.D., G.B., J.I. and V.D.; writing—review and editing, C.D., J.I. and G.B.; supervision, C.D. and A.A.; funding acquisition, A.A.; All authors participated in manuscript drafting, revision and approval before submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Associazione Italiana per la Ricerca sul Cancro (AIRC, Grant N° IG 21510 to AA by PRIN Italian Ministry of University and Research (MIUR)); “Leveraging basic knowledge of ion channel network in cancer for innovative therapeutic strategies (LIONESS)” 20174TB8KW to AA, ex 60% Università degli Studi di Firenze to AA. Claudia Duranti was supported by a AIRC fellowship for Italy, “Francesco Tonni” ID 24020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Becerra, J.; Andrades, J.A.; Guerado, E.; Zamora-Navas, P.; López-Puertas, J.M.; Reddi, A.H. Articular Cartilage: Structure and Regeneration. Tissue Eng. Part B Rev. 2010, 16, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar] [PubMed]
- Knutsen, G.; Drogset, J.O.; Engebretsen, L.; Grøntvedt, T.; Isaksen, V.; Ludvigsen, T.C.; Roberts, S.; Solheim, E.; Strand, T.; Johansen, O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J. Bone Jt. Surg. Am. 2007, 89, 2105–2112. [Google Scholar] [CrossRef]
- Revell, C.M.; Athanasiou, K.A. Success Rates and Immunologic Responses of Autogenic, Allogenic, and Xenogenic Treatments to Repair Articular Cartilage Defects. Tissue Eng. Part B Rev. 2009, 15, 5. [Google Scholar] [CrossRef]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef]
- Robert, A.W.; Marcon, B.H.; Dallagiovanna, B.; Shigunov, P. Adipogenesis, Osteogenesis, and Chondrogenesis of Human Mesenchymal Stem/Stromal Cells: A Comparative Transcriptome Approach. Front. Cell Dev. Biol. 2020, 8, 561. [Google Scholar] [CrossRef]
- Im, G.-I. Regeneration of articular cartilage using adipose stem cells. J. Biomed. Mater. Res. Part A 2016, 104, 1830–1844. [Google Scholar] [CrossRef]
- Wu, L.; Cai, X.; Zhang, S.; Karperien, M.; Lin, Y. Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: Perspectives from stem cell biology and molecular medicine. J. Cell Physiol. 2013, 228, 938–944. [Google Scholar] [CrossRef]
- Veronesi, F.; Maglio, M.; Tschon, M.; Aldini, N.N.; Fini, M. Adipose-derived mesenchymal stem cells for cartilage tissue engineering: State-of-the-art in in vivo studies. J. Biomed. Mater. Res. A 2014, 102, 2448–2466. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Kartolo, W.A.; Lee, S.H. Cartilage Regeneration in Human with Adipose Tissue-Derived Stem Cells: Current Status in Clinical Implications. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Funk, R.H.; Monsees, T.; Özkucur, N. Electromagnetic effects—From cell biology to medicine. Prog. Histochem. Cytochem. 2009, 43, 177–264. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Pagani, S.; Giavaresi, G.; De Mattei, M.; Ongaro, A.; Varani, K.; Vincenzi, F.; Massari, L.; Cadossi, M. Functional tissue engineering in articular cartilage repair: Is there a role for electro-magnetic biophysical stimulation? Tissue Eng. Part B Rev. 2013, 19, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lin, Y.-S.; Fu, Y.-C.; Wang, C.-K.; Wu, S.-C.; Wang, G.-J.; Eswaramoorthy, R.; Wang, Y.-H.; Wang, C.-Z.; Wang, Y.-H.; et al. Electromagnetic fields enhance chondrogenesis of human adipose-derived stem cells in a chondrogenic microenvironment in vitro. J. Appl. Physiol. 2013, 114, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Gengadharan, A.C.; Balachandran, C.; Manohar, B.M.; Puvanakrishnan, R. Low frequency pulsed electromagnetic field--a viable alternative therapy for arthritis. Indian J. Exp. Biol. 2009, 47, 939–948. [Google Scholar] [PubMed]
- Lottini, T.; Iorio, J.; Lastraioli, E.; Carraresi, L.; Duranti, C.; Sala, C.; Armenio, M.; Noci, I.; Pillozzi, S.; Arcangeli, A. Transgenic mice overexpressing the LH receptor in the female reproductive system spontaneously develop endometrial tumour masses. Sci. Rep. 2021, 11, 8847. [Google Scholar] [CrossRef]
- Duranti, C.; Iorio, J.; Lottini, T.; Lastraioli, E.; Crescioli, S.; Bagni, G.; Lulli, M.; Capitani, C.; Bouazzi, R.; Stefanini, M.; et al. Harnessing the hERG1/β1 Integrin Complex via a Novel Bispecific Single-chain An-tibody: An Effective Strategy against Solid Cancers. Mol. Cancer Ther. 2021, 20, 1338–1349. [Google Scholar] [CrossRef]
- Barry, F.; Boynton, R.E.; Liu, B.; Murphy, J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp. Cell. Res. 2001, 268, 189–200. [Google Scholar] [CrossRef]
- Liu, T.M.; Martina, M.; Hutmacher, D.W.; Hui, J.H.P.; Lee, E.H.; Lim, B. Identification of Common Pathways Mediating Differentiation of Bone Marrow- and Adipose Tissue-Derived Human Mesenchymal Stem Cells into Three Mesenchymal Lineages. Stem Cells 2007, 25, 750–760. [Google Scholar] [CrossRef]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mes-enchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef]
- Green, J.D.; Tollemar, V.; Dougherty, M.; Yan, Z.; Yin, L.-J.; Ye, J.; Collier, Z.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. Multifaceted signaling regulators of chondrogenesis: Implications in cartilage regeneration and tissue engineering. Genes Dis. 2015, 2, 307–327. [Google Scholar] [CrossRef] [Green Version]
- Kunze, K.N.; Burnett, R.A.; Wright-Chisem, J.; Frank, R.M.; Chahla, J. Adipose-Derived Mesenchymal Stem Cell Treatments and Available Formulations. Curr. Rev. Musculoskelet. Med. 2020, 13, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Dimitri, M.; Duranti, C.; Aquino, S.; Mazzantini, L.; Iorio, J.; Lulli, M.; Ricci, M.; Capineri, L.; Arcangeli, A.; Corvi, A. Biophysical and Biomechanical Effect of Low Intensity US Treatments on Pancreatic Adenocarcinoma 3D Cultures. Appl. Sci. 2022, 12, 666. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).