Production of Daidzein and Genistein from Seed and Root Extracts of Korean Wild Soybean (Glycine soja) by Thermostable β-Galactosidase from Thermoproteus uzoniensis
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains, Plasmids, and Gene Cloning
2.3. Preparation of β-Galactosidase
2.4. SDS-PAGE and Gel Filtration Chromatography
2.5. Preparation of Wild Soybean Extracts
2.6. Hydrolytic Activity
2.7. Biotransformation of Isoflavones
2.8. HPLC Analysis
3. Results
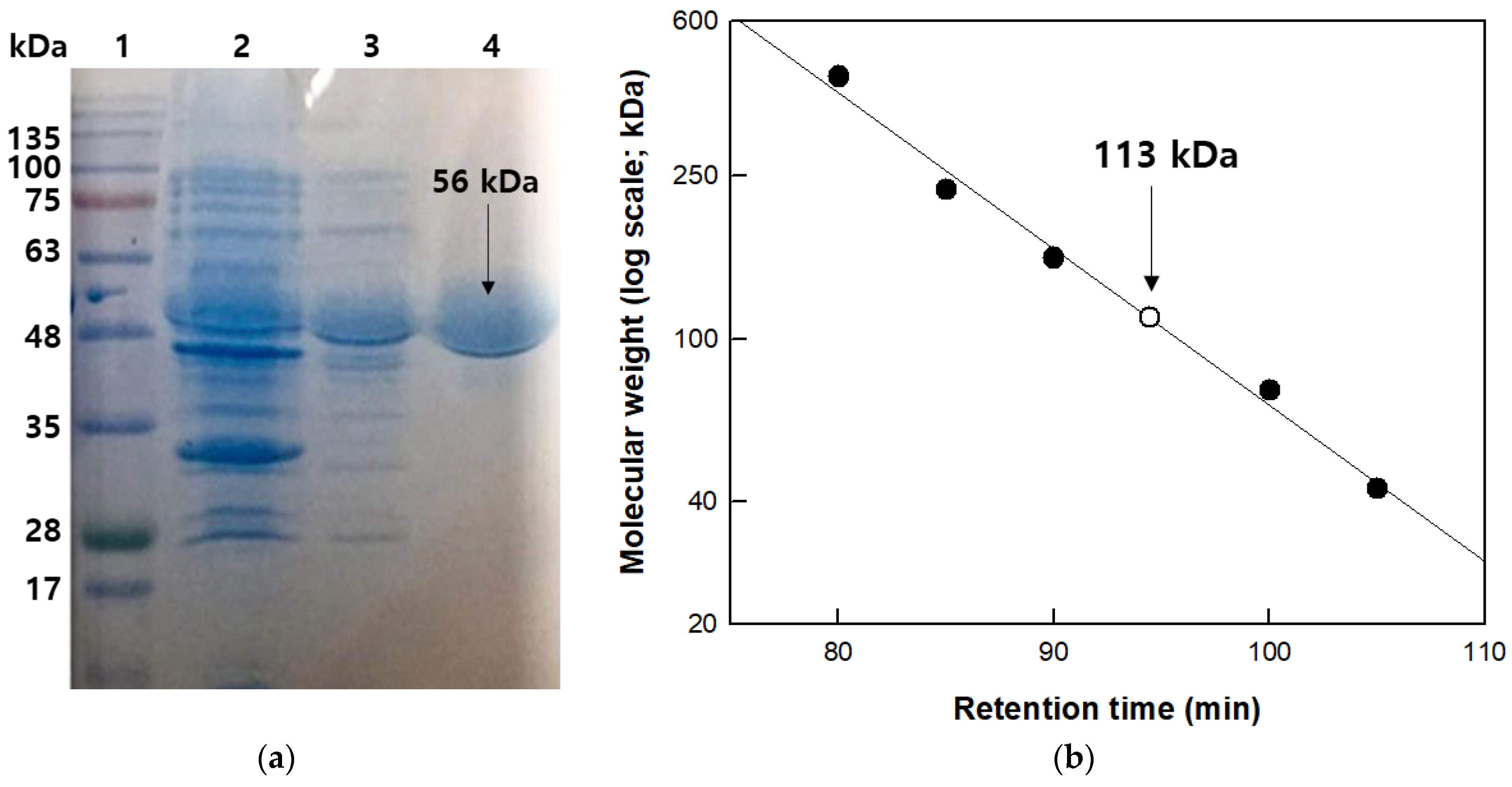
3.1. Cloning, Purification, and Molecular Mass Determination of β-Galactosidase from T. uzoniensis
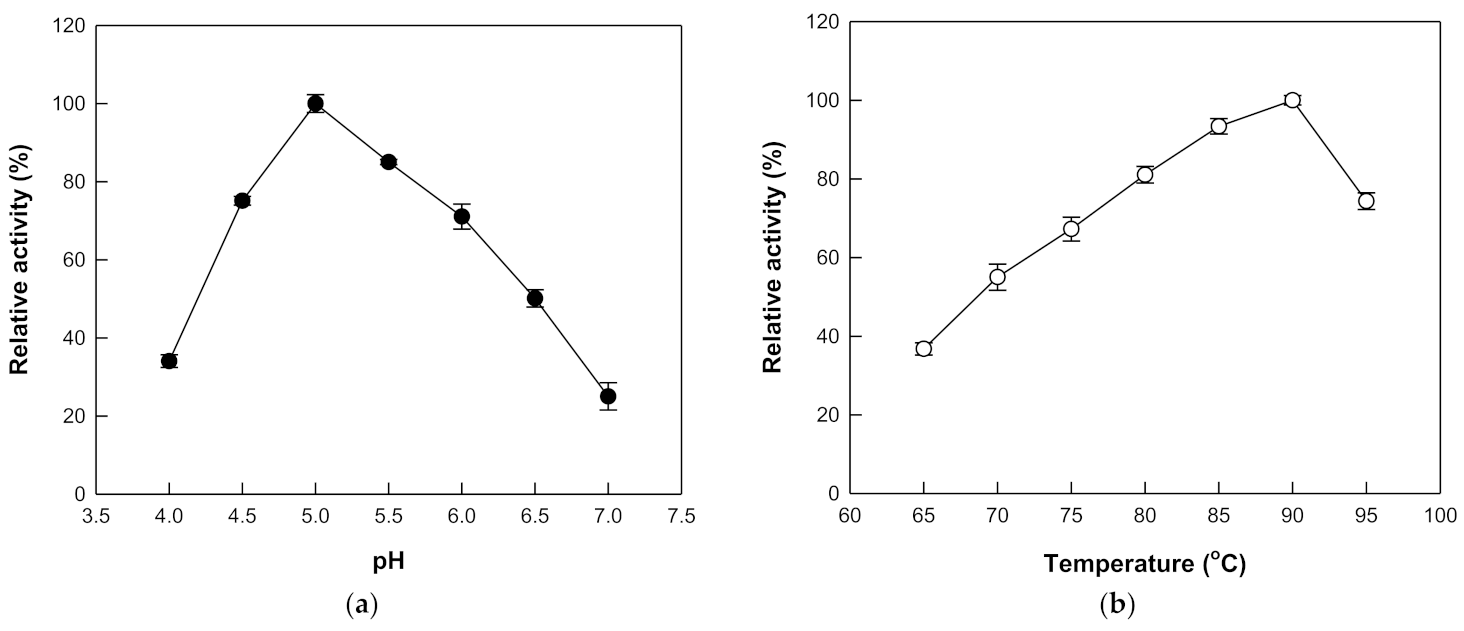
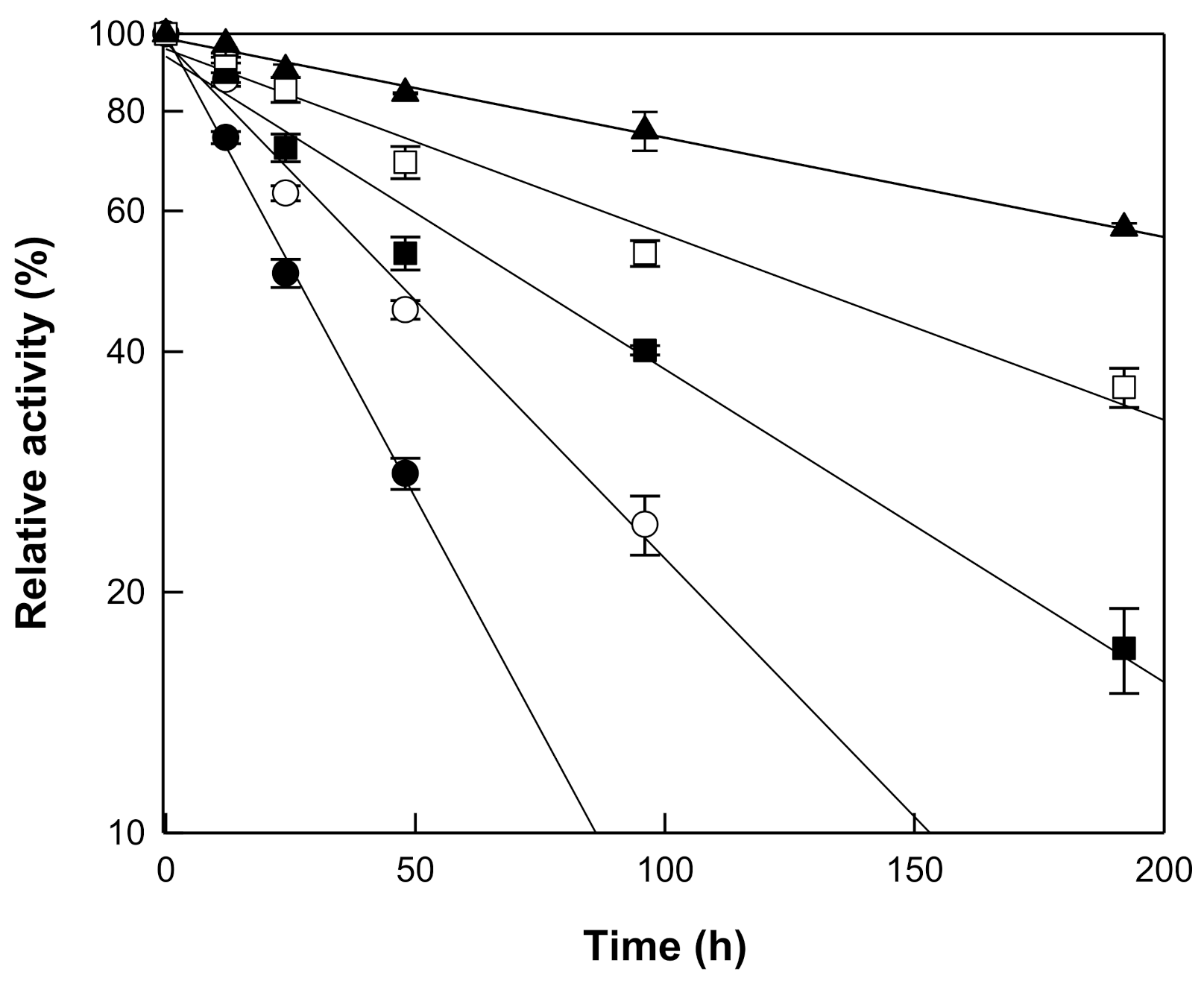
3.2. Effects of pH and Temperature on the Hydrolytic Activity of β-Galactosidase from T. uzoniensis
3.3. Substrate Specificity of β-Galactosidase from T. uzoniensis for Aryl Glycosides and Isoflavone Glycosides
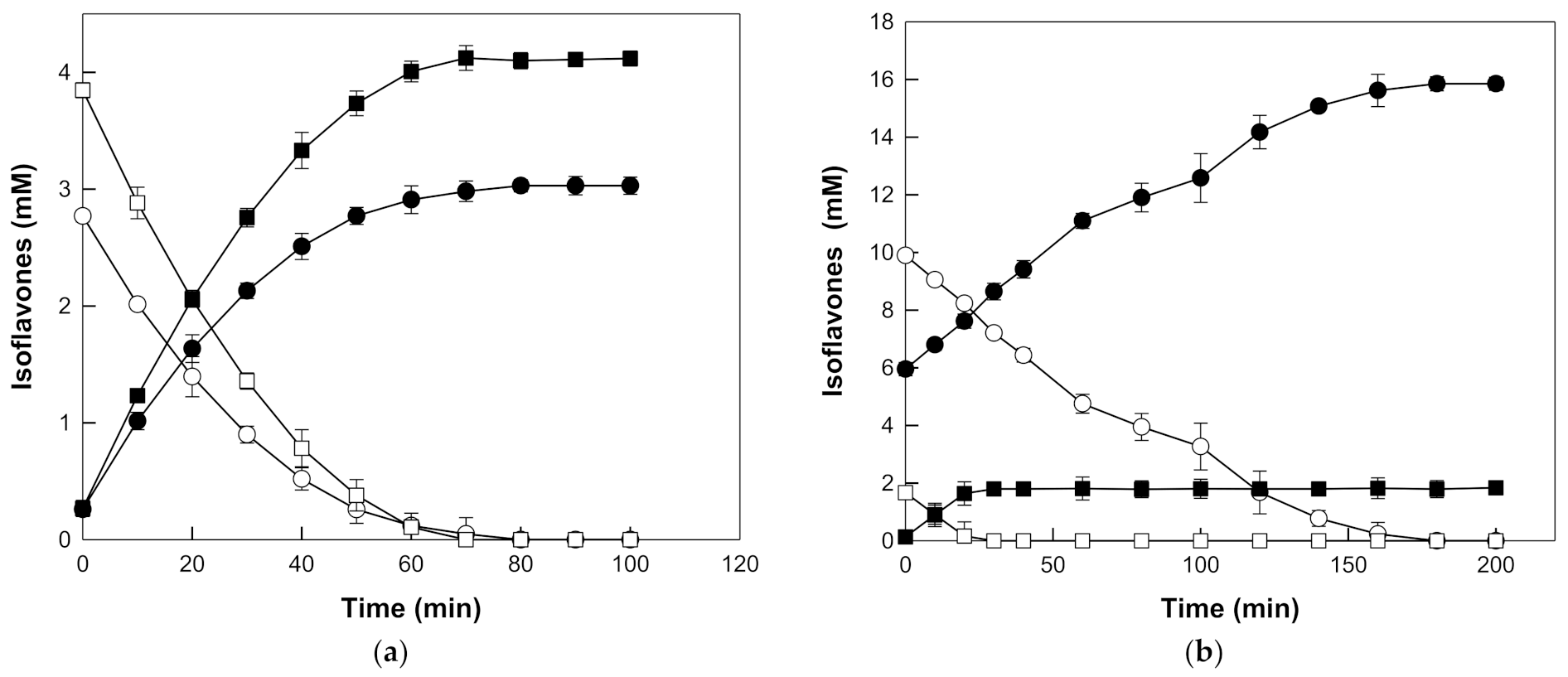
3.4. Conversion of Reagent Grade Isoflavone Glycosides into Isoflavone Aglycones by β-Galactosidase from T. uzoniensis
3.5. Production of Ioflavone Aglycones from Isoflavone Glycosides in Seed and Root Extracts of Korean Wild Soybeans by β-Galactosidase from T. uzoniensis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, T.T.; Jin, F.; Li, J.G.; Xu, Y.Y.; Dong, H.T.; Liu, Q.; Xing, P.; Zhu, G.L.; Xu, H.; Miao, Z.F. Dietary isoflavones or isoflavone-rich food intake and breast cancer risk: A meta-analysis of prospective cohort studies. Clin. Nutr. 2019, 38, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Barańska, A.; Błaszczuk, A.; Kanadys, W.; Baczewska, B.; Jędrych, M.; Wawryk-Gawda, E.; Polz-Dacewicz, M. Effects of soy protein containing of isoflavones and isoflavones extract on plasma lipid profile in postmenopausal women as a potential prevention factor in cardiovascular diseases: Systematic review and meta-analysis of randomized controlled trials. Nutrients 2021, 13, 2531. [Google Scholar] [CrossRef] [PubMed]
- Jagga, S.; Sharma, A.R.; Kim, E.J.; Nam, J.S. Isoflavone-enriched whole soy milk powder stimulates osteoblast differentiation. J. Food Sci. Technol. 2021, 58, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Chen, K.H. Utilization of isoflavones in soybeans for women with menopausal syndrome: An overview. Int. J. Mol. Sci. 2021, 22, 3212. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Tsurugasaki, W.; Nakamura, S.; Osada, K. Comparison of regulative functions between dietary soy isoflavones aglycone and glucoside on lipid metabolism in rats fed cholesterol. J. Nutr. Biochem. 2005, 16, 205–212. [Google Scholar] [CrossRef]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef] [Green Version]
- Chun, J.; Kim, G.M.; Lee, K.W.; Choi, I.D.; Kwon, G.H.; Park, J.Y.; Jeong, S.J.; Kim, J.S.; Kim, J.H. Conversion of isoflavone glucosides to aglycones in soymilk by fermentation with lactic acid bacteria. J. Food Sci. 2007, 72, M39–M44. [Google Scholar] [CrossRef]
- Kuo, L.C.; Cheng, W.Y.; Wu, R.Y.; Huang, C.J.; Lee, K.T. Hydrolysis of black soybean isoflavone glycosides by Bacillus subtilis natto. Appl. Microbiol. Biotechnol. 2006, 73, 314–320. [Google Scholar] [CrossRef]
- Ibe, S.; Kumada, K.; Yoshiba, M.; Onga, T. Production of natto which contains a high level of isoflavone aglycons. J. Jpn. Soc. Food Sci. Technol.-Nippon Shokuhin Kagaku Kogaku Kaishi 2001, 48, 27–34. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yeom, S.J.; Oh, D.K. Characterization of a GH3 family β-glucosidase from Dictyoglomus turgidum and its application to the hydrolysis of isoflavone glycosides in spent coffee grounds. J. Agric. Food Chem. 2011, 59, 11812–11818. [Google Scholar] [CrossRef]
- Pyeon, H.M.; Lee, Y.S.; Choi, Y.L. Cloning, purification, and characterization of GH3 β-glucosidase, MtBgl85, from Microbulbifer thermotolerans DAU221. PeerJ 2019, 7, e7106. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Ma, R.; Shi, P.; Huang, H.; Bai, Y.; Wang, Y.; Yang, P.; Fan, Y.; Yao, B. Molecular characterization of a highly-active thermophilic β-glucosidase from Neosartorya fischeri P1 and its application in the hydrolysis of soybean isoflavone glycosides. PLoS ONE 2014, 9, e106785. [Google Scholar] [CrossRef]
- Yeom, S.J.; Kim, B.N.; Kim, Y.S.; Oh, D.K. Hydrolysis of isoflavone glycosides by a thermostable β-glucosidase from Pyrococcus furiosus. J. Agric. Food Chem. 2012, 60, 1535–1541. [Google Scholar] [CrossRef]
- Kim, B.N.; Yeom, S.J.; Kim, Y.S.; Oh, D.K. Characterization of a β-glucosidase from Sulfolobus solfataricus for isoflavone glycosides. Biotechnol. Lett. 2012, 34, 125–129. [Google Scholar] [CrossRef]
- Li, X.; Xia, W.; Bai, Y.; Ma, R.; Yang, H.; Luo, H.; Shi, P. A novel thermostable GH3 β-glucosidase from Talaromyce leycettanus with broad substrate specificity and significant soybean isoflavone glycosides-hydrolyzing capability. Biomed Res. Int. 2018, 2018, 4794690. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Li, X.; Dang, W.; Tran, P.L.; Park, S.H.; Oh, B.C.; Hong, W.S.; Lee, J.S.; Park, K.H. Characterization and application of an acidophilic and thermostable β-glucosidase from Thermofilum pendens. J. Biosci. Bioeng. 2013, 115, 490–496. [Google Scholar] [CrossRef]
- Xue, Y.; Yu, J.; Song, X. Hydrolysis of soy isoflavone glycosides by recombinant β-glucosidase from hyperthermophile Thermotoga maritima. J. Ind. Microbiol. Biotechnol. 2009, 36, 1401–1408. [Google Scholar] [CrossRef]
- Bonch-Osmolovskaya, E.A.; Miroshnichenko, M.L.; Kostrikina, N.A.; Chernych, N.A.; Zavarzin, G.A. Thermoproteus uzoniensis sp. nov., a new extremely thermophilic archaebacterium from Kamchatka continental hot springs. Arch. Microbiol. 1990, 154, 556–559. [Google Scholar] [CrossRef]
- Aguirre, A.; Peiru, S.; Eberhardt, F.; Vetcher, L.; Cabrera, R.; Menzella, H.G. Enzymatic hydrolysis of steryl glucosides, major contaminants of vegetable oil-derived biodiesel. Appl. Microbiol. Biotechnol. 2014, 98, 4033–4040. [Google Scholar] [CrossRef]
- Tepavcevic, V.; Cvejic, J.; Posa, M.; Popovic, J. Isoflavone Content and Composition in Soybean; IntechOpen: London, UK, 2011. [Google Scholar]
- Hyten, D.L.; Song, Q.; Zhu, Y.; Choi, I.Y.; Nelson, R.L.; Costa, J.M.; Specht, J.E.; Shoemaker, R.C.; Cregan, P.B. Impacts of genetic bottlenecks on soybean genome diversity. Proc. Natl. Acad. Sci. USA 2006, 103, 16666–16671. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.A.; Golokhvast, K.S.; Rehman, H.M.; Tsukamoto, C.; Kim, H.S.; Yang, S.H.; Chung, G. Soyisoflavone diversity in wild soybeans (Glycine soja Sieb. & Zucc.) from the main centres of diversity. Biochem. Syst. Ecol. 2018, 77, 16–21. [Google Scholar]
- Lee, S.J.; Ahn, J.K.; Kim, S.H.; Kim, J.T.; Han, S.J.; Jung, M.Y.; Chung, I.M. Variation in isoflavone of soybean cultivars with location and storage duration. J. Agric. Food Chem. 2003, 51, 3382–3389. [Google Scholar] [CrossRef]
- Lee, Y.W.; Kim, J.D.; Zheng, J.; Row, K. Comparisons of isoflavones from Korean and Chinese soybean and processed product. Biochem. Eng. J. 2007, 36, 49–53. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.S.; Hwang, T.Y. Variation in protein and isoflavone contents of collected domestic and foreign soybean (Glycine max (L.) Merrill) germplasms in Korea. Agriculture 2021, 11, 735. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., 3rd; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Shin, K.C.; Seo, M.J.; Kim, D.W.; Yeom, S.J.; Kim, Y.S. Characterization of β-glycosidase from Caldicellulosiruptor owensensis and its application in the production of platycodin D from balloon flower leaf. Catalysts 2019, 9, 1025. [Google Scholar] [CrossRef] [Green Version]
- Song, T.; Barua, K.; Buseman, G.; Murphy, P.A. Soy isoflavone analysis: Quality control and a new internal standard. Am. J. Clin. Nutr. 1998, 68, 1474s–1479s. [Google Scholar] [CrossRef]
- Park, N.Y.; Cha, J.; Kim, D.O.; Park, C.S. Enzymatic characterization and substrate specificity of thermostable β-glycosidase from hyperthermophilic archaea, Sulfolobus shibatae, expressed in E. coli. J. Microbiol. Biotechnol. 2007, 17, 454–460. [Google Scholar] [PubMed]
- D’Auria, S.; Morana, A.; Febbraio, F.; Vaccaro, C.; De Rosa, M.; Nucci, R. Functional and structural properties of the homogeneous β-glycosidase from the extreme thermoacidophilic archaeon Sulfolobus solfataricus expressed in Saccharomyces cerevisiae. Protein Expr. Purif. 1996, 7, 299–308. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, S.; Nucci, R.; Rossi, M.; Bertoli, E.; Tanfani, F.; Gryczynski, I.; Malak, H.; Lakowicz, J.R. β-Glycosidase from the hyperthermophilic archaeon Sulfolobus solfataricus: Structure and activity in the presence of alcohols. J. Biochem. 1999, 126, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, M.; Kataoka, M.; Watanabe, M.; Ishikawa, K. Monomer structure of a hyperthermophilic β-glucosidase mutant forming a dodecameric structure in the crystal form. Acta Crystallogr. F-Struct. Biol. Commun. 2014, 70, 854–859. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.R.; Kim, Y.S.; Park, C.S.; Lee, J.K.; Kim, Y.S.; Oh, D.K. Characterization of a recombinant β-glucosidase from the thermophilic bacterium Caldicellulosiruptor saccharolyticus. J. Biosci. Bioeng. 2009, 108, 36–40. [Google Scholar] [CrossRef]
- Colussi, F.; da Silva, V.M.; Miller, I.; Cota, J.; de Oliveira, L.C.; de Oliveira Neto, M.; Squina, F.M.; Garcia, W. Oligomeric state and structural stability of two hyperthermophilic β-glucosidases from Thermotoga petrophila. Amino Acids 2015, 47, 937–948. [Google Scholar] [CrossRef]
- Chuankhayan, P.; Rimlumduan, T.; Svasti, J.; Cairns, J.R. Hydrolysis of soybean isoflavonoid glycosides by Dalbergia β-glucosidases. J. Agric. Food Chem. 2007, 55, 2407–2412. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Yan, Q.J.; Jiang, Z.; Li, L. Hydrolysis of soybean isoflavone glycosides by a thermostable β-glucosidase from Paecilomyces thermophila. Food Chem. 2009, 115, 1247–1252. [Google Scholar] [CrossRef]
- Kaya, M.; Ito, J.; Kotaka, A.; Matsumura, K.; Bando, H.; Sahara, H.; Ogino, C.; Shibasaki, S.; Kuroda, K.; Ueda, M.; et al. Isoflavone aglycones production from isoflavone glycosides by display of β-glucosidase from Aspergillus oryzae on yeast cell surface. Appl. Microbiol. Biotechnol. 2008, 79, 51–60. [Google Scholar] [CrossRef]
- Fang, W.; Song, R.; Zhang, X.; Zhang, X.; Zhang, X.; Wang, X.; Fang, Z.; Xiao, Y. Characterization of a novel β-glucosidase from Gongronella sp. W5 and its application in the hydrolysis of soybean isoflavone glycosides. J. Agric. Food Chem. 2014, 62, 11688–11695. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, Y.; Shi, P.; Ma, R.; Yang, H.; Xia, W.; Cui, Y.; Luo, H.; Bai, Y.; Yao, B. A highly glucose-tolerant GH1 β-glucosidase with greater conversion rate of soybean isoflavones in monogastric animals. J. Ind. Microbiol. Biotechnol. 2018, 45, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, M.; Obata, A. β-Glucosidases from soybeans hydrolyze daidzin and genistin. J. Food Sci. 1993, 58, 144–147. [Google Scholar] [CrossRef]
- Angelotti, J.A.F.; Dias, F.F.G.; Sato, H.H.; Fernandes, P.; Nakajima, V.M.; Macedo, J. Improvement of aglycone content in soy isoflavones extract by free and immobilized β-glucosidase and their effects in lipid accumulation. Appl. Biochem. Biotechnol. 2020, 192, 734–750. [Google Scholar] [CrossRef]
- Wu, J.; Muir, A.D. Isoflavone during protease hydrolysis of defatted soybean meal. Food Chem. 2010, 118, 328–332. [Google Scholar] [CrossRef]
- Abdella, A.; El-Baz, A.F.; Ibrahim, I.A.; Mahrous, E.E.; Yang, S.T. Biotransformation of soy flour isoflavones by Aspergillus niger NRRL 3122 β-glucosidase enzyme. Nat. Prod. Res. 2018, 32, 2382–2391. [Google Scholar] [CrossRef]
- Horii, K.; Adachi, T.; Matsuda, T.; Tanaka, T.; Sahara, H.; Shibasaki, S.; Ogino, C.; Hata, Y.; Ueda, M.; Kondo, A. Improvement of isoflavone aglycones production using β-glucosidase secretory produced in recombinant Aspergillus oryzae. J. Mol. Catal. B-Enzym. 2009, 59, 297–301. [Google Scholar] [CrossRef]
- Yan, F.Y.; Xia, W.; Zhang, X.X.; Chen, S.; Nie, X.Z.; Qian, L.C. Characterization of β-glucosidase from Aspergillus terreus and its application in the hydrolysis of soybean isoflavones. J. Zhejiang Univ. Sci. B 2016, 17, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Mai, Z.; Wang, L.; Zeng, Q. Characterization of a novel isoflavone glycoside-hydrolyzing β-glucosidase from mangrove soil metagenomic library. Biochem. Biophys. Res. Commun. 2021, 569, 61–65. [Google Scholar] [CrossRef]
- Maitan-Alfenas, G.P.; Lorena, G.A.D.; de Almeida, M.N.; Visser, E.M.; de Rezende, S.T.; Guimarães, V.M. Hydrolysis of soybean isoflavones by Debaryomyces hansenii UFV-1 immobilised cells and free β-glucosidase. Food Chem. 2014, 146, 429–436. [Google Scholar] [CrossRef] [Green Version]

| Substrate | Specific Activity (μmol/min/mg) |
|---|---|
| pNP-β-D-glucopyranoside | 1021 ± 10 |
| pNP-β-D-galactopyranoside | 1103 ± 13 |
| pNP-β-D-xylopyranoside | 105 ± 2.7 |
| pNP-α-L-arabinopyranoside | ND |
| pNP-α-L-rhamnopyranoside | ND |
| oNP-β-D-glucopyranoside | 830 ± 8.1 |
| oNP-β-D-galactopyranoside | 1208 ± 16 |
| daidzin | 304 ± 9.6 |
| genistin | 405 ± 5.5 |
| ononin | 197 ± 3.5 |
| glycitin | 96 ± 1.3 |
| Isoflavone | Content (mg/g) | ||
|---|---|---|---|
| Seed | Stem | Root | |
| daidzin | 5760 ± 63 | ND | 20,580 ± 135 |
| daidzein | 330 ± 0.9 | 210 ± 7.8 | 7560 ± 171 |
| genistin | 8310 ± 99 | 1320 ± 33 | 3600 ± 45 |
| genistein | 360 ± 2.7 | ND | 1770 ± 33 |
| glycitin | ND | ND | ND |
| glycitein | 510 ± 3.0 | ND | ND |
| Substrate | Strain | Substrate (mM) | Product (mM) | Molar Yield (%) | Productivity (mM/h) | Ref. |
|---|---|---|---|---|---|---|
| Soybean meal | Alicyclobacillus sp. | daidzin (NR a) | daidzein (0.21) | NR | 0.10 | [39] |
| genistin (NR) | genistein (0.32) | NR | 0.16 | |||
| Soybean milk | Soybean | daidzin (0.15) | daidzein (0.04) | 27 | 0.01 | [40] |
| genistin (NR) | genistein (NR) | 17 | NR | |||
| Soybean flour | Aspergillus niger | daidzin (NR) | daidzein (0.77 b) | NR | NR | [41] |
| genistin (NR) | genistein (0.36 b) | NR | NR | |||
| T. maritima | daidzin (NR) | daidzein (0.35 b) | 86 | NR | [17] | |
| genistin (NR) | genistein (0.38 b) | 94 | NR | |||
| Soybean flour extract | Almond | daidzin (NR) | daidzein (NR) | 81 | NR | [42] |
| genistin (NR) | genistein (NR) | 79 | NR | |||
| A. niger | genistin (NR) | genistein (9.38 b) | NR | NR | [43] | |
| A. oryzae | daidzin (2.50) | daidzein (1.80) | 73 | 0.61 | [44] | |
| genistin (1.20) | genistein (0.54) | 45 | 0.11 | |||
| A. oryzae | daidzin (10.00) | daidzein (8.40) | 84 | 0.17 | [37] | |
| genistin (5.00) | genistein (4.09) | 76 | 0.08 | |||
| Aspergillus terreus | daidzin (0.21) | daidzein (0.07) | 33 | 0.41 | [45] | |
| genistin (0.14) | genistein (0.04) | 28 | 0.24 | |||
| Gongronella sp. | daidzin (5.00) | daidzein (4.50) | 90 | 1.50 | [38] | |
| genistin (4.10) | genistein (3.70) | 86 | 1.23 | |||
| P. thermophila | daidzin (NR) | daidzein (NR) | 98 | NR | [36] | |
| genistin (NR) | genistein (NR) | 95 | NR | |||
| P. fusiosus | genistin (1.00) | genistein (1.00) | 100 | 1.00 | [13] | |
| Unculturable microbes | daidzin (NR) | daidzein (1.74) | NR | 0.87 | [46] | |
| genistin (NR) | genistein (1.18) | NR | 0.59 | |||
| Soy molasses | Debaryomyces hansenii | daidzin (NR) | daidzein (0.07 b) | NR | NR | [47] |
| genistin (NR) | genistein (6.73 b) | NR | NR | |||
| Spent coffee ground extract | D. turgidum | daidzin (1.20) | daidzein (1.20) | 100 | 0.60 | [10] |
| Wild soybean seed extract | T. uzoniensis | daidzin (2.76) | daidzein (2.76) | 100 | 1.86 | this study |
| genistin (3.84) | genistein (3.84) | 100 | 3.30 | |||
| Wild soybean root extract | T. uzoniensis | daidzin (9.90) | daidzein (9.90) | 100 | 3.30 | this study |
| genistin (1.68) | genistein (1.68) | 100 | 3.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, K.-C.; Kang, S.-H.; Oh, D.-K.; Kim, D.W.; Kim, S.H.; Na, C.S.; Kim, Y.-S. Production of Daidzein and Genistein from Seed and Root Extracts of Korean Wild Soybean (Glycine soja) by Thermostable β-Galactosidase from Thermoproteus uzoniensis. Appl. Sci. 2022, 12, 3481. https://doi.org/10.3390/app12073481
Shin K-C, Kang S-H, Oh D-K, Kim DW, Kim SH, Na CS, Kim Y-S. Production of Daidzein and Genistein from Seed and Root Extracts of Korean Wild Soybean (Glycine soja) by Thermostable β-Galactosidase from Thermoproteus uzoniensis. Applied Sciences. 2022; 12(7):3481. https://doi.org/10.3390/app12073481
Chicago/Turabian StyleShin, Kyung-Chul, Su-Hwan Kang, Deok-Kun Oh, Dae Wook Kim, Sae Hyun Kim, Chae Sun Na, and Yeong-Su Kim. 2022. "Production of Daidzein and Genistein from Seed and Root Extracts of Korean Wild Soybean (Glycine soja) by Thermostable β-Galactosidase from Thermoproteus uzoniensis" Applied Sciences 12, no. 7: 3481. https://doi.org/10.3390/app12073481
APA StyleShin, K.-C., Kang, S.-H., Oh, D.-K., Kim, D. W., Kim, S. H., Na, C. S., & Kim, Y.-S. (2022). Production of Daidzein and Genistein from Seed and Root Extracts of Korean Wild Soybean (Glycine soja) by Thermostable β-Galactosidase from Thermoproteus uzoniensis. Applied Sciences, 12(7), 3481. https://doi.org/10.3390/app12073481






