Abstract
d-Tagatose, a functional sweetener, is converted from d-galactose by l-arabinose isomerase, which catalyzes the conversion of l-arabinose to l-ribulose. In this study, the araA gene encoding l-arabinose isomerase from Klebsiella pneumoniae was cloned and expressed in Escherichia coli, and the expressed enzyme was purified and characterized. The purified l-arabinose isomerase, a soluble protein with 11.6-fold purification and a 22% final yield, displayed a specific activity of 1.8 U/mg for d-galactose and existed as a homohexamer of 336 kDa. The enzyme exhibited maximum activity at pH 8.0 and 40 °C in the presence of Mn2+ and relative activity for pentoses and hexoses in the order l-arabinose > d-galactose > l-ribulose > d-xylulose > d-xylose > d-tagatose > d-glucose. The thermal stability of recombinant E. coli cells expressing l-arabinose isomerase from K. pneumoniae was higher than that of the enzyme. Thus, the reaction conditions of the recombinant cells were optimized to pH 8.0, 50 °C, and 4 g/L cell concentration using 100 g/L d-galactose with 1 mM Mn2+. Under these conditions, 33.5 g/L d-tagatose was produced from d-galactose with 33.5% molar yield and 67 g/L/h productivity. Our findings will help produce d-tagatose using whole-cell reactions, extending its industrial application.
1. Introduction
d-Tagatose, a ketohexose monosaccharide contained in dairy products in small amounts, is an isomer of d-galactose [1,2]. d-Tagatose tastes similar to sucrose (92% sweetness); both sugars lack laxative and cooling effects and are involved in browning reactions [3]. However, d-tagatose possesses numerous health benefits, such as preventing weight gain, improving fetal development, and alleviating symptoms of type 2 diabetes, anemia, and hemophilia [4,5]. Moreover, it is known as a low-calorie and tooth-friendly sweetener, unlike sucrose [6]. Therefore, d-tagatose has attracted significant attention in the fields of foods, beverages, health foods, and dietary supplements [2,7].
d-Tagatose can be chemically synthesized from d-galactose using a calcium catalyst; however, it is undesirable due to disadvantages such as complicated purification steps and the formation of chemical waste and byproducts [8]. Thus, the biological production of d-tagatose from d-galactose using l-arabinose isomerase has been intensively studied. l-Arabinose isomerase, an enzyme catalyzing the conversion of l-arabinose to l-ribulose, can catalyze the conversion of d-galactose to d-tagatose because of its broad substrate specificity toward similarly configured substrates [9]. To date, d-galactose has been converted to d-tagatose using l-arabinose isomerases from various microorganisms, including Acidothermus cellulolyticus [10], Alicyclobacillus acidocaldarius [11], Arthrobacter sp. [12], Anoxybacillus flavithermus [13], Bacillus coagulans [14], Bacillus halodurans [15], Bacillus licheniformis [16], Bacillus stearothermophilus [17], Bacillus subtilis [18], Bacillus thermoglucosidasius [19], Bifidobacterium longum [20], Escherichia coli [21], Geobacillus kaustophilus [22], Geobacillus stearothermophilus [23], Geobacillus thermodenitrificans [24], Lactobacillus fermentum [25], Lactobacillus plantarum [26], Lactobacillus sakei [27], Pediococcus pentosaceus [28], Shewanella sp. [29], Thermoanaerobacter mathranii [30], Thermoanaerobacterium saccharolyticum [31], Thermotoga maritima [32], Thermotoga neapolitana [33], and Thermus sp. [34].
l-arabinose isomerases from thermophilic microorganisms, such as A. cellulolyticus, A. acidocaldarius, B. thermoglucosidasius, G. kaustophilus, G. stearothermophilus, G. thermodenitrificans, T. mathranii, T. saccharolyticum, T. maritima, T. neapolitana, and Thermus sp., have exhibited higher activity or thermostability than those from mesophilic microorganisms. Therefore, the production of d-tagatose from d-galactose using thermostable l-arabinose isomerase from thermophilic microorganisms has been mainly studied so far. Despite having advantages, thermostable l-arabinose isomerase has limited ability in showing the highest activity in industrial processes, such as fermentation and whole-cell reaction using an intrinsic system of cells, because cells are inactivated at their optimum temperature, which is too high [35,36,37,38]. Therefore, additional studies are needed to discover and characterize l-arabinose isomerase from a mesophilic microorganism that has been neglected in recent years. In addition, the production of d-tagatose from d-galactose using whole cells expressing l-arabinose isomerase needs to be evaluated.
In this study, we cloned and expressed l-arabinose isomerase derived from Klebsiella pneumoniae, a lactose-fermenting mesophilic bacterium, in E. coli. The l-arabinose isomerase was purified and characterized, and E. coli cells expressing the enzyme were used for producing d-tagatose from d-galactose.
2. Materials and Methods
2.1. Microorganism, Plasmid, and Gene Cloning
The DNA template and expression vector for l-arabinose isomerase gene (GenBank accession no. CP000964) cloning and host cells of enzyme expression were K. pneumoniae DSM 681 (DSMZ, Braunschweig, Germany), pET-28a (+) vector (Novagen, Darmstadt, Germany), and E. coli ER2566 strain (New England Biolabs, Ipswich, MA, USA), respectively. The l-arabinose isomerase gene was amplified from K. pneumoniae genomic DNA by polymerase chain reaction (PCR) using the following primers from Bioneer (Daejeon, Korea): Forward (5′-CCCGCTAGCATGACGATTTTTGATAACTATGAAGTATG-3′) and reverse (5′-GGGAAGCTTTTAGCGTTTTGAACTGTAATACACTTCATT-3′), introduced into NheI and HindIII restriction sites (underlined), respectively. The amplified DNA fragments obtained using PCR were purified and inserted into the pET-28a (+) vector digested with the same restriction enzymes. The E. coli ER2566 strain was transformed with the ligation mixture using an electroporator (MicroPulser; Bio-Rad Laboratories, Hercules, CA, USA), and the transformed cells were plated on Luria-Bertani (LB) agar containing 20 μg/mL kanamycin. A kanamycin-resistant colony was selected, and plasmid DNA from the transformant was isolated using a plasmid purification kit (Promega, Madison, WI, USA). DNA sequencing was performed at a Macrogen facility (Seoul, Korea).
2.2. Culture Conditions and Enzyme Purification
Transformed E. coli cells were grown in a 2000 mL flask containing 500 mL of LB medium supplemented with 20 μg/mL of kanamycin at 37 °C with shaking at 200 rpm. When the optical density of the bacterial culture suspension reached 0.5 at 600 nm, 0.1 mM isopropyl-β-d-thiogalactopyranoside was added to the culture medium and incubated at 16 °C for an additional 16 h with shaking at 150 rpm. After cell growth, the induced cells were harvested and resuspended in 50 mM of phosphate buffer (pH 7.0) containing 300 mM NaCl, 10 mM imidazole, and 1 mg/mL lysozyme. The resuspended cells were disrupted by sonication on ice for 25 min. The unbroken cells and cell debris were removed by centrifugation at 13,000× g at 4 °C for 20 min. The cell-free supernatant was loaded on a His-Trap HP affinity chromatography column equipped with a fast protein liquid chromatography system (Bio-Rad Laboratories). The bound protein was eluted at 1 mL/min using 50 mM phosphate buffer (pH 7.0) containing 250 mM imidazole at 4 °C. The eluted active fractions were dialyzed against 50 mM phosphate buffer (pH 7.0) without other components at 4 °C for 16 h. The obtained protein was used as purified l-arabinose isomerase.
2.3. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis and Gel Filtration
The subunit molecular mass of l-arabinose isomerase was determined by SDS-PAGE using a prestained ladder (MBI Fermentas, Glen Burnie, MD, USA) as a reference. Coomassie brilliant blue was used to stain protein bands for visualization. The molecular mass of the native l-arabinose isomerase from K. pneumoniae was determined by gel filtration chromatography using a Sephacryl S-300 HR 16/60 column (GE Healthcare, Piscataway, NJ, USA). Purified l-arabinose isomerase was applied to the column and eluted at 1 mL/min with 50 mM phosphate buffer (pH 7.0) containing 150 mM NaCl. The column was calibrated using conalbumin (75 kDa), aldolase (158 kDa), ferritin (400 kDa), and thyroglobulin (669 kDa) as reference proteins (GE Healthcare), and the molecular mass of the native enzyme was calculated by comparing its retention time with that of the reference proteins.
2.4. Enzyme Assay
The activity of l-arabinose isomerase was determined by measuring d-tagatose converted from d-galactose as a substrate. The reaction was performed with 50 mM d-galactose at 40 °C in 50 mM phosphate buffer (pH 8.0) for 10 min. Then, the enzyme solution was centrifuged, filtered, and used for analyzing the substrate and product. One unit of the activity was defined as the amount of enzyme producing 1 μmol of d-tagatose per minute at 40 °C and pH 8.0. To measure the specific activity, the enzyme reactions were performed in 50 mM phosphate buffer (pH 8.0) containing 50 mM sugar and 0.8 U/mL (0.4 mg/mL) of enzyme at 40 °C for 10 min.
2.5. Effects of pH, Temperature, and Metal Ions
To examine the effects of pH and temperature on l-arabinose isomerase activity, the pH values were varied from 4.0 to 10.0 using 50 mM citrate buffer (pH 4.0–6.0), phosphate buffer (pH 6.0–8.0), and Tris-HCl buffer (pH 8.0–9.0) at a constant temperature of 35 °C, and temperatures were varied from 30 to 60 °C in 50 mM phosphate buffer at a constant pH of 8.0. The reactions were performed using 50 mM d-galactose and 0.8 U/mL enzymes for 10 min. The effect of temperature on the stability of the enzyme and E. coli cells expressing the enzyme was monitored as a function of incubation time by incubating the reaction solutions of the enzyme and cells at 40 °C and 50 °C, respectively, in 50 mM phosphate buffer (pH 8.0) containing 50 mM and 20 g/L d-galactose.
The purified enzyme was treated with 10 mM ethylenediaminetetraacetic acid (EDTA) and dialyzed against 50 mM phosphate buffer (pH 8.0) to prepare an EDTA-treated enzyme. To investigate the effect of metal ions on l-arabinose isomerase activity, the reactions were performed at 40 °C in 50 mM phosphate buffer (pH 8.0) containing 50 mM d-galactose, 0.8 U/mL EDTA-treated enzymes, and 1 mM of various metal ions such as Ba2+, Ca2+, Co2+, Cu2+, Fe2+, Mg2+, and Mn2+.
2.6. Optimization of Reaction Conditions Using E. coli Cells Expressing l-Arabinose Isomerase
Unless otherwise stated, the reaction using transformed E. coli cells expressing l-arabinose isomerase was performed with 20 g/L of d-galactose and 1 g/L of cell at 50 °C in 50 mM phosphate buffer (pH 8.0) for 10 min. The effects of pH and temperature were investigated to optimize the production of d-tagatose from d-galactose using E. coli cells expressing l-arabinose isomerase as in the above enzymatic methods. To determine the optimal concentration of E. coli cells expressing l-arabinose isomerase, the reactions were performed by varying E. coli cell concentration from 0.5 to 5 g/L supplemented with 100 g/L d-galactose. The reactions were performed in 50 mM phosphate buffer (pH 8.0) at 50 °C for 30 min. The production of d-tagatose was performed in 50 mM phosphate buffer (pH 8.0) containing 4 g/L cells and 100 g/L d-galactose at 50 °C for 45 min.
2.7. Analytical Methods
The concentrations of protein and cells were determined using the Bradford method with bovine serum albumin as the protein standard and a calibration curve derived from the correlation between dry cell weight and optical density at 600 nm. The concentrations of monosaccharides were determined using a Bio-LC system (Dionex ICS-3000, Sunnyvale, CA, USA) equipped with an electrochemical detector and a CarboPac PA1 column, which was eluted at 30 °C with 200 mM sodium hydroxide at a flow rate of 1 mL/min.
3. Results and Discussion
3.1. Gene Cloning, Purification, and Molecular Mass Determination of l-Arabinose Isomerase from K. pneumoniae
The 1503 bp of araA gene from K. pneumoniae DSM 681 with the same sequence in GenBank (Accession No., ABR75521) was cloned and expressed in E. coli ER2566. The amino acid sequence of the expressed enzyme showed 91.4%, 59.5%, 59.4%, 59.2%, 52.0%, and 49.0% identities with those of l-arabinose isomerase from E. coli (AAA23463) [39], A. acidocaldarius (AAY68209) [11], G. kaustophilus (BAD76189) [22], G. stearothermophilus (AAD45718) [40], B. subtilis (CAA61585) [18], and Arthrobacter sp. (ABK01625) [12], respectively.
l-Arabinose isomerase from K. pneumoniae was purified using His-Trap affinity chromatography into a soluble protein with an 11.6-fold purification, a final yield of 22%, and a specific activity of 1.8 U/mg for d-galactose. The molecular mass of the purified enzyme was approximately 56 kDa on SDS-PAGE (Figure 1a), which was consistent with the calculated value of 57,131 Da based on 493 amino acids combined with six histidine residues. The native enzyme was determined to be a homohexamer with a molecular mass of 336 kDa by gel filtration chromatography (Figure 1b). l-Arabinose isomerases from E. coli [39], G. kaustophilus [22], L. plantarum [26], and L. fermentum [41] with 91.4%, 59.4%, 43.6%, and 42.0% sequence identities to l-arabinose isomerase from K. pneumoniae, respectively, were hexamers. In contrast, l-arabinose isomerases from B. licheniformis [16] and B. subtilis [18] with 48.0% and 52.0% sequence identities, respectively, were dimers, and l-arabinose isomerases from A. acidocaldarius [11] and E. faecium [42] with 59.5% and 47.4% sequence identities, respectively, were tetramers. These results indicate that the level of sequence identity within l-arabinose isomerases does not correlate with determining the association form.
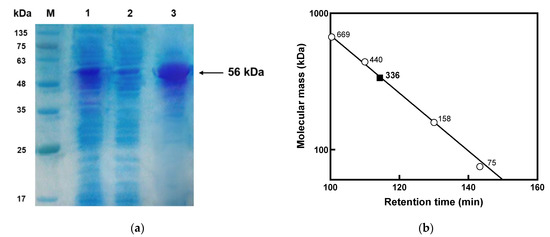
Figure 1.
SDS-PAGE and gel filtration analyses of l-arabinose isomerase from K. pneumoniae. (a) SDS-PAGE of l-arabinose isomerase from K. pneumoniae. M, molecular weight size marker proteins; lane 1, pellet; lane 2, crude enzyme extract; and lane 3, purified l-arabinose isomerase from K. pneumoniae. (b) Gel filtration chromatography of l-arabinose isomerase from K. pneumoniae. The solution of purified l-arabinose isomerase from K. pneumoniae (filled square) was applied to a Sephacryl column. The column was calibrated using the reference proteins (empty circle) conalbumin, aldolase, ferritin, and thyroglobulin, corresponding to 75, 158, 400, and 669 kDa, respectively.
3.2. Effects of Environmental Factors on the Activity of l-Arabinose Isomerase from K. pneumoniae
The effects of pH and temperature on l-arabinose isomerase activity were investigated using varying pH (4.0–9.0) at a constant temperature of 35 °C and varying temperatures (30–60 °C) at a constant pH value of 8.0, respectively. The maximum activity was observed at pH 8.0 and 40 °C (Figure 2). The thermal stability of l-arabinose isomerase from K. pneumoniae was evaluated by measuring the residual activity after incubating for 1, 2, 3, 4, and 5 h at 40 °C (Figure 3). The enzyme activity decreased as the time increased. The activity decreased to less than 50% of the initial activity between 3 and 4 h and dropped to less than 20% at 5 h. l-Arabinose isomerase from K. pneumoniae showed lower thermostability than other mesophilic l-arabinose isomerases from B. thermoglucosidasius [19] and L. sakei [27], which displayed residual activities of 80% and 95% for 2 h at 40 °C, respectively, and than from B. longum [20], which had a half-life of 52 h at 45 °C. However, the activity of the recombinant E. coli cells expressing l-arabinose isomerase from K. pneumoniae was more than 60% after incubation for 5 h at 50 °C (Figure 3), which indicated that the recombinant cells were more stable in producing d-tagatose from d-galactose than the enzyme.

Figure 2.
Effects of pH and temperature on the activity of l-arabinose isomerase from K. pneumoniae. (a) Effect of pH. The reactions were performed in 50 mM citrate (pH 4.0–6.0), phosphate (pH 6.0–8.0), and Tris-HCl (pH 8.0–9.0) buffers containing 50 mM d-galactose and 0.8 U/mL enzyme at 35 °C for 10 min. (b) Effect of temperature. The reactions were performed in 50 mM phosphate buffer (pH 8.0) containing 50 mM d-galactose and 0.8 U/mL enzyme at different temperatures, ranging from 30 °C to 60 °C for 10 min. Data represent the mean of values from three experiments, and error bars represent standard deviation.

Figure 3.
Thermal inactivation of l-arabinose isomerase from K. pneumoniae (closed square) and E. coli cells expressing the enzyme (closed circle). l-Arabinose isomerase or E. coli cells were incubated at 40 °C or 50 °C, respectively, withdrawn at each time point, and assayed in 50 mM phosphate buffer (pH 8.0) containing 0.8 U/mL enzyme or 1 g/L cells and 50 mM or 20 g/L d-galactose at 40 °C or 50 °C, respectively, for 10 min. Data represent the mean of values from three experiments, and error bars represent standard deviation.
The effects of metal ions on l-arabinose isomerase activity were evaluated at a concentration of 1 mM (Table 1). Mg2+ and Mn2+ enhanced the activity by 15% and 28%, respectively, and Ca2+ and Co2+ showed similar activity to the negative control (not treated with EDTA), whereas Ba2+, Cu2+, and Fe2+ inhibited d-galactose isomerization. Enzyme activity was the highest when Mn2+ was used, similar to most enzymes, including l-arabinose isomerases from T. neapolitana [33], T. maritima [43], T. saccharolyticum [31], T. mathranii [44], L. fermentum [25], L. sakei [27], and G. thermodenitrificans [24].

Table 1.
Effects of metal ions on the activity of l-arabinose isomerase from K. pneumoniae.
3.3. Substrate Specificity of l-Arabinose Isomerase from K. pneumoniae
The substrate specificity of l-arabinose isomerase from K. pneumoniae was investigated using various pentoses such as l-arabinose, d-xylose, l-ribulose, and d-xylulose and hexoses such as d-galactose, d-glucose, d-tagatose, and d-fructose (Table 2). The enzyme activity was the highest for l-arabinose, followed by d-galactose, l-ribulose, d-xylulose, d-xylose, d-tagatose, and d-glucose. The activity was generally higher for pentose than hexose except for d-galactose; the activity for l-arabinose was 3.15 times higher than that for d-galactose. However, the activity against d-tagatose, a product of d-galactose, was only 4.3% of that of d-galactose.

Table 2.
Substrate specificity of l-arabinose isomerase from K. pneumoniae.
3.4. Optimization of Reaction Conditions Using E. coli Cells Expressing l-Arabinose Isomerase from K. pneumoniae
To produce d-tagatose from d-galactose, reaction conditions such as pH, temperature, and cell concentration were optimized using recombinant E. coli cells expressing l-arabinose isomerase from K. pneumoniae. The maximum activity of the recombinant E. coli cells was observed at pH 8.0, the same as the enzyme, and 50 °C (Figure 4), 10 °C higher than that of the enzyme (Figure 2).

Figure 4.
Investigation of pH and temperature to optimize the production of d-tagatose from d-galactose by E. coli cells expressing l-arabinose isomerase from K. pneumoniae. (a) Investigation of pH. The reactions were performed in 50 mM citrate (pH 4.0–6.0), phosphate (pH 6.0–8.0), and Tris-HCl (pH 8.0–9.0) buffers containing 20 g/L d-galactose and 1 g/L cells at 40 °C for 10 min. (b) Investigation of temperature. The reactions were performed in 50 mM phosphate buffer (pH 8.0) containing 20 g/L d-galactose and 1 g/L cells at different temperatures, ranging from 30 °C to 60 °C for 10 min. Data represent the mean of values from three experiments, and error bars represent standard deviation.
After incubation for 5 h at 50 °C, the activity of the recombinant E. coli cells was more than 60%, which was approximately three-fold higher than that of the enzyme after 5 h at 40 °C (Figure 3). Therefore, the optimal temperature for producing d-tagatose from d-galactose using recombinant E. coli cells was determined to be 50 °C.
The production of d-tagatose from d-galactose was further optimized by varying the concentration of the recombinant cells (Figure 5a). The production increased with an increase in the concentration of cells up to 4 g/L and reached a plateau above that concentration, indicating that the optimum concentration for the recombinant E. coli cells was 4 g/L.
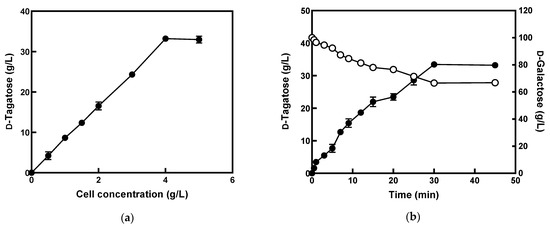
Figure 5.
Production of d-tagatose from d-galactose by E. coli cells expressing l-arabinose isomerase from K. pneumoniae. (a) Effect of the concentration of E. coli cells expressing l-arabinose isomerase from K. pneumoniae on d-tagatose production. The reactions were performed in 50 mM phosphate buffer (pH 8.0) containing 100 g/L d-galactose at 50 °C with varying concentrations of cells from 0.5 to 5 g/L. (b) Time-course reactions for the production of d-tagatose (black circle) from d-galactose (white circle) by E. coli cells expressing l-arabinose isomerase from K. pneumoniae. The reactions were performed in 50 mM phosphate buffer (pH 8.0) containing 100 g/L d-galactose and 5 g/L cells at 50 °C. Data represent the mean of values from three experiments, and error bars represent standard deviation.
3.5. Production of d-Tagatose from d-Galactose Using E. coli Cells Expressing l-Arabinose Isomerase from K. pneumoniae
The optimal reaction conditions for producing d-tagatose from d-galactose were pH 8.0, 50 °C, and 60 g/L recombinant E. coli cells using 100 g/L d-galactose with 1 mM Mn2+. Under these conditions, the time-course reaction for producing d-tagatose from d-galactose was performed by E. coli cells expressing l-arabinose isomerase from K. pneumoniae (Figure 5b). The recombinant E. coli cells produced 33.5 g/L d-tagatose for 30 min with a molar yield of 33.5% and a productivity of 67 g/L/h.
l-Arabinose isomerases from G. stearothermophilus and T. neapolitana have been applied to recombinant E. coli cells and immobilized for better d-tagatose production [45,46]. In addition, d-tagatose has been produced from milk whey powder using recombinant E. coli cells expressing l-arabinose isomerase from Lactobacillus plantarum [47] and from maltodextrin by multienzyme coexpression system of E. coli cells containing l-arabinose isomerase [48]. Therefore, further studies on applying immobilization and multienzyme system to E. coli cells expressing l-arabinose isomerase from K. pneumoniae will enhance its industrial applicability.
4. Conclusions
In this study, we cloned and characterized l-arabinose isomerase from K. pneumoniae as a purified enzyme, which existed as a homohexamer of 336 kDa and exhibited a specific activity of 1.8 U/mg for d-galactose and maximal activity at pH 8.0 and 40 °C with 1.0 mM Mn2+. Recombinant E. coli cells expressing l-arabinose isomerase from K. pneumoniae, displaying higher stability than the enzyme, were used to produce d-tagatose from d-galactose; they produced 33.5 g/L d-tagatose with a molar yield of 33.5% and a productivity of 67 g/L/h under the optimal conditions. These findings will be helpful for the industrial production of d-tagatose using a whole-cell reaction.
Author Contributions
Conceptualization, C.-S.P. and Y.-S.K.; methodology, K.-C.S. and M.-J.S.; validation, K.-C.S.; formal analysis, M.-J.S. and S.J.K.; investigation, K.-C.S. and S.J.K.; resources C.-S.P.; data curation, C.-S.P.; writing—original draft preparation, K.-C.S.; writing—review and editing, Y.-S.K. and C.-S.P.; visualization, S.J.K.; supervision, C.-S.P. and Y.-S.K.; project administration, C.-S.P. and Y.-S.K.; funding acquisition, C.-S.P. and Y.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2019R1F1A1059906) and R&D Program for Forest Science Technology (Project No. 2020197A00-2022-BA01) provided by the Korea Forest Service (Korea Forestry Promotion Institute).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This paper was supported by the KU Research Professor Program of Konkuk University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mendoza, M.R.; Olano, A.; Villamiel, M. Chemical indicators of heat treatment in fortified and special milks. J. Agric. Food Chem. 2005, 53, 2995–2999. [Google Scholar] [CrossRef]
- Oh, D.K. Tagatose: Properties, applications, and biotechnological processes. Appl. Microbiol. Biotechnol. 2007, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V.; Zehner, L.R.; Saunders, J.P.; Beadle, J.R. Sugar substitutes: Their energy values, bulk characteristics, and potential health benefits. Am. J. Clin. Nutr. 1995, 62, 1161s–1168s. [Google Scholar] [CrossRef]
- Levin, G.V. Tagatose, the new GRAS sweetener and health product. J. Med. Food 2002, 5, 23–36. [Google Scholar] [CrossRef]
- Espinosa, I.; Fogelfeld, L. Tagatose: From a sweetener to a new diabetic medication? Expert Opin. Investig. Drugs 2010, 19, 285–294. [Google Scholar] [CrossRef]
- Kim, P. Current studies on biological tagatose production using l-arabinose isomerase: A review and future perspective. Appl. Microbiol. Biotechnol. 2004, 65, 243–249. [Google Scholar] [CrossRef]
- Lu, Y.; Levin, G.V.; Donner, T.W. Tagatose, a new antidiabetic and obesity control drug. Diabetes Obes. Metab. 2008, 10, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Beadle, J.R.; Saunders, J.P.; Wajda, T.J. Process for Manufacturing Tagatose. U.S. Patent US5078796A, 7 January 1992. [Google Scholar]
- Xu, Z.; Li, S.; Feng, X.; Liang, J.; Xu, H. l-Arabinose isomerase and its use for biotechnological production of rare sugars. Appl. Microbiol. Biotechnol. 2014, 98, 8869–8878. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Mu, W.; Zhang, T.; Jiang, B. An l-arabinose isomerase from Acidothermus cellulolytics ATCC 43068: Cloning, expression, purification, and characterization. Appl. Microbiol. Biotechnol. 2010, 86, 1089–1097. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.W.; Choe, E.A.; Hong, Y.H.; Kim, S.B.; Kim, B.C.; Pyun, Y.R. Characterization of a thermoacidophilic l-arabinose isomerase from Alicyclobacillus acidocaldarius: Role of Lys-269 in pH optimum. Appl. Environ. Microbiol. 2005, 71, 7888–7896. [Google Scholar] [CrossRef] [Green Version]
- Wanarska, M.; Kur, J. A method for the production of d-tagatose using a recombinant Pichia pastoris strain secreting β-D-galactosidase from Arthrobacter chlorophenolicus and a recombinant l-arabinose isomerase from Arthrobacter sp. 22c. Microb. Cell Fact. 2012, 11, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhu, Y.; Liu, A.; Sun, Y. Identification and characterization of a novel l-arabinose isomerase from Anoxybacillus flavithermus useful in d-tagatose production. Extrem. Life Under Extrem. Cond. 2011, 15, 441–450. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, J.C. Heterologous expression and characterization of Bacillus coagulans l-arabinose isomerase. World J. Microbiol. Biotechnol. 2012, 28, 2205–2212. [Google Scholar] [CrossRef]
- Lee, D.W.; Choe, E.A.; Kim, S.B.; Eom, S.H.; Hong, Y.H.; Lee, S.J.; Lee, H.S.; Lee, D.Y.; Pyun, Y.R. Distinct metal dependence for catalytic and structural functions in the l-arabinose isomerases from the mesophilic Bacillus halodurans and the thermophilic Geobacillus stearothermophilus. Arch. Biochem. Biophys. 2005, 434, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, P.; Tiwari, M.K.; Jeya, M.; Gunasekaran, P.; Kim, I.W.; Lee, J.K. Cloning and characterization of a novel l-arabinose isomerase from Bacillus licheniformis. Appl. Microbiol. Biotechnol. 2008, 81, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, M.; Bejar, S. Cloning, purification and biochemical characterization of metallic-ions independent and thermoactive L-arabinose isomerase from the Bacillus stearothermophilus US100 strain. Biochim. Biophys. Acta 2006, 1760, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Prabhu, P.; Jeya, M.; Tiwari, M.K.; Moon, H.J.; Singh, R.K.; Lee, J.K. Characterization of an l-arabinose isomerase from Bacillus subtilis. Appl. Microbiol. Biotechnol. 2010, 85, 1839–1847. [Google Scholar] [CrossRef]
- Seo, M.J. Characterization of an l-arabinose isomerase from Bacillus thermoglucosidasius for d-tagatose production. Biosci. Biotechnol. Biochem. 2013, 77, 385–388. [Google Scholar] [CrossRef]
- Salonen, N.; Nyyssölä, A.; Salonen, K.; Turunen, O. Bifidobacterium longum l-arabinose isomerase--overexpression in Lactococcus lactis, purification, and characterization. Appl. Biochem. Biotechnol. 2012, 168, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Kim, P.; Oh, D.K. Properties of l-arabinose isomerase from Escherichia coli as biocatalyst for tagatose production. World J. Microbiol. Biotechnol. 2003, 19, 47–51. [Google Scholar] [CrossRef]
- Cao, T.P.; Choi, J.M.; Lee, S.J.; Lee, Y.J.; Lee, S.K.; Jun, Y.; Lee, D.W.; Lee, S.H. Crystallization and preliminary X-ray crystallographic analysis of l-arabinose isomerase from thermophilic Geobacillus kaustophilus. Acta Crystallogr. F-Struct. Biol. Commun. 2014, 70, 108–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Kim, J.H.; Oh, H.J.; Oh, D.K. Characterization of a mutated Geobacillus stearothermophilus l-arabinose isomerase that increases the production rate of d-tagatose. J. Appl. Microbiol. 2006, 101, 213–221. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, D.K. Purification and characterization of an l-arabinose isomerase from an isolated strain of Geobacillus thermodenitrificans producing d-tagatose. J. Biotechnol. 2005, 120, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qing, Y.; Li, S.; Feng, X.; Xu, H.; Ouyang, P. A novel l-arabinose isomerase from Lactobacillus fermentum CGMCC2921 for d-tagatose production: Gene cloning, purification and characterization. J. Mol. Catal. B-Enzym. 2011, 70, 1–7. [Google Scholar] [CrossRef]
- Chouayekh, H.; Bejar, W.; Rhimi, M.; Jelleli, K.; Mseddi, M.; Bejar, S. Characterization of an l-arabinose isomerase from the Lactobacillus plantarum NC8 strain showing pronounced stability at acidic pH. FEMS Microbiol. Lett. 2007, 277, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Rhimi, M.; Ilhammami, R.; Bajic, G.; Boudebbouze, S.; Maguin, E.; Haser, R.; Aghajari, N. The acid tolerant l-arabinose isomerase from the food grade Lactobacillus sakei 23K is an attractive d-tagatose producer. Bioresour. Technol. 2010, 101, 9171–9177. [Google Scholar] [CrossRef]
- Men, Y.; Zhu, Y.; Zhang, L.; Kang, Z.; Izumori, K.; Sun, Y.; Ma, Y. Enzymatic conversion of d-galactose to d-tagatose: Cloning, overexpression and characterization of l-arabinose isomerase from Pediococcus pentosaceus PC-5. Microbiol. Res. 2014, 169, 171–178. [Google Scholar] [CrossRef]
- Rhimi, M.; Bajic, G.; Ilhammami, R.; Boudebbouze, S.; Maguin, E.; Haser, R.; Aghajari, N. The acid-tolerant l-arabinose isomerase from the mesophilic Shewanella sp. ANA-3 is highly active at low temperatures. Microb. Cell Fact. 2011, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, F.; Hansen, O.C.; Stougaard, P. Enzymatic conversion of d-galactose to d-tagatose: Heterologous expression and characterisation of a thermostable l-arabinose isomerase from Thermoanaerobacter mathranii. Appl. Microbiol. Biotechnol. 2004, 64, 816–822. [Google Scholar] [CrossRef]
- Lin, C.J.; Tseng, W.C.; Lin, T.H.; Liu, S.M.; Tzou, W.S.; Fang, T.Y. Characterization of a thermophilic l-rhamnose isomerase from Thermoanaerobacterium saccharolyticum NTOU1. J. Agric. Food Chem. 2010, 58, 10431–10436. [Google Scholar] [CrossRef]
- Lee, D.W.; Jang, H.J.; Choe, E.A.; Kim, B.C.; Lee, S.J.; Kim, S.B.; Hong, Y.H.; Pyun, Y.R. Characterization of a thermostable l-arabinose (d-galactose) isomerase from the hyperthermophilic eubacterium Thermotoga maritima. Appl. Environ. Microbiol. 2004, 70, 1397–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.C.; Lee, Y.H.; Lee, H.S.; Lee, D.W.; Choe, E.A.; Pyun, Y.R. Cloning, expression and characterization of l-arabinose isomerase from Thermotoga neapolitana: Bioconversion of d-galactose to d-tagatose using the enzyme. FEMS Microbiol. Lett. 2002, 212, 121–126. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, Y.W.; Roh, H.J.; Kim, H.Y.; Cha, J.H.; Park, K.H.; Park, C.S. Production of tagatose by a recombinant thermostable l-arabinose isomerase from Thermus sp. IM6501. Biotechnol. Lett. 2003, 25, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Mordukhova, E.A.; Lee, H.S.; Pan, J.G. Improved thermostability and acetic acid tolerance of Escherichia coli via directed evolution of homoserine o-succinyltransferase. Appl. Environ. Microbiol. 2008, 74, 7660–7668. [Google Scholar] [CrossRef] [Green Version]
- Gur, E.; Biran, D.; Gazit, E.; Ron, E.Z. In vivo aggregation of a single enzyme limits growth of Escherichia coli at elevated temperatures. Mol. Microbiol. 2002, 46, 1391–1397. [Google Scholar] [CrossRef]
- van de Vossenberg, J.L.; Ubbink-Kok, T.; Elferink, M.G.; Driessen, A.J.; Konings, W.N. Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol. Microbiol. 1995, 18, 925–932. [Google Scholar] [CrossRef] [Green Version]
- Ron, E.Z.; Davis, B.D. Growth rate of Escherichia coli at elevated temperatures: Limitation by methionine. J. Bacteriol. 1971, 107, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Manjasetty, B.A.; Chance, M.R. Crystal structure of Escherichia coli l-arabinose isomerase (ECAI), the putative target of biological tagatose production. J. Mol. Biol. 2006, 360, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, S.J.; Kim, S.B.; Lee, S.J.; Lee, S.H.; Lee, D.W. Structural insights into conserved l-arabinose metabolic enzymes reveal the substrate binding site of a thermophilic l-arabinose isomerase. FEBS Lett. 2014, 588, 1064–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Li, S.; Liang, J.; Feng, X.; Xu, H. Protein purification, crystallization and preliminary X-ray diffraction analysis of l-arabinose isomerase from Lactobacillus fermentum CGMCC2921. Acta Crystallogr. F-Struct. Biol. Commun. 2015, 71, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Torres, P.R.; Manzo, R.M.; Rubiolo, A.C.; Batista-Viera, F.D.; Mammarella, E.J. Purification of an l-arabinose isomerase from Enterococcus faecium DBFIQ E36 employing a biospecific affinity strategy. J. Mol. Catal. B-Enzym. 2014, 102, 99–105. [Google Scholar] [CrossRef]
- Hong, Y.H.; Lee, D.W.; Pyun, Y.R.; Lee, S.H. Creation of metal-independent hyperthermophilic l-arabinose isomerase by homologous recombination. J. Agric. Food Chem. 2011, 59, 12939–12947. [Google Scholar] [CrossRef]
- Liang, M.; Chen, M.; Liu, X.; Zhai, Y.; Liu, X.W.; Zhang, H.; Xiao, M.; Wang, P. Bioconversion of d-galactose to d-tagatose: Continuous packed bed reaction with an immobilized thermostable l-arabinose isomerase and efficient purification by selective microbial degradation. Appl. Microbiol. Biotechnol. 2012, 93, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.S.; Kim, H.J.; Oh, D.K. Tagatose production by immobilized recombinant Escherichia coli cells containing Geobacillus stearothermophilus l-arabinose isomerase mutant in a packed-bed bioreactor. Biotechnol. Prog. 2005, 21, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Lee, D.W.; Lee, S.J.; Choe, E.A.; Kim, S.B.; Lee, Y.H.; Cheigh, C.I.; Pyun, Y.R. Production of d-tagatose at high temperatures using immobilized Escherichia coli cells expressing l-arabinose isomerase from Thermotoga neapolitana. Biotechnol. Lett. 2007, 29, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zabed, H.M.; Yun, J.; Yuan, J.; Zhang, Y.; Wang, Y.; Qi, X. Two-stage biosynthesis of d-tagatose from milk whey powder by an engineered Escherichia coli strain expressing l-arabinose isomerase from Lactobacillus plantarum. Bioresour. Technol. 2020, 305, 123010. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Li, M.; Jiang, B.; Zhang, T.; Chen, J. Whole-cell biosynthesis of d-tagatose from maltodextrin by engineered Escherichia coli with multi-enzyme co-expression system. Enzyme Microb. Technol. 2021, 145, 109747. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).