Root Canal Obturation by Electrochemical Precipitation of Calcium Phosphates
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Precipitation Experiments
2.3. Analysis of Precipitation Products
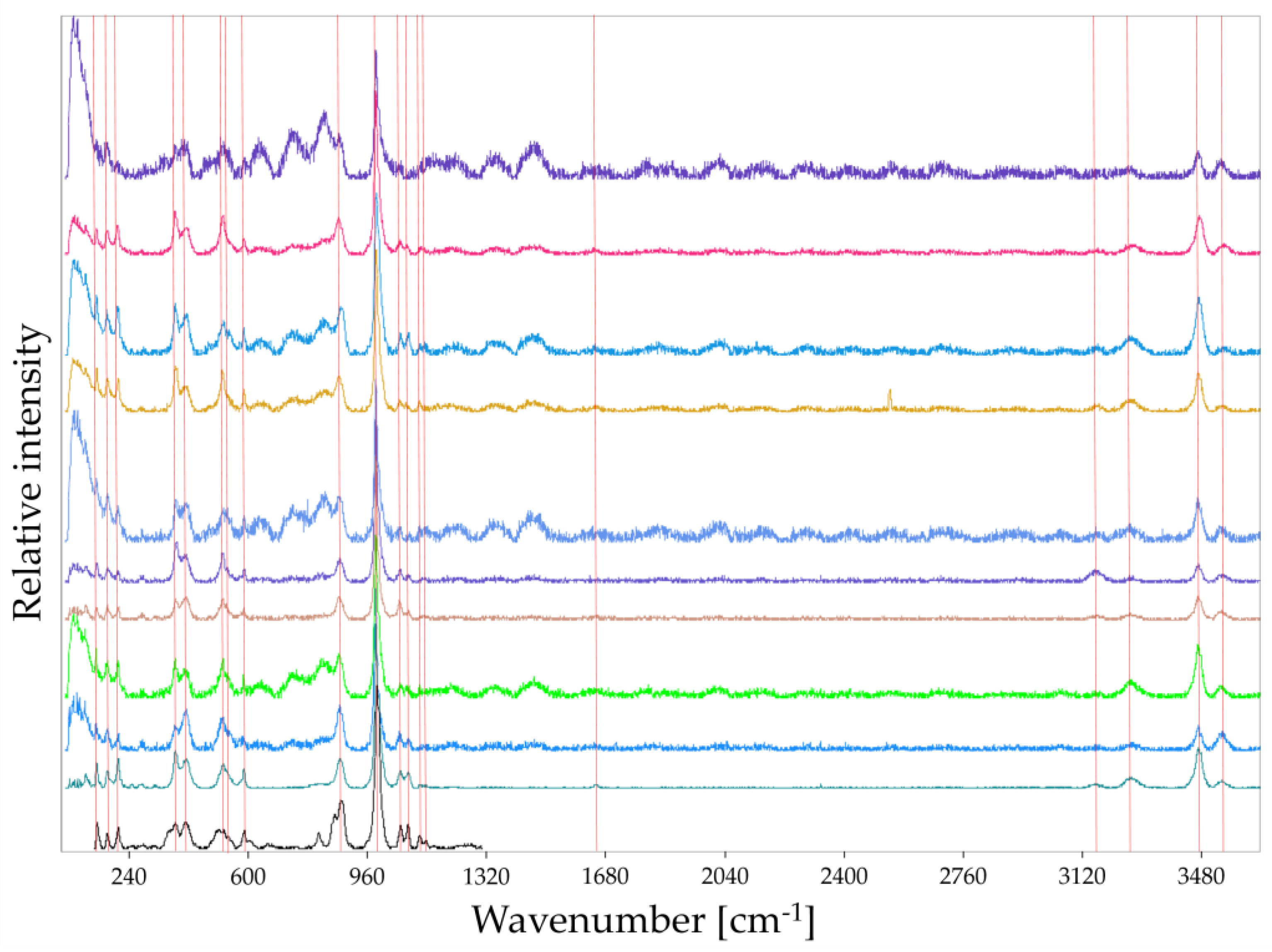
2.3.1. RAMAN Spectroscopy
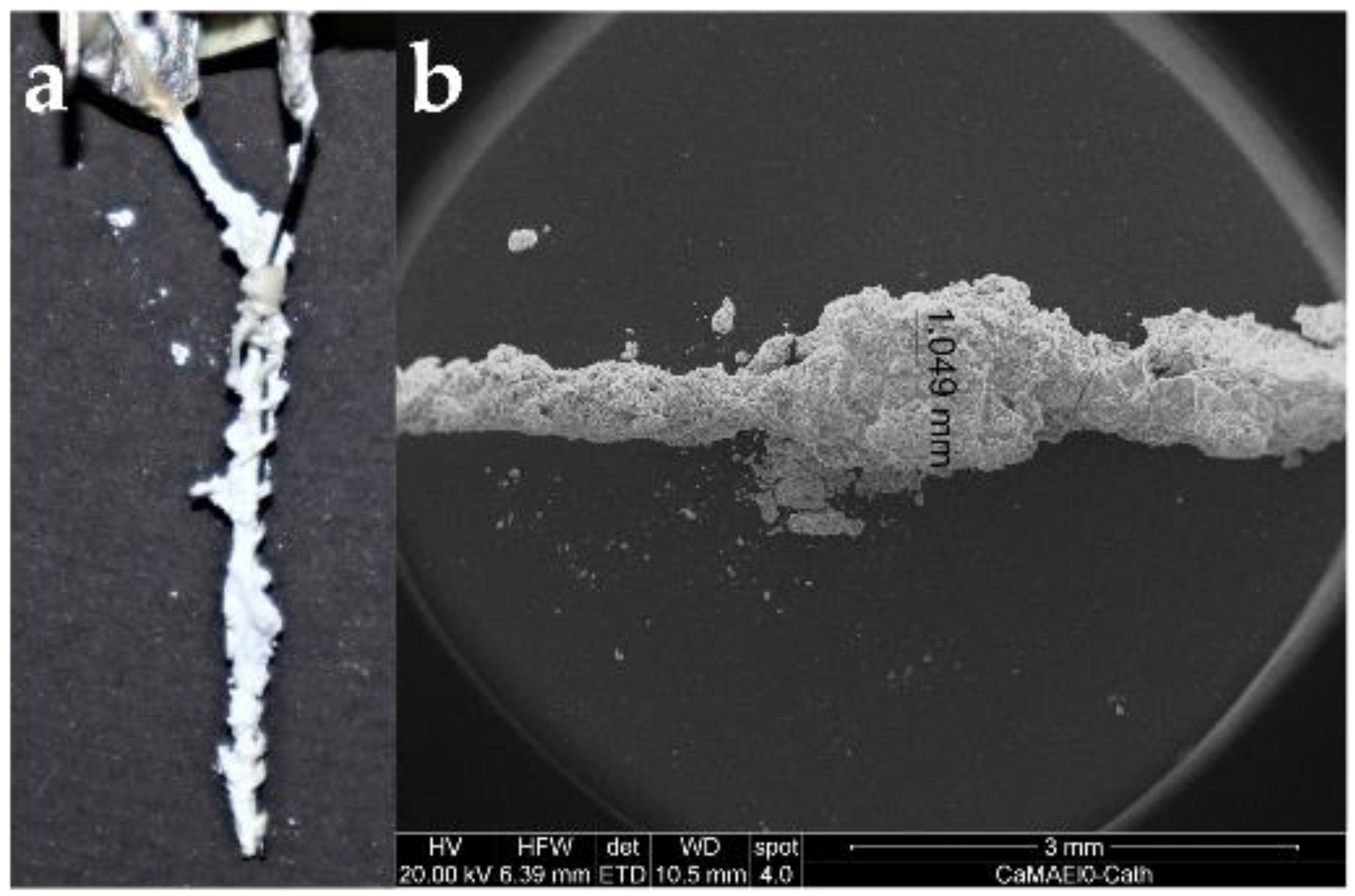
2.3.2. SEM/EDX Spectroscopy
2.4. Analysis of Maleate Degradation
2.5. Characterization of Sealing Quality
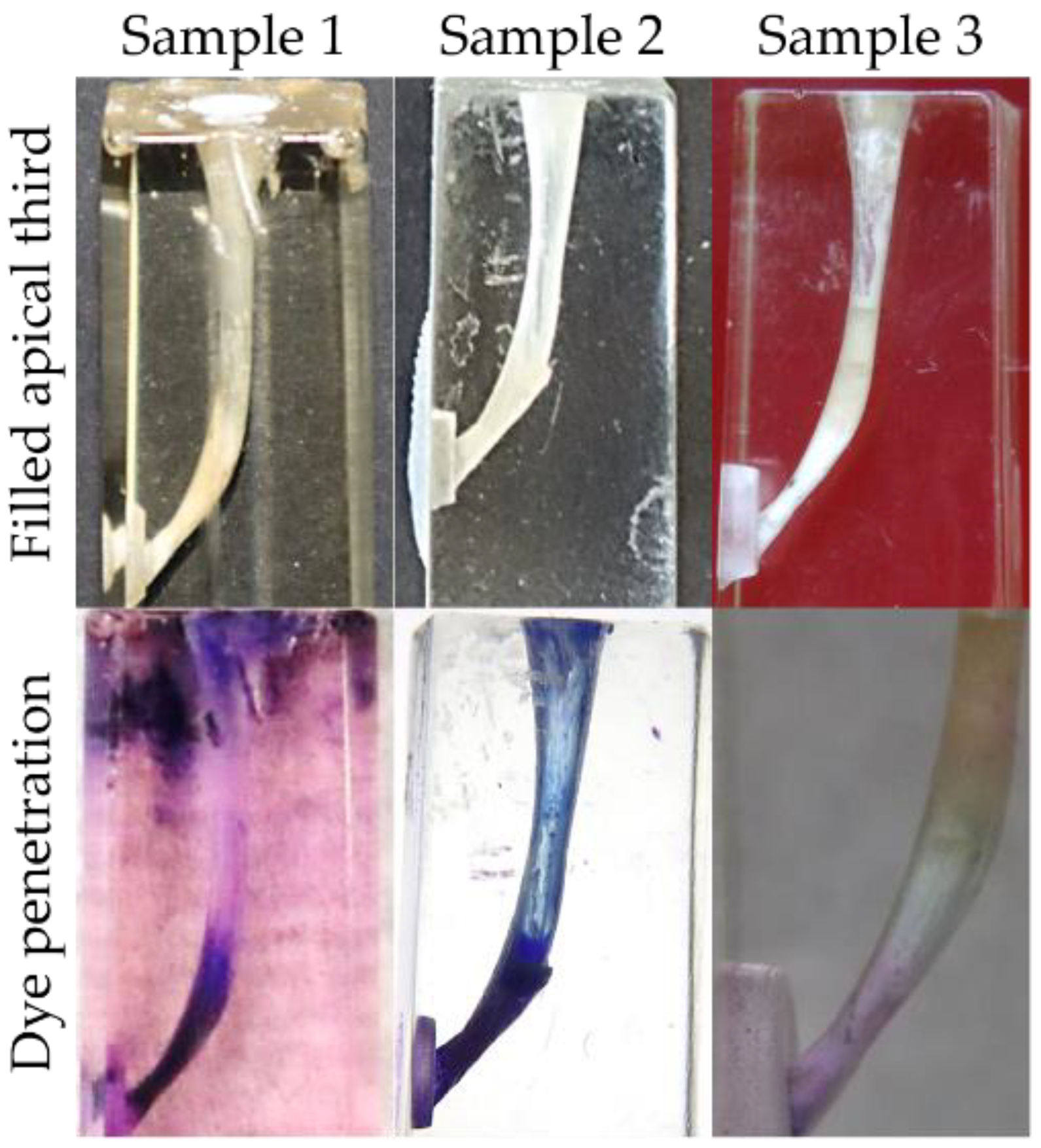
2.5.1. Dye Penetration Test
2.5.2. Imaging Analysis of Precipitate Density
3. Results
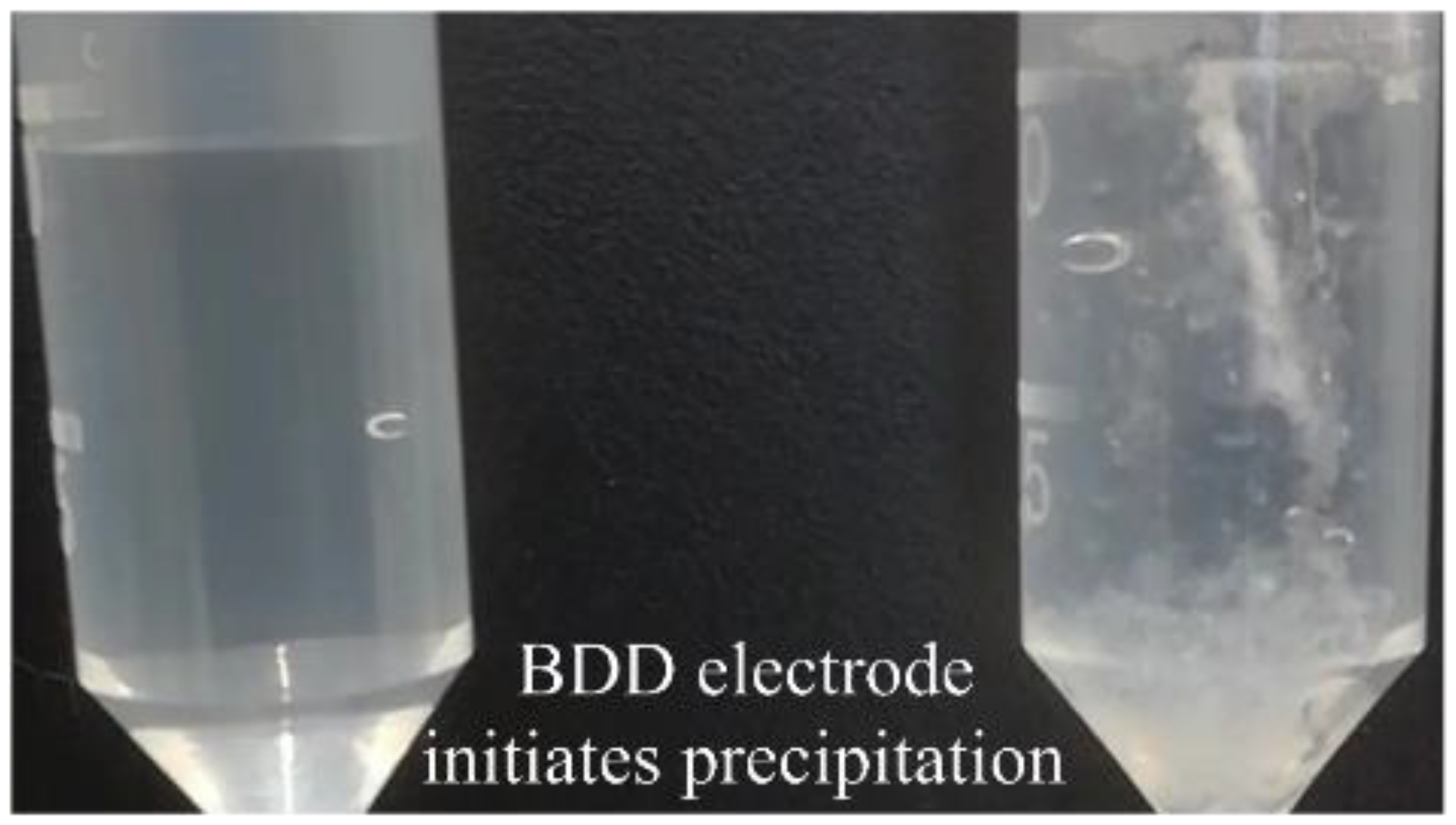
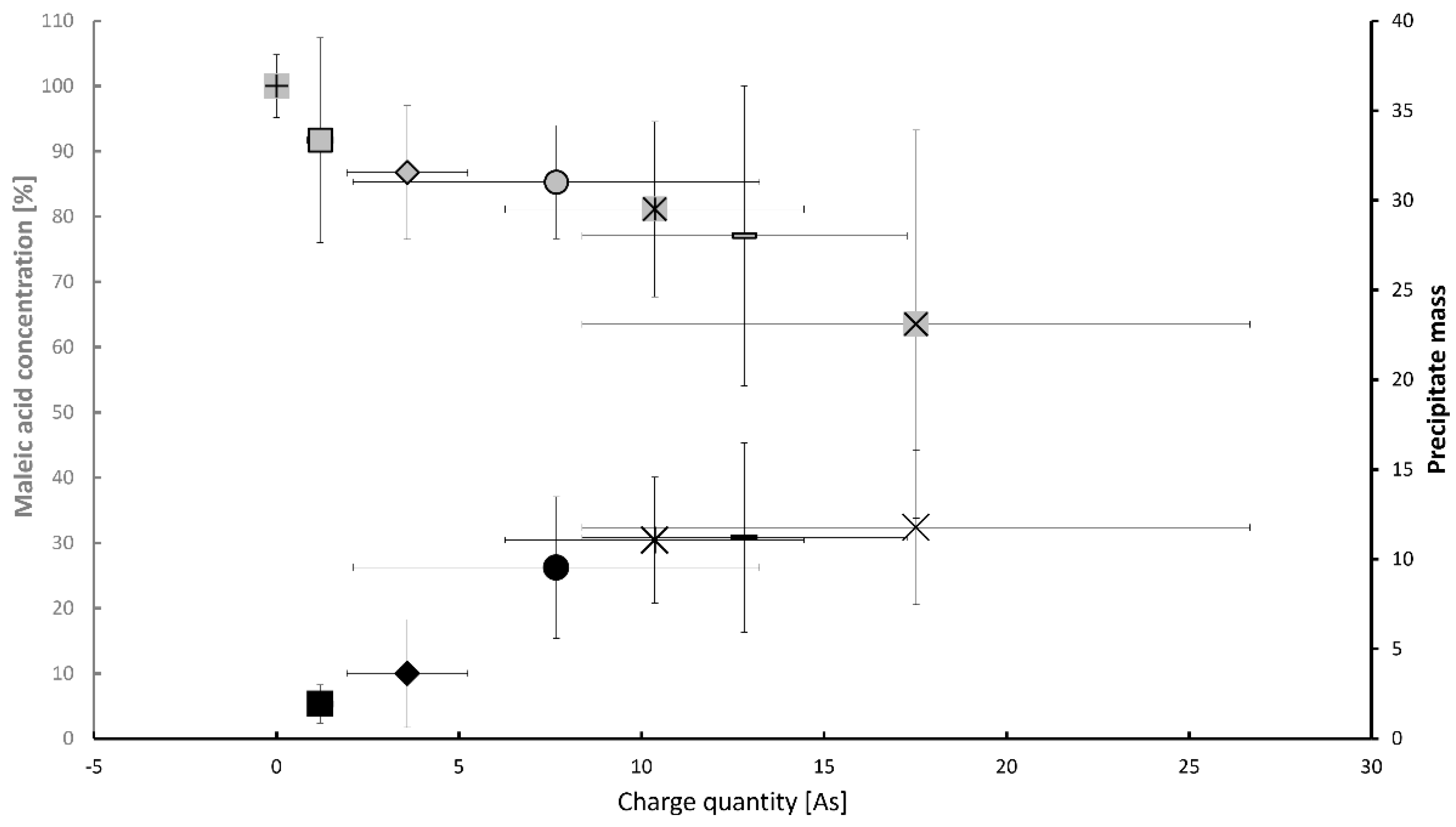
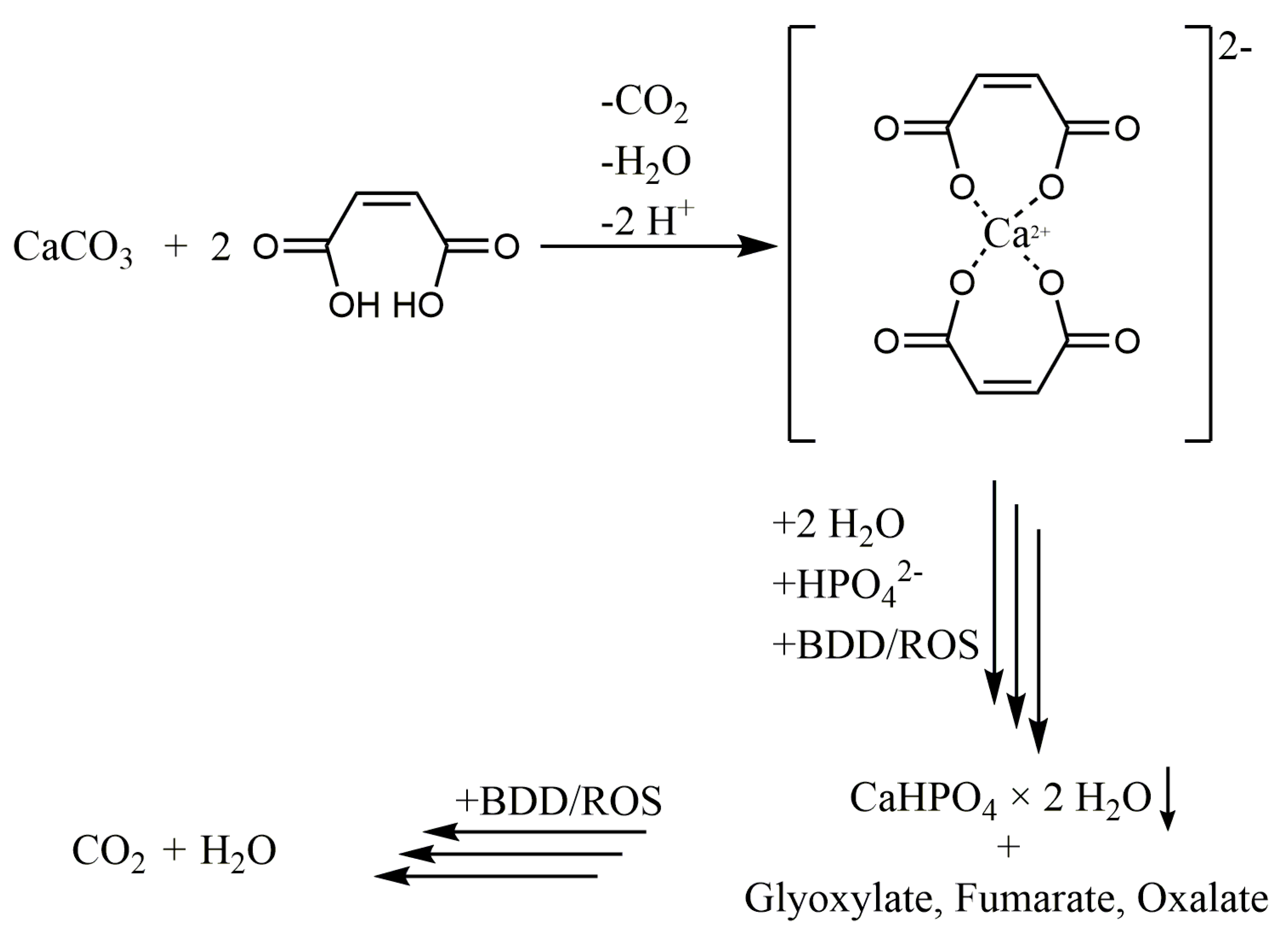
3.1. Maleic Acid as BDD Electrode-Degradable Retardant
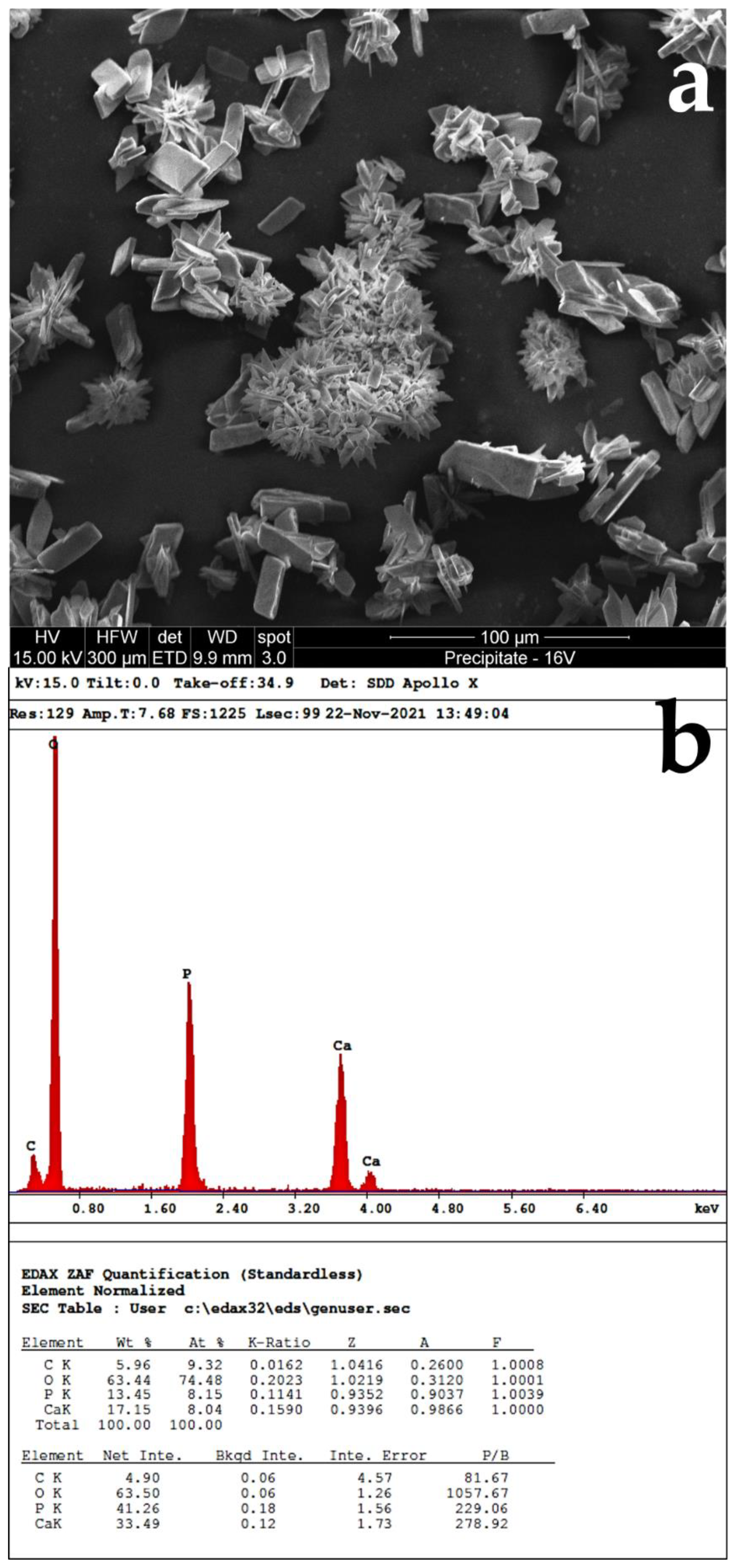
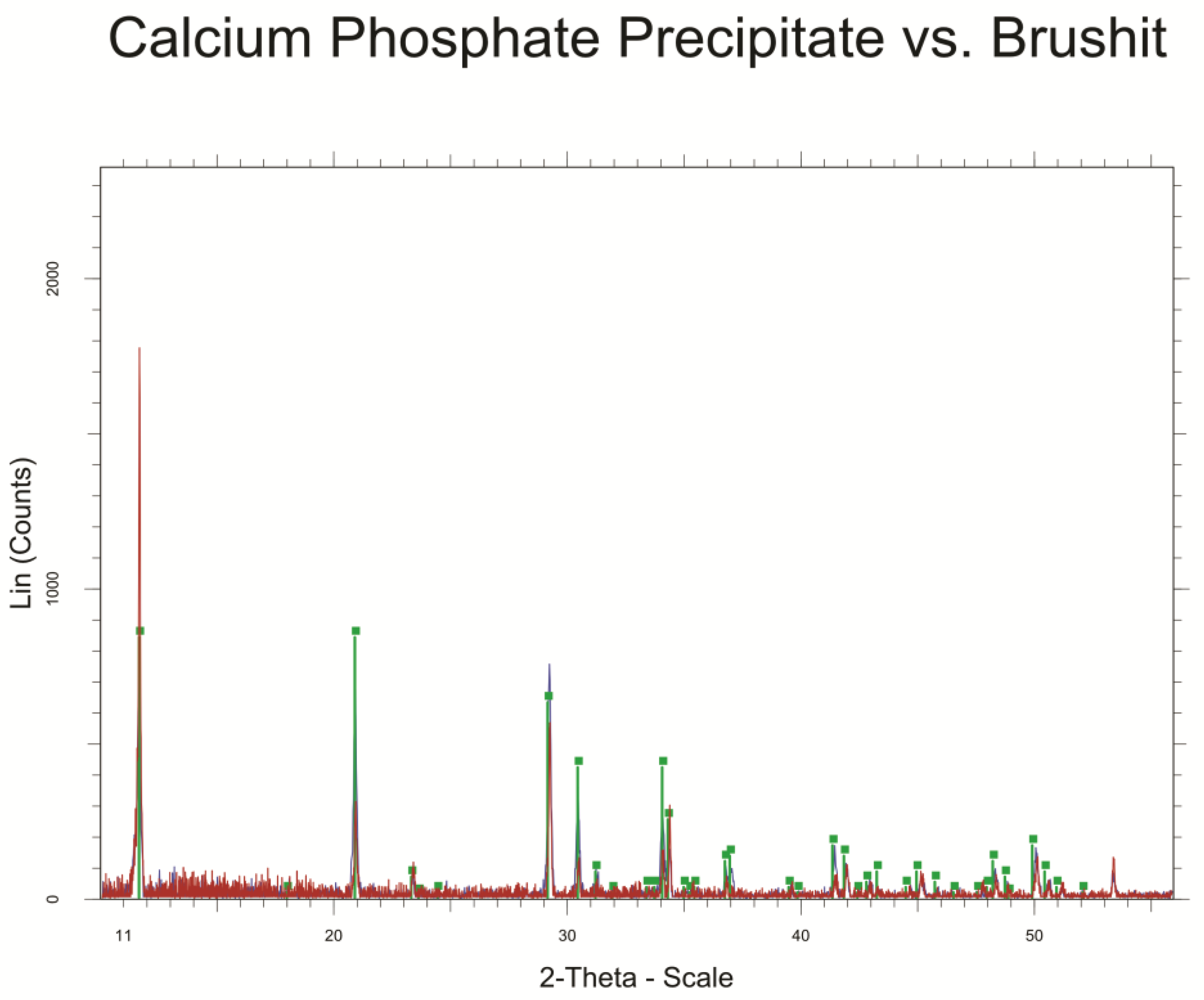
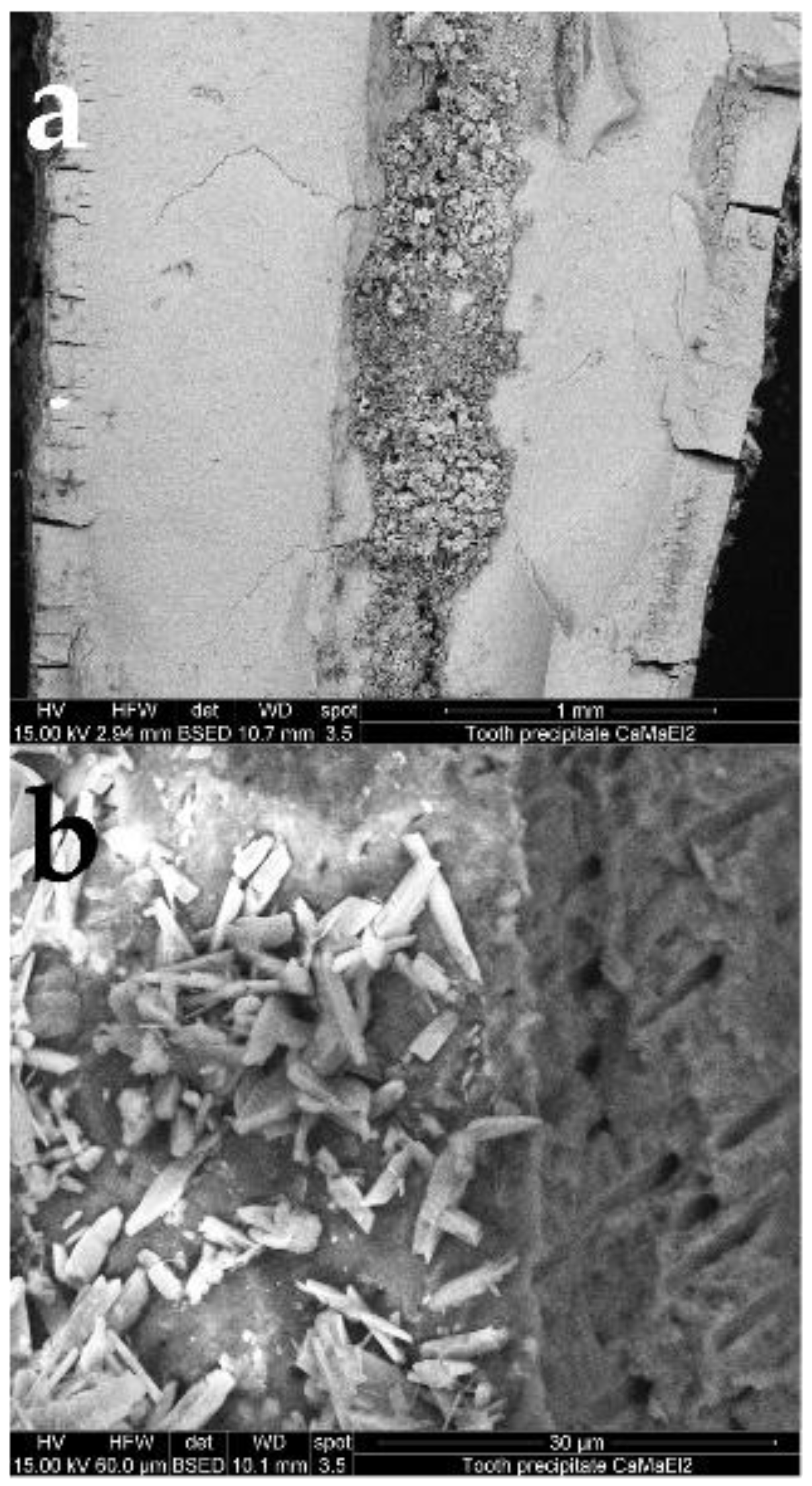
3.2. Analysis of Precipitated Calcium Phosphate Salts
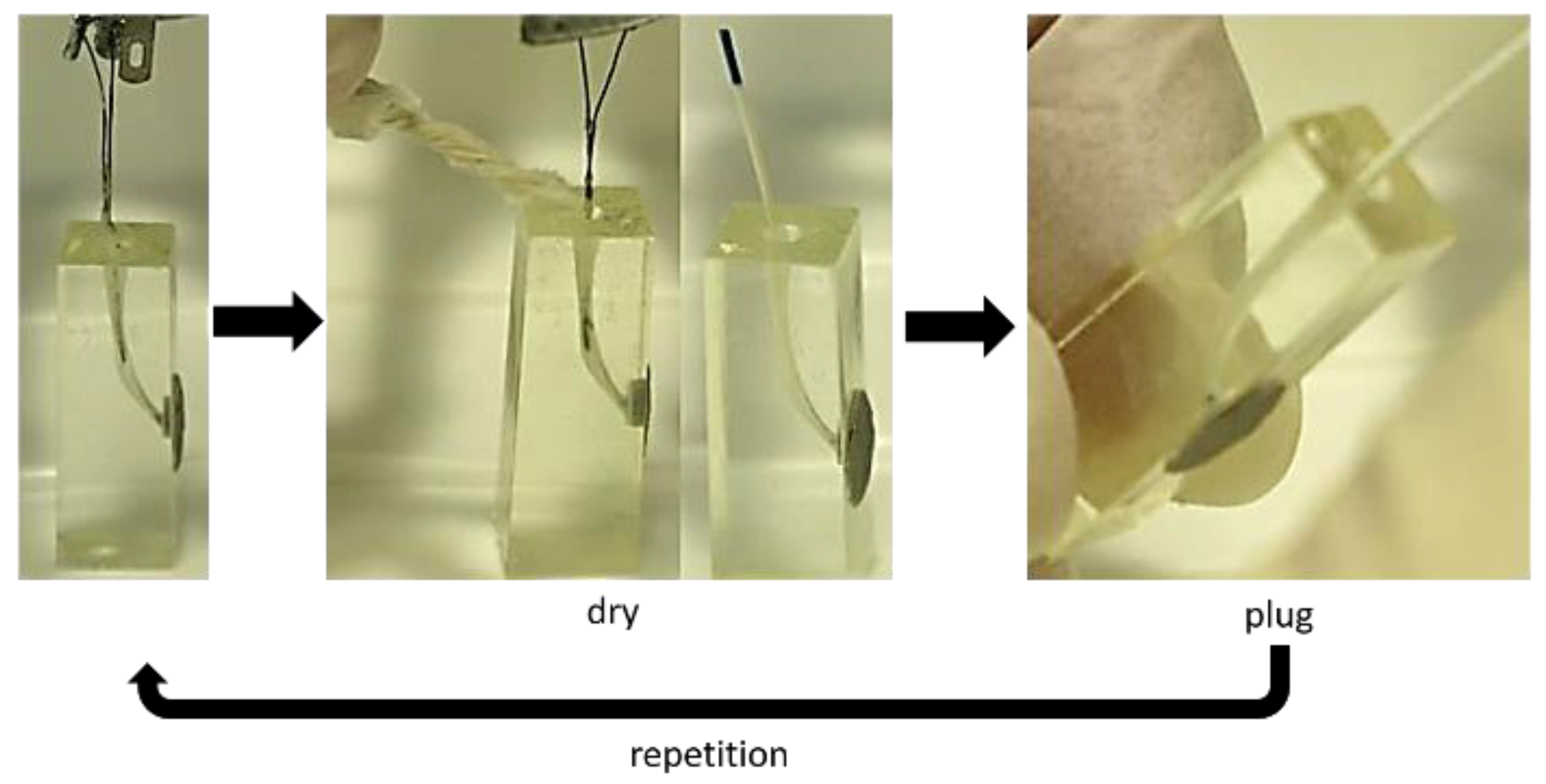
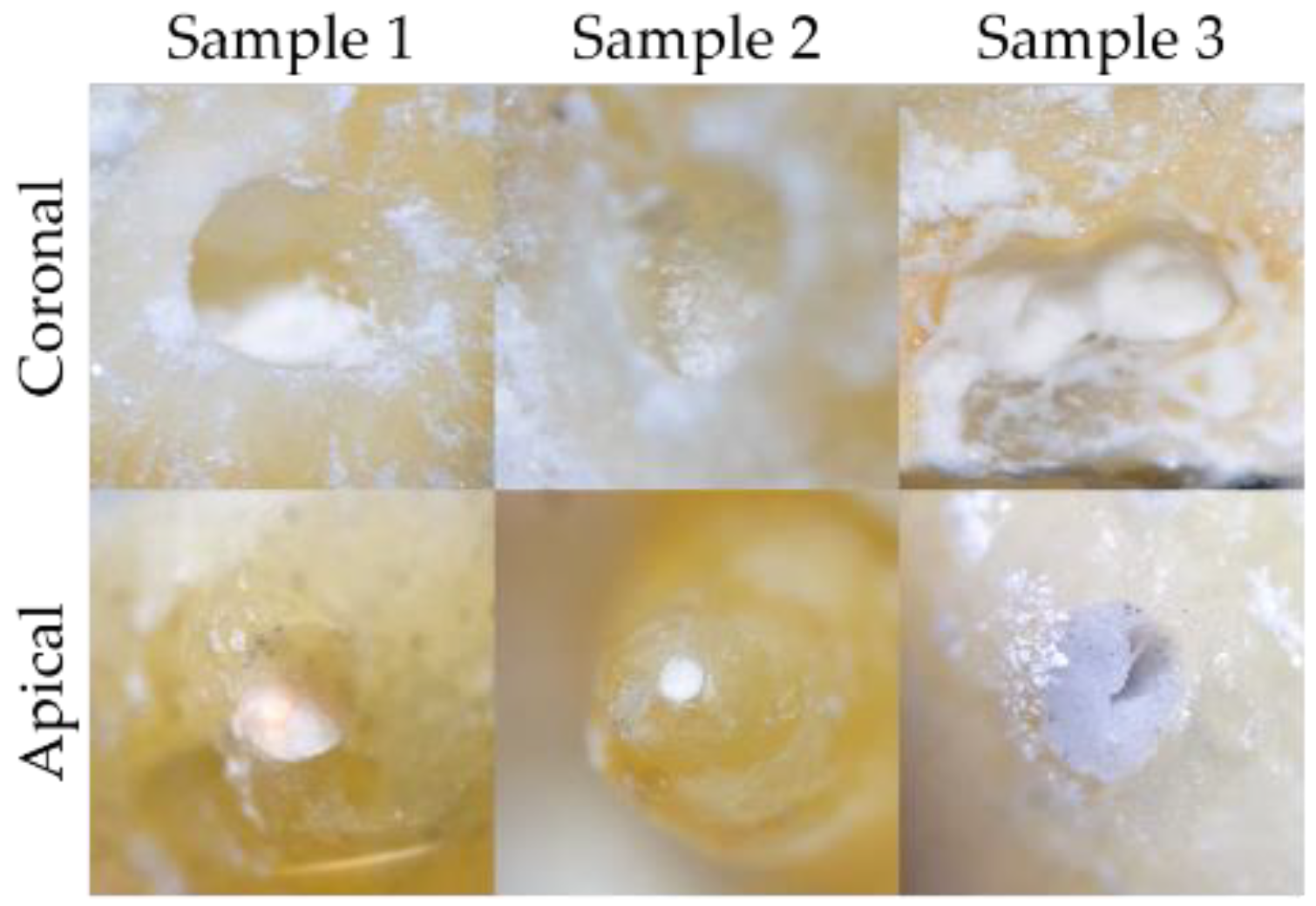
3.3. Obturation of Root Canals
3.4. Dye Penetration Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bergenholtz, G.; Spångberg, L. Controversies in endodontics. Crit. Rev. Oral Biol. Med. 2004, 15, 99–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.-S.; Kim, J.R.; Ling, J.; Choi, K.K.; Pashley, D.H.; Tay, F.R. Review of contemporary irrigant agitation techniques and devices. J. Endod. 2009, 35, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Löst, C. Quality guidelines for endodontic treatment: Consensus report of the european society of endodontology. Int. Endod. J. 2006, 39, 921–930. [Google Scholar]
- Pedrazzi, V.; Oliveira-Neto, J.M.; Sequeira-Byron, P.; Fedorowicz, Z.; Nasser, M. Hand and ultrasonic instrumentation for orthograde root canal treatment of permanent teeth. Cochrane Database Syst. Rev. 2019, 2, CD006384. [Google Scholar] [CrossRef]
- Peters, O.A. Current challenges and concepts in the preparation of root canal systems: A review. J. Endod. 2004, 30, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, J.F.; Rôças, I.N.; Ricucci, D. Internal tooth anatomy and root canal instrumentation. In The Root Canal Anatomy in Permanent Dentition, 1st ed.; Versiani, M.A., Basrani, B., Sousa-Neto, M.D., Eds.; Springer: Cham, Switzerland, 2018; pp. 277–302. [Google Scholar]
- Burkovski, A.; Karl, M. Lack of evidence for the necessity of root canal obturation. Quintessence Int. 2019, 50, 22–28. [Google Scholar]
- Hamedy, R.; Shakiba, B.; White, S.N. Essential elder endodontics. Gerodontology 2016, 33, 433. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, E.; Vehkalahti, M.M.; Kotiranta, A.K. Radiographic outcome of root canal treatment in general dental practice: Tooth type and quality of root filling as prognostic factors. Acta Odontol. Scand. 2021, 79, 37–42. [Google Scholar] [CrossRef]
- Zandi, H.; Petronijevic, N.; Mdala, I.; Kristoffersen, A.K.; Enersen, M.; Rôças, I.N.; Siqueira, J.F.; Ørstavik, D. Outcome of endodontic retreatment using 2 root canal irrigants and influence of infection on healing as determined by a molecular method: A randomized clinical trial. J. Endod. 2019, 45, 1089–1098.e5. [Google Scholar] [CrossRef]
- Sabeti, M.A.; Nekofar, M.; Motahhary, P.; Ghandi, M.; Simon, J.H. Healing of apical periodontitis after endodontic treatment with and without obturation in dogs. J. Endod. 2006, 32, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Keskin, G.; Çiloğlu, M. Efficacy of antimicrobial photodynamic therapy and Er, Cr:YSGG laser-activated irrigation on dentinal tubule penetration of MTA-based root canal sealer: A confocal microscopy study. Photodiagn. Photodyn. Ther. 2021, 36, 102584. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Everett, J.; Sidow, S.; Bergeron, B.E.; Tian, F.; Ma, J.; Tay, F.R. In vitro evaluation of efficacy of two endodontic sonic-powered irrigant agitation systems in killing single-species intracanal biofilms. J. Dent. 2021, 36, 103859. [Google Scholar] [CrossRef] [PubMed]
- Böhm, A.L.; Koch, M.; Rosiwal, S.; Burkovski, A.; Karl, M.; Grobecker-Karl, T. Electrochemical disinfection of experimentally infected teeth by boron-doped diamond electrode treatment. J.Clin. Med. 2019, 8, 2037. [Google Scholar] [CrossRef] [Green Version]
- Kolokuris, I.; Beltes, P.; Economides, N.; Vlemmas, I. Experimental study of the biocompatibility of a new glass-Ionomer root canal sealer (Ketac-Endo). J. Endod. 1996, 22, 395–398. [Google Scholar] [CrossRef]
- Scarparo, R.K.; Soares Grecca, F.; Vianna, E.; Fachin, F. Analysis of tissue reactions to methacrylate resin-based, epoxy resin-based, and zinc oxide-eugenol endodontic sealers. J. Endod. 2008, 35, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Niu, L.N.; Zhang, W.; Olsen, M.; De-Deus, G.; Eid, A.A.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Ability of new obturation materials to improve the seal of the root canal system—A review. Acta Biomater. 2014, 10, 1050. [Google Scholar] [CrossRef] [Green Version]
- Signoretti, F.G.C.; Endo, M.S.; Gomes, B.P.F.A.; Montagner, F.; Tosello, F.B.; Erio, R.; Jacinto, C. Persistent extraradicular infection in root-filled asymptomatic human tooth: Scanning electron microscopic analysis and microbial investigation after apical microsurgery. J. Endod. 2011, 37, 1696–1700. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Sun, W.-B.; Zhang, H. Amorphous calcium phosphate and its application in dentistry. Chem. Cent. J. 2011, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Lima, P.P.d.C.S.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative Efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 18. [Google Scholar] [CrossRef]
- Jung, S.; Sielker, S.; Hanisch, M.R.; Libricht, V.; Schäfer, E.; Dammaschke, T. Cytotoxic effects of four different root canal sealers on human osteoblasts. PLoS ONE 2018, 13, e0194467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepla, E. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Pobloth, A.M.; Mersiowsky, M.J.; Kliemt, L.; Schell, H.; Dienelt, A.; Pfitzner, B.M.; Burgkart, R.; Detsch, R.; Wulsten, D.; Boccaccini, A.R.; et al. Bioactive coating of zirconia toughened alumina ceramic implants improves cancellous osseointegration. Sci. Rep. 2019, 9, 16692. [Google Scholar] [CrossRef] [PubMed]
- Bayani, M.; Torabi, S.; Shahnaz, A.; Pourali, M. Main properties of nanocrystalline hydroxyapatite as a bone graft material in treatment of periodontal defects. A review of literature. Biotechnol. Biotechnol. Equip. 2017, 31, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Abbassy, M.A.; Bakry, A.S.; Almoabady, E.H.; Almusally, S.M.; Hassan, A.H. Characterization of a novel enamel sealer for bioactive remineralization of white spot lesions. J. Dent. 2021, 109, 103663. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.S.; Takahashi, H.; Otsuki, M.; Tagami, J. Evaluation of new treatment for incipient enamel demineralization using 45S5 bioglass. Dent. Mater. 2014, 30, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, S.-A.; Nancollas, G.H. The role of brushite and octacalcium phosphate in apatite formation. Crit. Rev. Oral. Biol. Med. 1992, 3, 61–82. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Peacock, C.L.; Benning, L.G. Carboxylic acids: Effective inhibitors for calcium sulfate precipitation? Mineral. Mag. 2014, 78, 1465–1472. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, H.; Huang, X.; Yi, L. Zinc maleate and calcium stearate as a complex thermal stabilizer for Poly(Vinyl Chloride). J. Vinyl Addit. Technol. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Bensalah, N.; Louhichi, B.; Abdel-Wahab, A. Electrochemical oxidation of succinic acid in aqueous solutions using boron doped diamond anodes. Int. J. Environ. Sci. Technol. 2012, 9, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Griesbach, U.; Malkowsky, I.M.; Waldvogel, S.R. Green electroorganic synthesis using BDD electrodes. In Electrochemistry for the Environment, 1st ed.; Comninellis, C., Chen, G., Eds.; Springer: New York, NY, USA, 2010; pp. 125–141. [Google Scholar]
- McBeath, S.T.; Wilkinson, D.P.; Graham, N.J.D. Application of boron-doped diamond electrodes for the anodic oxidation of pesticide micropollutants in a water treatment process: A critical review. Environ. Sci. Water Res. Technol. 2019, 5, 2090–2107. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Divyapriya, G.; Oturan, N.; Trellu, C.; Oturan, M.A. Environmental applications of boron-doped diamond electrodes: 1. Applications in water and wastewater treatment. ChemElectroChem 2019, 6, 2124–2142. [Google Scholar] [CrossRef]
- Dirany, A.; Sirés, I.; Oturan, N.; Özcan, A.; Oturan, M.A. Electrochemical treatment of the antibiotic sulfachloropyridazine: Kinetics, reaction pathways, and toxicity evolution. Environ. Sci. Technol. 2012, 46, 4074–4082. [Google Scholar] [CrossRef]
- Fu, B.; Yuan, J.; Qian, W.; Shen, Q.; Sun, X.; Hannig, M. Evidence of chemisorption of maleic acid to enamel and hydroxyapatite. Eur. J. Oral Sci. 2004, 112, 362–367. [Google Scholar] [CrossRef]
- Göltz, M.; Koch, M.; Detsch, R.; Karl, M.; Burkovski, A.; Rosiwal, S. Influence of in-situ electrochemical oxidation on implant surface and Colonizing Microorganisms Evaluated by Scanning Electron Microscopy. Materials 2019, 12, 3977. [Google Scholar] [CrossRef] [Green Version]
- Koch, M.; Göltz, M.; Xiangjun, M.; Karl, M.; Rosiwal, S.; Burkovski, A. Electrochemical disinfection of dental implants experimentally contaminated with microorganisms as a model for periimplantitis. J. Clin. Med. 2020, 9, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuscó, R.; Guitián, F.; de Aza, A.; Artús, L. Differentiation between hydroxyapatite and β-tricalcium phosphate by means of μ-raman spectroscopy. J. Eur. Ceram. Soc. 1998, 18, 1301–1305. [Google Scholar] [CrossRef]
- Miller, M.A.; Kendall, M.R.; Jain, M.K.; Larson, P.R.; Madden, A.S.; Tas, A.C. Testing of brushite (CaHPO4·2 H2O) in synthetic biomineralization solutions and in situ crystallization of brushite micro-granules. J. Am. Ceram. Soc. 2012, 95, 2178–2188. [Google Scholar] [CrossRef]
- Boanini, E.; Silingardi, F.; Gazzano, M.; Bigi, A. Synthesis and hydrolysis of brushite (DCPD): The role of ionic substitution. Cryst. Growth Des. 2021, 21, 1689–1697. [Google Scholar] [CrossRef]
- Kalantar Motamedi, M.R.; Mortaheb, A.; Zare Jahromi, M.; Gilbert, B.E. Micro-CT evaluation of four root canal obturation techniques. Scanning 2021, 2021, 6632822. [Google Scholar] [CrossRef] [PubMed]
- Zuolo, M.L.; Zaia, A.A.; Belladonna, F.G.; Silva, E.J.N.L.; Souza, E.M.; Versiani, M.A.; Lopes, R.T.; De-Deus, G. Micro-CT assessment of the shaping ability of four root canal instrumentation systems in oval-shaped canals. Int. Endod. J. 2018, 51, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, A.; Williams, R.L.; Cox, S.C.; Grover, L.M. Visualising phase change in a brushite-based calcium phosphate ceramic. Sci. Rep. 2016, 6, 32671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography, 1st ed.; Armbruster, T., Danisi, R.M., Eds.; De Gruyter: Berlin, Germany; Munich, Germany; Boston, MA, USA, 2015; pp. 1–30. [Google Scholar]
- Montes-Hernandez, G. Nucleation of brushite and hydroxyapatite from amorphous calcium phosphate phases revealed by dynamic in situ raman spectroscopy. J. Phys. Chem. C 2020, 124, 15302–15311. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, N.; van Landuyt, P.; Flautre, B.; Hardouin, P.; Lemaître, J.; Leroy, G. Raman microspectrometry studies of brushite cement: In vivo evolution in a sheep model. Bone 1999, 25, 81–84. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, M.; Palarie, V.; Göltz, M.; Kurzer, M.; Zulla, M.; Rosiwal, S.; Willner, M.; Burkovski, A.; Karl, M. Root Canal Obturation by Electrochemical Precipitation of Calcium Phosphates. Appl. Sci. 2022, 12, 2956. https://doi.org/10.3390/app12062956
Koch M, Palarie V, Göltz M, Kurzer M, Zulla M, Rosiwal S, Willner M, Burkovski A, Karl M. Root Canal Obturation by Electrochemical Precipitation of Calcium Phosphates. Applied Sciences. 2022; 12(6):2956. https://doi.org/10.3390/app12062956
Chicago/Turabian StyleKoch, Maximilian, Victor Palarie, Maximilian Göltz, Marvin Kurzer, Manuel Zulla, Stefan Rosiwal, Marian Willner, Andreas Burkovski, and Matthias Karl. 2022. "Root Canal Obturation by Electrochemical Precipitation of Calcium Phosphates" Applied Sciences 12, no. 6: 2956. https://doi.org/10.3390/app12062956
APA StyleKoch, M., Palarie, V., Göltz, M., Kurzer, M., Zulla, M., Rosiwal, S., Willner, M., Burkovski, A., & Karl, M. (2022). Root Canal Obturation by Electrochemical Precipitation of Calcium Phosphates. Applied Sciences, 12(6), 2956. https://doi.org/10.3390/app12062956







