The Improvement of Reserve Polysaccharide Glycogen Level and Other Quality Parameters of S. cerevisiae Brewing Dry Yeasts by Their Rehydration in Water, Treated with Low-Temperature, Low-Pressure Glow Plasma (LPGP)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Plasma-Treated Water under Air Atmosphere (PTWAir)
2.2.2. Plasma-Treated Water under Nitrogen (PTWN)
2.2.3. Rehydration of Dry Yeast
2.2.4. Determination of Yeast Cell Count Using Thoma Chamber
2.2.5. Determination of Yeast Viability
2.2.6. Determination of Yeast Acidity
2.2.7. Determination of Maltolytic and Saccharolytic Activity (Indirect Method)
2.2.8. Determination of Yeast Flocculation
2.2.9. Determination of Trehalose Content
2.3. Determination of Glycogen Content
2.3.1. Preparation of Samples for Observation with Scanning Electron Microscopy
2.3.2. Determination of Calcium, Magnesium and Zinc Ions by Atomic Absorption Spectrometry (AAS)
2.3.3. Fermentation Trials
2.4. Statistical Analyses
3. Results and Discussion
3.1. Number of Yeast
3.2. Viability
3.3. Floccualtion
3.4. Acidity
3.5. Maltolytic and Saccharolytic Activity
3.6. Trehalose and Glycogen Concentration
3.7. Cation Content in Yeast Biomass
| Parameters | Number of Yeast (log CFU/mL) | Viability (%) | Flocculation (%) | Acidity (mL of 1 M NaOH per 100 g of Dry Yeast Mass) | Maltolytic Activity (mL of CO2 per 0.1 g of Dry Yeast mass for 12 h) | Saccharolytic Activity (mL of CO2 per 0.1 g of Dry Yeast Mass for 12 h) | Trehalose (% ww) | Glycogen (% ww) | Mg (mg/g of Dry Yeast Mass) | Zn (mg/g of Dry Yeast Mass) | Ca (mg/g of Dry Yeast Mass) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae Safbrew US-05 (fresh strain) | PTWAir | 8.16 ± 1.25 | 72.3 ± 4.74 a | 32.3 ± 2.06 a | 4.76 ± 0.06 a | 24.7 ± 1.05 a | 37.8 ± 1.4 a | 2.07 ± 0.01 a | 18.2 ± 0.66 | 1.15 ± 0.04 | 0.55 ± 0.02 | 0.32 ± 0.03 |
| PTWN | 8.05 ± 0.73 | 49.3 ± 5.63 b | 20.6 ± 1.25 b | 6.23 ± 0.06 b | 20.8 ± 1.36 b | 30.4 ± 0.77 b | 4.03 ± 0.02 b | 18.9 ± 0.56 | 1.00 ± 0.1 | 0.40 ± 0.4 | 0.29 ± 0.05 | |
| CW | 8.15 ± 0.94 | 76.6 ± 4.19 aA | 24.5 ± 2.00 cA | 5.06 ± 0.15 aA | 27.6 ± 1.65 aA | 37.2 ± 1.3 aA | 4.16 ± 0.01 cA | 18.4 ± 0.38 A | 1.07 ± 0.04 A | 0.53 ± 0.04 A | 0.35 ± 0.04A | |
| Sig. | Water 2 | ns | ** | * | ** | ** | ** | *** | ns | ns | ns | ns |
| Saccharomyces cerevisiae Safbrew T58 (poor quality) | PTWAir | 8.55 ± 0.89 a | 58.8 ± 8.02 a | 29.9 ± 1.74 a | 5.96 ± 0.15 | 23.2 ± 1.1 a | 51.0 ± 1.24 a | 4.14 ± 0.02 a | 15.6 ± 0.14 a | 0.64 ± 0.03 | 0.07 ± 0.006 | 0.25 ± 0.04 |
| PTWN | 7.66 ± 0.29 b | 32.3 ± 5.6 b | 45.6 ± 3.79 b | 5.63 ± 0.25 | 16.8 ± 0.86 b | 46.9 ± 1.75 b | 4.42 ± 0.02 b | 16.7 ± 0.35 b | 0.73 ± 0.02 | 0.05 ± 0.06 | 0.38 ± 0.05 | |
| CW | 8.51 ± 0.9 a | 43.8 ± 2.41 cB | 20.9 ± 7.5 Aa | 5.76 ± 0.25 B | 21.9 ± 1.78 aB | 53.5 ± 1.04 aB | 4.71 ± 0.01 cA | 11.4 ± 0.17 cB | 0.67 ± 0.03 A | 0.08 ± 0.003 B | 0.30 ± 0.03A | |
| Sig. | Water 2 | ** | *** | ** | ns | ** | * | ** | *** | ns | ns | ns |
| Saccharomyces cerevisiae Safbrew S33 (poor quality) | PTWAir | 8.00 ± 0.47 a | 41.9 ± 5.12 a | 2.73 ± 0.1 a | 4.83 ± 0.06 | 24.4 ± 1.1 a | 28.3 ± 0.95 a | 6.25 ± 0.05 a | 12.2 ± 0.33 a | 0.88 ± 0.02 | 0.24 ± 0.01 | 0.17 ± 0.04 |
| PTWN | 7.41 ± 0.34 b | 18.6 ± 3.72 b | 11.6 ± 0.79 b | 5.06 ± 0.06 | 18.5 ± 0.86 b | 23.2 ± 0.96 b | 6.93 ± 1.18 a | 10.7 ± 0.59 b | 1.09 ± 0.08 | 0.28 ± 0.03 | 0.17 ± 0.02 | |
| CW | 8.39 ± 0.58 c | 22.8 ± 3.2 Cb | 1.26 ± 0.02 cB | 4.46 ± 0.21 C | 24.3 ± 1.78 aA | 28.3 ± 1.17 Ca | 8.33 ± 0.03 bB | 10.3 ± 0.22 bB | 0.97 ± 0.05 B | 0.24 ± 0.01 C | 0.15 ± 0.04 B | |
| Sig. | Water2 | ** | *** | *** | ns | ** | * | * | * | ns | ns | ns |
| Saccharomyces pastorianus Saflager W30/70 (poor quality) | PTWAir | 7.12 ± 0.39 a | 55.6 ± 8.01 a | 60.2 ± 0.19 a | 3.26 ± 0.44 | 31.5 ± 2.85 a | 34.9 ± 2.23 a | 10.2 ± 0.05 a | 15.3 ± 0.03 a | 0.69 ± 0.03 | 0.20 ± 0.01 | 0.14 ± 0.01 |
| PTWN | 8.81 ± 0.58 b | 46.7 ± 4.49 a | 46.1 ± 0.73 b | 3.69 ± 0.16 | 25.5 ± 0.56 b | 29.0 ± 0.95 b | 8.23 ± 0.03 b | 7.37 ± 0.1 b | 0.68 ± 0.3 | 0.12 ± 0.09 | 0.12 ± 0.005 | |
| CW | 8.19 ± 0.49 b | 61.8 ± 3.36Db | 70.2 ± 0.06 cC | 3.52 ± 0.27D | 31.9 ± 1.66 cB | 34.7 ± 0.75 aA | 4.12 ± 0.02 cA | 14.3 ± 0.05 cC | 0.62 ± 0.02 A | 0.22 ± 0.03 C | 0.11 ± 0.02 B | |
| Sig. | Water 2 | *** | * | * | ns | * | ** | *** | *** | ns | ns | ns |
| Strain 1 | ns | *** | *** | *** | ** | ** | ** | *** | ** | ** | ** | |
| −1 | −0.5 | 0 | 0.5 | 1 | ||||||||
| Parameters | Viability of Biomass (%) | Trehalose (% ww) | Glycogen (% ww) | ||||
|---|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae Safbrew US-05 (fresh strain) | PTWAir | 83.3 ± 1.91 a | 6.76 ± 1.02 a | 17.1 ± 0.3 a | |||
| PTWN | 69.7 ± 1.43 b | 8.32 ± 0.00 b | 18.7 ± 0.43 b | ||||
| CW | 84.5 ± 3.48 aA | 4.17 ± 0.00 cA | 17.2 ± 0.15 aA | ||||
| Sig. | Water 2 | ** | *** | * | |||
| Saccharomyces cerevisiae Safbrew T58 (poor quality) | PTWAir | 71.3 ± 1.24 a | 15.1 ± 1.00 a | 14.2 ± 0.05 a | |||
| PTWN | 50.2 ±1.78 b | 16.1 ± 1.01 a | 9.8 ± 0.24 b | ||||
| CW | 70.1 ± 1.52 aB | 11.4 ± 0.65 bB | 11.6 ± 0.35 cB | ||||
| Sig. | Water 2 | ** | * | *** | |||
| Saccharomyces cerevisiae Safbrew S33 (poor quality) | PTWAir | 65.6 ± 2.87 a | 6.76 ± 1.02 a | 13.2 ± 0.1 a | |||
| PTWN | 54.5 ± 6.61 b | 9.35 ± 1.21 b | 11.6 ± 0.02 b | ||||
| CW | 68.8 ± 1.57 aB | 6.25 ± 0.00 aC | 10.3 ± 0.32 cC | ||||
| Sig. | Water 2 | * | * | ** | |||
| Saccharomyces pastorianus Saflager W30/70 (poor quality) | PTWAir | 74.3 ± 2.58 a | 15.6 ± 1.21 a | 13.4 ± 0.15 a | |||
| PTWN | 61.1 ± 2.18 b | 17.5 ± 0.59 b | 9.2 ± 0.01 b | ||||
| CW | 77.5 ± 2.09 aC | 10.4 ± 0.00 cD | 15.2 ± 0.05 cD | ||||
| Sig. | Water 2 | ** | *** | *** | |||
| Strain 1 | *** | *** | *** | ||||
| −1 | −0.5 | 0 | 0.5 | 1 | |||
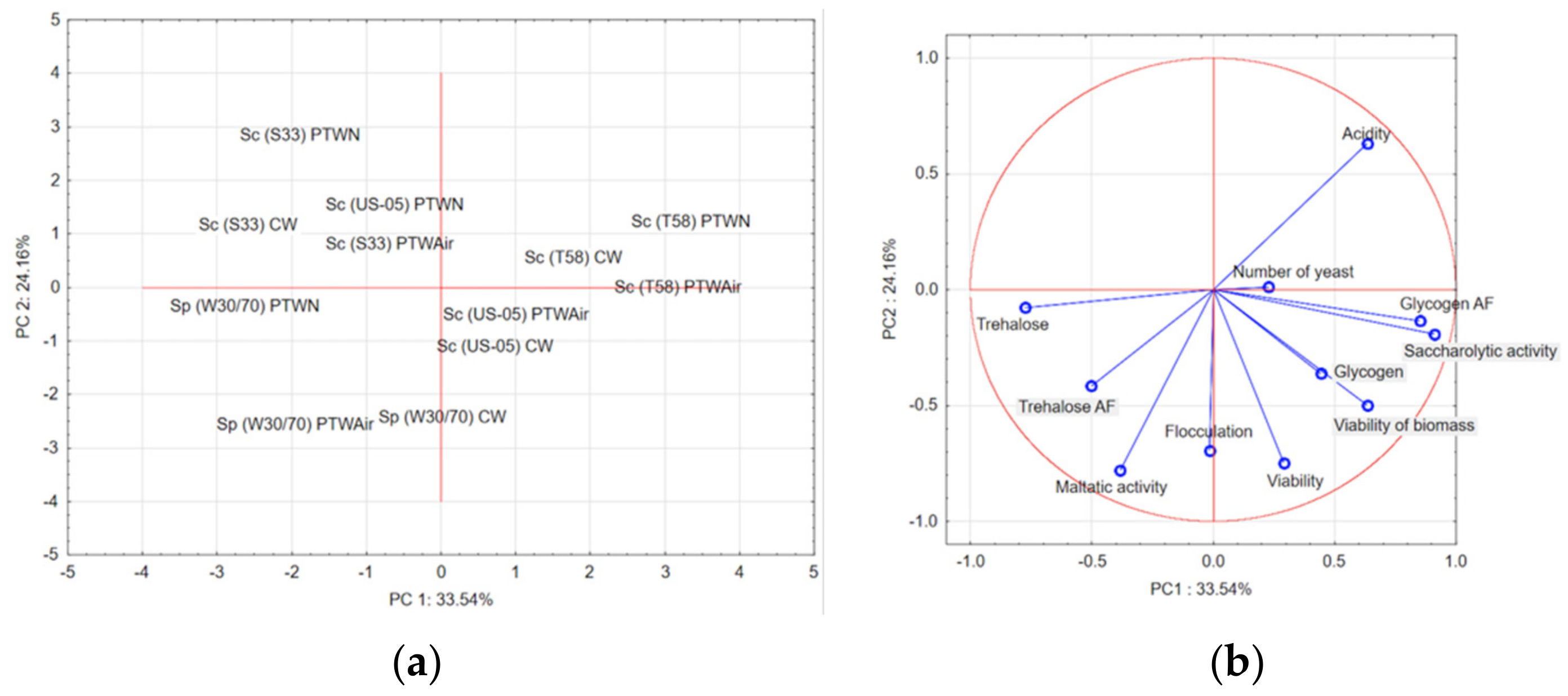
3.8. Principal Component Analyses (PCA)
3.9. Scanning Electron Microscopy (SEM) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canonico, L.; Galli, E.; Ciani, E.; Comitini, F.; Ciani, M. Exploitation of three non-conventional yeast species in the brewing process. Microorganisms 2019, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Kerby, C.; Vriesekoop, F. An Overview of the Utilisation of Brewery By-Products as Generated by British Craft Breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef]
- Finn, D.A.; Stewart, G.G. Fermentation characteristics of dried brewers yeast: Effect of drying on flocculation and fermentation. J. Am. Soc. Brew. Chem. 2002, 60, 135–139. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Satora, P. Rehydratacja drożdży piwowarskich®. Technol. Prog. Food Processing 2018, 2, 79–84. [Google Scholar]
- Diniz-Mendes, L.; Bernardes, E.; De Araujo, P.S.; Panek, A.D.; Paschoalin, V.M.F. Preservation of frozen yeast cells by trehalose. Biotechnol. Bioeng. 1999, 65, 572–578. [Google Scholar] [CrossRef]
- Bolat, I.; Turtoi, M.; Walsh, M. Influence of yeast drying process on different lager brewing strains viability. J. Agroaliment. Process. Technol. 2009, 15, 370–377. [Google Scholar]
- Luna-Solano, G.; Salgado-Cervantes, M.A.; Rodríguez-Jimenes, G.C.; García-Alvarado, M.A. Optimization of brewer’s yeast spray drying process. J. Food Eng. 2005, 68, 9–18. [Google Scholar] [CrossRef]
- Jenkins, D.M.; Powell, C.D.; Fischborn, T.; Smart, K.A. Rehydration of active dry brewing yeast and its effect on cell viability. J. Inst. Brew. 2011, 117, 377–382. [Google Scholar] [CrossRef]
- Walker, G.M.; Van Dijck, P. Physiological and Molecular Responses of Yeasts to the Environment. In Yeasts in Food and Beverages; Springer: Berlin/Heidelberg, Germany, 2006; pp. 111–152. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Comparing the efficiency of protein and maltodextrin on spray drying of bayberry juice. Food Res. Int. 2012, 48, 478–483. [Google Scholar] [CrossRef]
- Poirier, I.; Maréchal, P.A.; Richard, S.; Gervais, P. Saccharomyces cerevisiae viability is strongly dependant on rehydration kinetics and the temperature of dried cells. J. Appl. Microbiol. 1999, 86, 87–92. [Google Scholar] [CrossRef]
- Laroche, C.; Gervais, P. Achievement of rapid osmotic dehydration at specific temperatures could maintain high Saccharomyces cerevisiae viability. Appl. Microbiol. Biotechnol. 2003, 60, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Pater, A.; Zdaniewicz, M.; Satora, P.; Khachatryan, G.; Oszczęda, Z. Application of water treated with low-temperature low-pressure glow plasma for quality improvement of barley and malt. Biomolecules 2020, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Elkin, I. Pl 216025 B1. 2009. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.389626 (accessed on 10 January 2022).
- Białopiotrowciz, T.; Ciesielski, W.; Domanski, J.; Doskocz, M.; Khachatryan, K.; Fiedorowicz, M.; Graz, K.; Kołoczek, H.; Kozak, A.; Oszczeda, Z. Structure and Physicochemical Properties of Water Treated with Low-Temperature Low-Frequency Glow Plasma. Curr. Phys. Chem. 2016, 6, 312–320. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Kwak, H.S.; Park, J.H.; Uhm, H.S.; Bogaerts, A.; Choi, E.H.; Attri, P. Bacterial inactivation by plasma treated water enhanced by reactive nitrogen species. Sci. Rep. 2018, 8, 11268. [Google Scholar] [CrossRef]
- Ciesielski, W.; Gąstoł, M.; Kulawik, D.; Oszczęda, Z.; Pisulewska, E.; Tomasik, P. Specific controlling essential oil composition of basil (Ocimum basilicum l.) involving low-temperature, low-pressure glow plasma of low frequency. Water 2020, 12, 3332. [Google Scholar] [CrossRef]
- Murawski, M.; Schwarz, T.; Grygier, J.; Patkowski, K.; Oszczęda, Z.; Jelkin, I.; Kosiek, A.; Gruszecki, T.M.; Szymanowska, A.; Skrzypek, T.; et al. The utility of nanowater for ram semen cryopreservation. Exp. Biol. Med. 2015, 240, 611–617. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Szymanowicz, J.; Fryc, K.; Schwarz, T.; Murawski, M.; Bartlewski, P.M. Bovine in vitro embryo production using media prepared with Milli-Q® Water or nanowater. Reprod. Biol. 2020, 20, 580–583. [Google Scholar] [CrossRef]
- Szymanowicz, J.; Schwarz, T.; Murawski, M.; Małopolska, M.; Oszczeda, Z.; Tuz, R.; Nowicki, J.; Bartlewski, P.M. Storage of boar semen at 16–18 °C in the long-term commercial extender prepared with deionized water or nanowater. Anim. Reprod. 2019, 16, 864–870. [Google Scholar] [CrossRef]
- Jaworska, M.; Oszczȩda, Z.; Tomasik, P. Stimulation of pathogenicity of entomopathogenic fungi with water treated with low-temperature, low-pressure glow plasma of low frequency. Polish J. Nat. Sci. 2018, 33, 561–568. [Google Scholar]
- Uchida, G.; Takenaka, K.; Takeda, K.; Ishikawa, K.; Hori, M.; Setsuhara, Y. Selective production of reactive oxygen and nitrogen species in the plasma-treated water by using a nonthermal high-frequency plasma jet. Jpn. J. Appl. Phys. 2018, 57. [Google Scholar] [CrossRef]
- Boulton, C. Fermentation Systems; BSL: Oxford, UK, 2007; ISBN 063-2054-751. [Google Scholar]
- Polčic, P.; Machala, Z. Effects of non-thermal plasma on yeast saccharomyces cerevisiae. Int. J. Mol. Sci. 2021, 22, 2247. [Google Scholar] [CrossRef]
- ASBC Technical Committe. Yeast–11 Flocculation. ASBC Methods Anal. 2011, 5, 11–12. [Google Scholar] [CrossRef]
- Somani, A.; Bealin-Kelly, F.; Axcell, B.; Smart, K.A. Impact of storage temperature on lager brewing yeast viability, glycogen, trehalose, and fatty acid content. J. Am. Soc. Brew. Chem. 2012, 70, 123–130. [Google Scholar] [CrossRef]
- Attfield, P.V.; Kletsas, S.; Veal, D.A.; Van Rooijen, R.; Bell, P.J.L. Use of flow cytometry to monitor cell damage and predict fermentation activity of dried yeasts. J. Appl. Microbiol. 2000, 89, 207–214. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, H.; Shioya, S. Physiological analysis of yeast cells by flow cytometry during serial-repitching of low-malt beer fermentation. J. Biosci. Bioeng. 2007, 103, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.A.; Whisker, S. Effect of serial repitching on the fermentation properties and condition of brewing yeast. J. Am. Soc. Brew. Chem. 1996, 54, 41–44. [Google Scholar] [CrossRef]
- Nagodawithana, T.W.; Steinkraus, K.H. Influence of the rate of ethanol production and accumulation on the viability of Saccharomyces cerevisiae in “rapid fermentation”. Appl. Environ. Microbiol. 1976, 31, 158–162. [Google Scholar] [CrossRef]
- Stewart, G.G. Yeast flocculation—sedimentation and flotation. Fermentation 2018, 4, 28. [Google Scholar] [CrossRef]
- Bony, M.; Thines-Sempoux, D.; Barre, P.; Blondin, B. Localization and cell surface anchoring of the Saccharomyces cerevisiae flocculation protein Flo1p. J. Bacteriol. 1997, 179, 4929–4936. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Verachtert, H.; Delvaux, F.R. Yeast flocculation: What brewers should know. Appl. Microbiol. Biotechnol. 2003, 61, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.G.; Russell, I.; Garrison, I.F. Some Considerations of the Flocculation Characteristics of Ale and Lager Yeast Strains. J. Inst. Brew. 1975, 81, 248–257. [Google Scholar] [CrossRef]
- Crabtree, B. The Vital Force: A Study of Bioenergetics. Biochem. Soc. Trans. 1987, 15, 183. [Google Scholar] [CrossRef]
- Rowe, S.M.; Simpson, W.J.; Hammond, J.R.M. Intracellular pH of yeast during brewery fermentation. Lett. Appl. Microbiol. 1994, 18, 135–137. [Google Scholar] [CrossRef]
- Gibson, B.R.; Lawrence, S.J.; Leclaire, J.P.R.; Powell, C.D.; Smart, K.A. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol. Rev. 2007, 31, 535–569. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Xu, H.; Feng, L.; Yu, Z.; Zhao, H.; Zhao, M. Fermentation performance of lager yeast in high gravity beer fermentations with different sugar supplementations. J. Biosci. Bioeng. 2016, 122, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Alizadeh, P.; Harding, T.; Hefner-Gravink, A.; Klionsky, D.J. Disruption of the yeast ATH1 gene confers better survival after dehydration, freezing, and ethanol shock: Potential commercial applications. Appl. Environ. Microbiol. 1996, 62, 1563–1569. [Google Scholar] [CrossRef]
- Magalhães, R.S.S.; Popova, B.; Braus, G.H.; Outeiro, T.F.; Eleutherio, E.C.A. The trehalose protective mechanism during thermal stress in Saccharomyces cerevisiae: The roles of Ath1 and Agt1. FEMS Yeast Res. 2018, 18, foy066. [Google Scholar] [CrossRef] [PubMed]
- Aranda, J.S.; Salgado, E.; Taillandier, P. Trehalose accumulation in Saccharomyces cerevisiae cells: Experimental data and structured modeling. Biochem. Eng. J. 2004, 17, 129–140. [Google Scholar] [CrossRef]
- Bolat, I. The importance of trehalose in brewing yeast survival. Innov. Rom. Food Biotechnol. 2008, 2, 1–10. [Google Scholar]
- Silljé, H.H.W.; Paalman, J.W.G.; Ter Schure, E.G.; Olsthoorn, S.Q.B.; Verkleij, A.J.; Boonstra, J.; Verrips, C.T. Function of trehalose and glycogen in cell cycle progression and cell viability in Saccharomyces cerevisiae. J. Bacteriol. 1999, 181, 396–400. [Google Scholar] [CrossRef]
- Anderson, C.; Tatchell, K. Hyperactive glycogen synthase mutants of Saccharomyces cerevisiae suppress the glc7-1 protein phosphatase mutant. J. Bacteriol. 2001, 183, 821–829. [Google Scholar] [CrossRef][Green Version]
- Deshpande, P.S.; Sankh, S.N.; Arvindekar, A.U. Study of two pools of glycogen in saccharomyces cerevisiae and their role in fermentation performance. J. Inst. Brew. 2011, 117, 113–119. [Google Scholar] [CrossRef]
- Sall, C.J.; Seipp, J.F.; Pringle, A.T. Changes in Brewer’s Yeast during Storage and the Effect of These Changes on Subsequent Fermentation Performance. J. Am. Soc. Brew. Chem. 1988, 46, 23–25. [Google Scholar] [CrossRef][Green Version]
- Brosnan, M.P.; Donnelly, D.; James, T.C.; Bond, U. The stress response is repressed during fermentation in brewery strains of yeast. J. Appl. Microbiol. 2000, 88, 746–755. [Google Scholar] [CrossRef]
- Walker, M.D. The influence of metal ions on concentrations of flavour-active sulphur compounds measured in beer using dynamic headspace sampling. J. Sci. Food Agric. 1995, 67, 25–28. [Google Scholar] [CrossRef]
- Walker, G.M.; Maynard, A.I. Magnesium-limited growth of Saccharomyces cerevisiae. Enzyme Microb. Technol. 1996, 18, 455–459. [Google Scholar] [CrossRef]
- Miki, B.L.A.; Poon, N.H.; James, A.P.; Seligy, V.L. Possible mechanism for flocculation interactions governed by gene FLO1 in Saccharomyces cerevisiae. J. Bacteriol. 1982, 150, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Rees, E.M.R.; Stewart, G.G. Effects of magnesium, calcium and wort oxygenation on the fermentative performance of ale and lager strains fermenting normal and high gravity worts. J. Inst. Brew. 1999, 105, 211–218. [Google Scholar] [CrossRef]
- Trofimova, Y.; Walker, G.; Rapoport, A. Anhydrobiosis in yeast: Influence of calcium and magnesium ions on yeast resistance to dehydration-rehydration. FEMS Microbiol. Lett. 2010, 308, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mochaba, F.; O’Connor-Cox, E.S.C.; Axcell, B.C. Metal ion concentration and release by a brewing yeast: Characterization and implications. J. Am. Soc. Brew. Chem. 1996, 54, 155–163. [Google Scholar] [CrossRef]
- Bromberg, S.K.; Bower, P.A.; Duncombe, G.R.; Fehring, J.; Gerber, L.A.; Lau, V.K.; Tata, M. Requirements for zinc, manganese, calcium, and magnesium in wort. J. Am. Soc. Brew. Chem. 1997, 55, 123–128. [Google Scholar] [CrossRef]
- Bergmans, L.; Moisiadis, P.; Van Meerbeek, B.; Quirynen, M.; Lambrechts, P. Microscopic observation of bacteria: Review highlighting the use of environmental SEM. Int. Endod. J. 2005, 38, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, E.H.; Kim, K.M.; Kim, K.N. Effect of non-thermal air atmospheric pressure plasma jet treatment on gingival wound healing. J. Phys. D. Appl. Phys. 2016, 49, 75402. [Google Scholar] [CrossRef]
- Attfield, P.V. Stress tolerance: The key to effective strains of industrial baker’s yeast. Nat. Biotechnol. 1997, 15, 1351–1357. [Google Scholar] [CrossRef] [PubMed]

| Strain | Source | Manufacturer’s Recommendations | Storage Method |
|---|---|---|---|
| Saccharomyces cerevisiae SafAle T58 | Lesaffre Fermentis | After opening, the packaging should be resealed and stored in a dry room at 4 °C for a period not longer than 7 days | After opening, the yeast was resealed and stored at 4 °C for one year |
| Saccharomyces cerevisiae SafAle US-05 | Lesaffre Fermentis | Fresh yeast | |
| Saccharomyces cerevisiae SafAle S33 | Lesaffre Fermentis | After opening, the yeast was resealed and stored at 4 °C for one year | |
| Saccharomyces pastorianus SafLager W30/70 | Lesaffre Fermentis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pater, A.; Satora, P.; Zdaniewicz, M.; Makarewicz, M.; Khachatryan, K. The Improvement of Reserve Polysaccharide Glycogen Level and Other Quality Parameters of S. cerevisiae Brewing Dry Yeasts by Their Rehydration in Water, Treated with Low-Temperature, Low-Pressure Glow Plasma (LPGP). Appl. Sci. 2022, 12, 2909. https://doi.org/10.3390/app12062909
Pater A, Satora P, Zdaniewicz M, Makarewicz M, Khachatryan K. The Improvement of Reserve Polysaccharide Glycogen Level and Other Quality Parameters of S. cerevisiae Brewing Dry Yeasts by Their Rehydration in Water, Treated with Low-Temperature, Low-Pressure Glow Plasma (LPGP). Applied Sciences. 2022; 12(6):2909. https://doi.org/10.3390/app12062909
Chicago/Turabian StylePater, Aneta, Paweł Satora, Marek Zdaniewicz, Małgorzata Makarewicz, and Karen Khachatryan. 2022. "The Improvement of Reserve Polysaccharide Glycogen Level and Other Quality Parameters of S. cerevisiae Brewing Dry Yeasts by Their Rehydration in Water, Treated with Low-Temperature, Low-Pressure Glow Plasma (LPGP)" Applied Sciences 12, no. 6: 2909. https://doi.org/10.3390/app12062909
APA StylePater, A., Satora, P., Zdaniewicz, M., Makarewicz, M., & Khachatryan, K. (2022). The Improvement of Reserve Polysaccharide Glycogen Level and Other Quality Parameters of S. cerevisiae Brewing Dry Yeasts by Their Rehydration in Water, Treated with Low-Temperature, Low-Pressure Glow Plasma (LPGP). Applied Sciences, 12(6), 2909. https://doi.org/10.3390/app12062909








