In Silico Pesticide Discovery for New Anti-Tobacco Mosaic Virus Agents: Reactivity, Molecular Docking, and Molecular Dynamics Simulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecule Library Preparation
2.2. Receptor Preparation
2.3. Global Reactivity Descriptors
2.4. Molecular Docking
2.5. Molecular Dynamics Simulations
3. Results and Discussion
3.1. Global Reactivity Descriptors
3.2. Molecular Docking
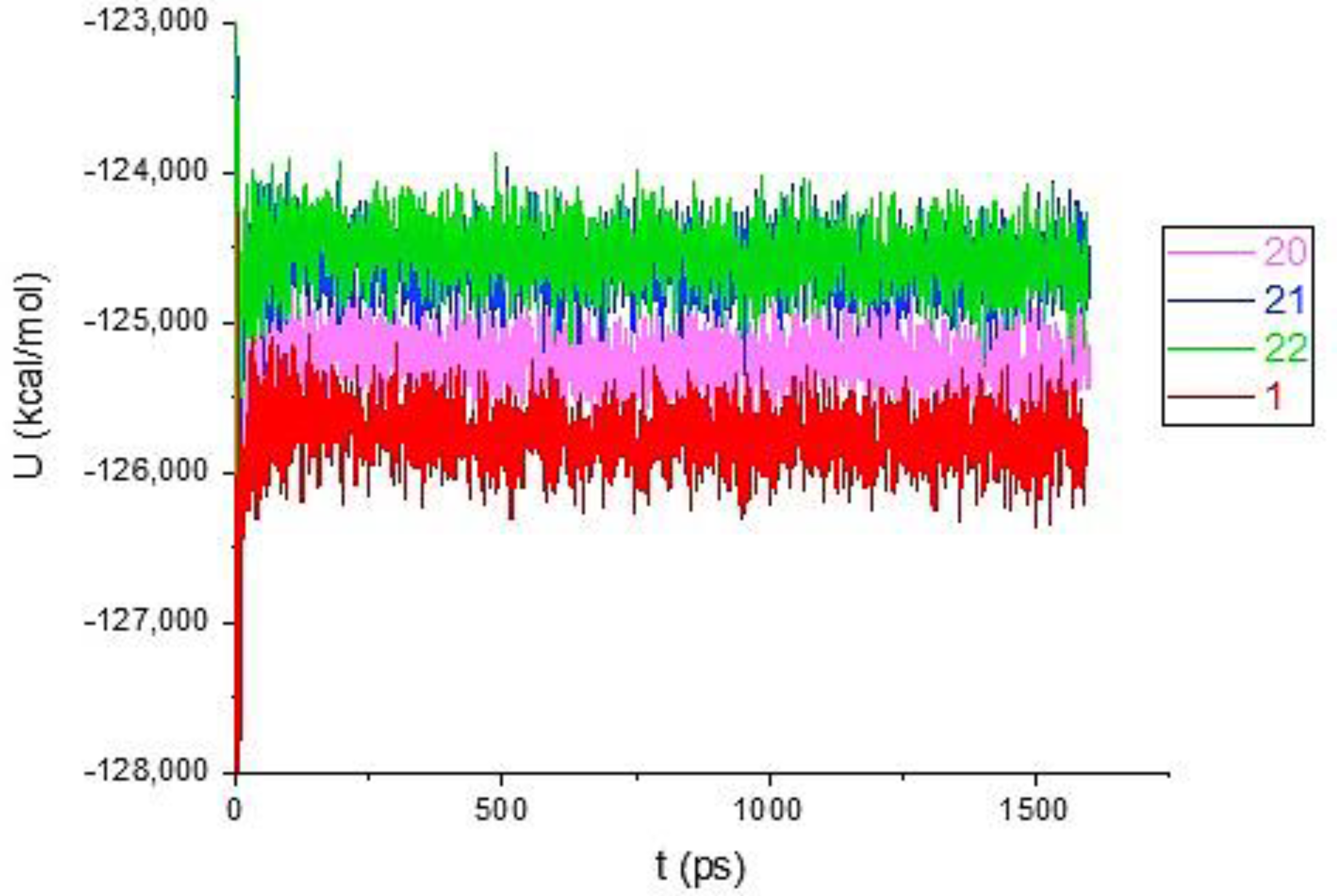
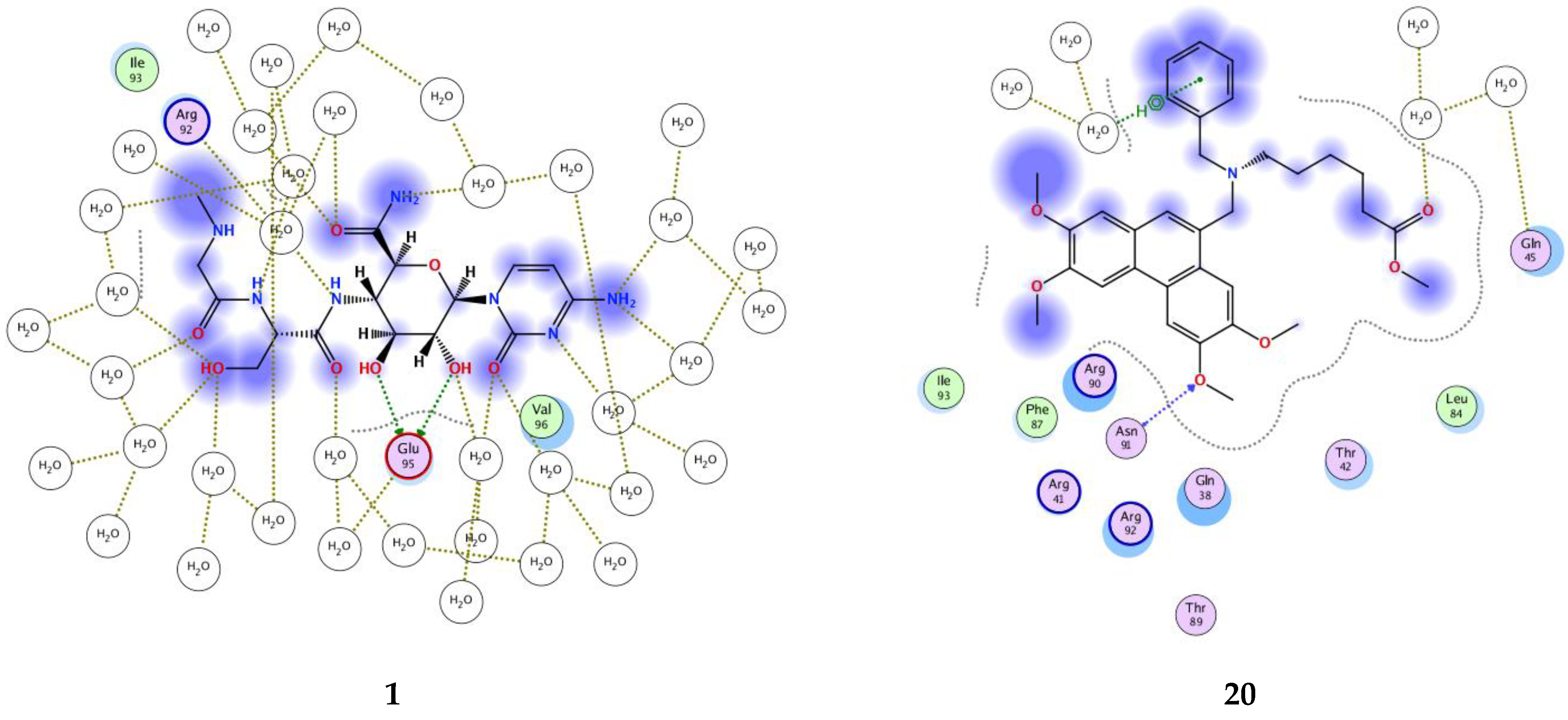
3.3. Molecular Dynamics Simulations
Binding Free Energy Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubio, L.; Galipienso, L.; Ferriol, I. Detection of plant viruses and disease management: Relevance of genetic diversity and evolution. Front. Plant Sci. 2020, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, P.A.; Baleev, D.N.; Ivanova, M.I.; Sokolova, L.M.; Karakozova, M.V. Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Nat. 2020, 12, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Mehetre, G.T.; Leo, V.V.; Singh, G.; Sorokan, A.; Maksimov, I.; Yadav, M.K.; Upadhyaya, K.; Hashem, A.; Alsaleh, A.N.; Dawoud, T.M.; et al. Current developments and challenges in plant viral diagnostics: A systematic review. Viruses 2021, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.C. Global plant virus disease pandemics and epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef]
- Lomonossoff, G.P.; Wege, C. TMV Particles: The journey from fundamental studies to bionanotechnology applictions. Adv. Virus. Res. 2018, 102, 149–176. [Google Scholar] [CrossRef]
- Knapp, E.; Lewandowski, D.J. Tobacco mosaic virus, not just a single component virus anymore. Mol. Plant Pathol. 2001, 2, 117–123. [Google Scholar] [CrossRef]
- Adams, M.J.; Adkins, S.; Bragard, C.; Gilmer, D.; Li, D.; MacFarlane, S.A.; Wong, S.-M.; Melcher, U.; Ratti, C.; Ryu, K.H.; et al. ICTV Virus taxonomy profile: Virgaviridae. J. Gen. Virol. 2017, 98, 1999–2000. [Google Scholar] [CrossRef]
- Lv, X.; Xiang, S.; Wang, X.; Wu, L.; Liu, C.; Yuan, M.; Gong, W.; Win, H.; Hao, C.; Xue, Y.; et al. Synthetic chloroinconazide compound exhibits highly efficient antiviral activity against tobacco mosaic virus. Pest. Manag. Sci. 2020, 76, 3636–3648. [Google Scholar] [CrossRef]
- Su, B.; Chen, F.; Wang, L.; Wang, Q. Design, synthesis, antiviral activity, and structure–activity relationships (SARs) of two types of structurally novel phenanthroindo/quinolizidine analogues. J. Agric. Food Chem. 2014, 62, 1233–1239. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, C.H.; Hou, C.T.; Hu, L.Y.; Wang, Q.C.; Wu, Y.F. First discovery of acetone extract from cottonseed oil sludge as a novel antiviral agent against plant viruses. PLoS ONE 2015, 10, e0117496. [Google Scholar] [CrossRef]
- Gan, X.H.; Hu, D.Y.; Wang, Y.J.; Yu, L.; Song, B.A. Novel trans-ferulic acid derivatives containing a chalcone moiety as potential activator for plant resistance induction. J. Agric. Food Chem. 2017, 65, 4367–4377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, A.; Wang, T.; Hui, H.; Wei, X.; Cui, W.; Zhou, C.; Li, H.; Wang, Z.; Guo, J.; Ma, D.; et al. Natural products for drug discovery: Discovery of gramines as novel agents against a plant virus. J. Agric. Food Chem. 2019, 67, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Loustaud-Ratti, V.; Debette-Gratien, M.; Jacques, J.; Alain, S.; Marquet, P.; Sautereau, D.; Rousseau, A.; Carrier, P. Ribavirin: Past, present and future. World, J. Hepatol. 2016, 8, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Cai, C.; Deng, M.; Wang, Q. Spatial configuration and three-dimensional conformation directed design, synthesis, antiviral activity, and structure–activity relationships of phenanthroindolizidine analogues. J. Agric. Food Chem. 2016, 64, 2039–2045. [Google Scholar] [CrossRef]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Alnaggar, A.E.-A.M.; Soliman, A.M.; El-Dougdoug, N.K. Ameliorating the adverse effects of tomato mosaic Tobamovirus infecting tomato plants in Egypt by boosting immunity in tomato plants using zinc oxide nanoparticles. Molecules 2021, 26, 1337. [Google Scholar] [CrossRef]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Refaey, E.E.; Mohamed, H.I.; El-Dougdoug, N.K. Molecular characterization of the alfalfa mosaic virus infecting Solanum melongena in Egypt and the control of its deleterious effects with melatonin and salicylic acid. Plants 2021, 10, 459. [Google Scholar] [CrossRef]
- Song, P.; Yu, X.; Yang, W.; Wang, Q. Natural phytoalexin stilbene compound resveratrol and its derivatives as anti-tobacco mosaic virus and anti-phytopathogenic fungus agents. Sci. Rep. 2021, 11, 16509. [Google Scholar] [CrossRef]
- Guo, W.; Xiang Lu, X.; Liu, B.; Yan, H.; Feng, J. Anti-TMV activity and mode of action of three alkaloids isolated from Chelidonium majus. Pest. Manag. Sci. 2021, 77, 510–517. [Google Scholar] [CrossRef]
- Hu, Z.-X.; Zou, J.-B.; An, Q.; Yi, P.; Yuan, C.-M.; Gu, W.; Zhao, L.-H.; Hao, X.-J. Anti-tobacco mosaic virus (TMV) activity of chemical constituents from the seeds of Sophora tonkinensis. J. Asian Nat. Prod. Res. 2021, 23, 644–651. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Wu, K.; Yan, H.; Hao, X.; Wu, Y. Application of fatty acids as antiviral agents against tobacco mosaic virus. Pestic. Biochem. Physiol. 2017, 139, 87–91. [Google Scholar] [CrossRef]
- Chen, M.-H.; Chen, Z.; Song, B.-A.; Bhadury, P.S.; Yang, S.; Cai, X.-J.; Hu, D.-Y.; Xue, W.; Zeng, S. Synthesis and antiviral activities of chiral thiourea derivatives containing an α-aminophosphonate moiety. J. Agric. Food Chem. 2009, 57, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Chen, Z.; Cai, X.-J.; Song, B.-A.; Bhadury, P.S.; Yang, S.; Jin, L.-H.; Xue, W.; Hu, D.-Y.; Zeng, S. Synthesis and antiviral activity of novel pyrazole derivatives containing oxime esters group. Bioorg. Med. Chem. 2008, 16, 9699–9707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; He, F.; Gan, X.; Song, B.; Hu, D. Design, synthesis, bioactivity and mechanism of dithioacetal derivatives containing dioxyether moiety. Bioorg. Med. Chem. Lett. 2019, 29, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, P.; Liu, Y.; Wand, Q. D and E rings may not be indispensable for antofine: Discovery of phenanthrene and alkylamine chain containing antofine derivatives as novel antiviral agents against tobacco mosaic virus (TMV) based on interaction of antofine and TMV RNA. J. Agric. Food Chem. 2014, 62, 10393–10404. [Google Scholar] [CrossRef]
- Wu, M.; Han, G.; Wang, Z.; Liu, Y.; Wang, Q. Synthesis and antiviral activities of antofine analogues with different C-6 substituent groups. J. Agric. Food Chem. 2013, 61, 1030–1035. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, P.; Xizhi, X.; Liu, Y.; Wang, L.; Wang, Q. Design, synthesis, and antiviral activity evaluation of phenanthrene-based antofine derivatives. J. Agric. Food Chem. 2012, 60, 8544–8551. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Ma, S.; Liu, Y.; Wang, L.; Wang, Q. Design, synthesis, antiviral activity, and SARs of 14-aminophenanthroindolizidines. J. Agric. Food Chem. 2012, 60, 5825–5831. [Google Scholar] [CrossRef]
- Nagalakshmamma, V.; Venkataswamy, M.; Pasala, C.; Umamaheswari, A.; Thyagaraju, K.; Nagaraju, C.; Chalapathi, P.V. Design, synthesis, anti-tobacco mosaic viral and molecule docking simulations of urea/thiourea derivatives of 2-(piperazine-1-yl)-pyrimidine and 1-(4-fluoro/4-chloroPhenyl)-piperazine and 1-(4-chlorophenyl)-piperazine-A study. Bioorg. Chem. 2020, 102, 104084. [Google Scholar] [CrossRef]
- Wang, D.; Huang, M.; Gao, D.; Chen, K.; Xu, W.; Li, X. Screening anti-TMV agents targeting tobacco mosaic virus Helicase Protein. Pestic. Biochem. Physiol. 2020, 166, 104449. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Yu, G.; Wang, Y.-Y.; Xu, J.-H.; Xu, F.-Z.; Fu, H.; Zhao, Y.-H.; Wu, J. Antiviral activity and molecular docking of active constituents from the root of Aconitum Carmichaelii. Chem. Nat. Compd. 2019, 55, 189–193. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, P.; Wang, L.; Wang, Q. Design, synthesis, and anti-tobacco mosaic virus (TMV) activity of phenanthroindolizidines and their analogues. J. Agric. Food Chem. 2012, 60, 10212–10219. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision, A.02, Inc., Wallingford CT, Wallingford. 2016. Available online: https://gaussian.com/g09citation/ (accessed on 24 November 2021).
- Molecular Operating Environment (MOE). 2015. Available online: http://www.chemcomp.com (accessed on 20 November 2021).
- Becke, A.D. Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals. J. Chem. Phys. 1997, 107, 8554–8560. [Google Scholar] [CrossRef]
- Yongqin, X.; Schiele, B.; Akata, Z. Zero-shot learning the good, the bad and the ugly. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Salt Lake City, UT, USA, 21–26 July 2017; pp. 4582–4591. [Google Scholar]
- Asurmendi, S.; Berg, R.H.; Koo, J.C. Coat protein regulates formation of replication complexes during tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 2004, 101, 1415–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichel, C.; Beachy, R.N. Tobacco mosaic virus infection induces severe morphological changes of the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1998, 95, 11169–11174. [Google Scholar] [CrossRef] [Green Version]
- Sachse, C.; Chen, J.Z.; Coureux, P.D.; Stroupe, M.E.; Fändrich, M.; Grigorieff, N. High-resolution electron microscopy of Helical specimens: A fresh look at tobacco mosaic virus. J. Mol. Biol. 2007, 371, 812–835. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional Theory indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, T.C.; Caetano, M.S.; da Cunha, E.F.F.; Souza, T.C.S.; Rocha, M.V.J. Construction and assessment of reaction models of class I EPSP synthase: Molecular docking and density functional theoretical calculations. J. Biomol. Struct. Dyn. 2009, 27, 195–207. [Google Scholar] [CrossRef]
- Kellenberger, E.; Rodrigo, J.; Muller, P.; Rognan, D. Comparative evaluation of eight docking tools for docking and virtual screening accuracy. Proteins 2004, 57, 225–242. [Google Scholar] [CrossRef]
- Sturgeon, J.B.; Laird, B.B. Symplectic algorithm for constant-pressure molecular dynamics using a Nosé–Poincaré thermostat. J. Chem. Phys. 2000, 112, 3474–3482. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R. Chemical reactivity theory (CRT) study of small drug-like biologically active molecules. J. Biomol. Struct. Dyn. 2021, 39, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.; Engdahl, A.L.; Dunbar, J.B., Jr.; Carlson, H.A. Biophysical limits of protein–ligand binding. J. Chem. Inf. Model. 2012, 52, 2098–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Compd | Structure | Smiles | Inhibition Rate (%) | Ref |
|---|---|---|---|---|
| 1 | 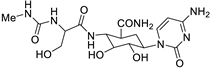 | O=C(N[C@H]1[C@H](O)[C@@H](O)[C@H](N2C(=O)N=C(N)C=C2)O[C@@H]1C(=O)N)[C@@H](NC(=O)CNC)CO | 69.3 | [14] |
| 2 | 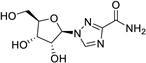 | O=C(N)c1nn([C@H]2[C@H](O)[C@H](O)[C@@H](CO)O2)cn1 | 41.3 | [14] |
| 3 | 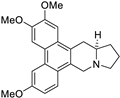 | O(C)c1c(OC)cc2c(c3c(c4c2C[C@@H]2[N@@](C4)CCC2)ccc(OC)c3)c1 | 65.8 | [14] |
| 4 | 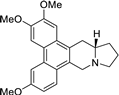 | O(C)c1c(OC)cc2c(c3c(c4c2C[C@H]2[N@@](C4)CCC2)ccc(OC)c3)c1 | 60.2 | [14] |
| 5 | 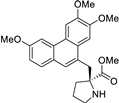 | O=C(OC)[C@]1(Cc2c3c(c4c(c2)ccc(OC)c4)cc(OC)c(OC)c3)NCCC1 | 62.5 | [14] |
| 6 | 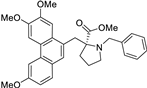 | O=C(OC)[C@]1(Cc2c3c(c4c(c2)ccc(OC)c4)cc(OC)c(OC)c3)[N@](Cc2ccccc2)CCC1 | 60.0 | [14] |
| 7 | 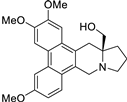 | O(C)c1c(OC)cc2c(c3c(c4c2C[C@@]2(CO)[N@@](C4)CCC2)ccc(OC)c3)c1 | 58.9 | [14] |
| 8 | 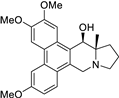 | O(C)c1c(OC)cc2c(c3c(c4c2[C@@H](O)[C@@]2(C)[N@@](C4)CCC2)ccc(OC)c3)c1 | 61.4 | [14] |
| 9 |  | O(C)c1c(OC)cc2c(c3c(c4c2[C@H](O)[C@@]2(C)[N@@](C4)CCC2)ccc(OC)c3)c1 | 54.3 | [14] |
| 10 |  | O=C(OC)[C@]12[N@@](Cc3c4c(c5c(c3C1)cc(OC)c(OC)c5)cc(OC)cc4)CCC2 | 53.8 | [14] |
| 11 | 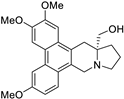 | O(C)c1c(OC)cc2c(c3c(c4c2C[C@]2(CO)[N@@](C4)CCC2)ccc(OC)c3)c1 | 85.3 | [14] |
| 12 |  | O(C)c1c(OC)cc2c(c3c(c4c2C[C@]2(C)[N@@](C4)CCC2)ccc(OC)c3)c1 | 55.5 | [14] |
| 13 | 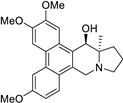 | O(C)c1c(OC)cc2c(c3c(c4c2[C@@H](O)[C@]2(C)[N@@](C4)CCC2)ccc(OC)c3)c1 | 63.8 | [14] |
| 14 | 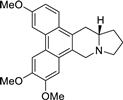 | O(C)c1c(OC)cc2c(c3c(c4c2C[N@@]2[C@H](C4)CCC2)ccc(OC)c3)c1 | 71.2 | [31] |
| 15 | 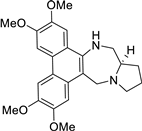 | O(C)c1c(OC)cc2c(c3c(c4c2NC[C@H]2[N@@](C4)CCC2)cc(OC)c(OC)c3)c1 | 40.4 | [31] |
| 16 | 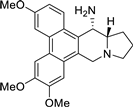 | O(C)c1c(OC)cc2c(c3c(c4[C@H](N)[C@H]5[N@@](Cc24)CCC5)ccc(OC)c3)c1 | 72.4 | [27] |
| 17 | 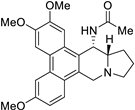 | O=C(N[C@@H]1[C@H]2[N@@](Cc3c4c(c5c(c13)cc(OC)c(OC)c5)cc(OC)cc4)CCC2)C | 75.1 | [27] |
| 18 | 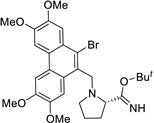 | Brc1c(C[N@@]2[C@H](C(OC(C)(C)C)=N)CCC2)c2c(c3c1cc(OC)c(OC)c3)cc(OC)c(OC)c2 | 39.7 | [26] |
| 19 | 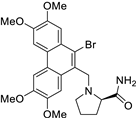 | Brc1c(C[N@@]2[C@@H](C(=O)N)CCC2)c2c(c3c1cc(OC)c(OC)c3)cc(OC)c(OC)c2 | 44.3 | [26] |
| 20 | 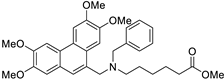 | O=C(OC)CCCCC[N@](Cc1c2c(c3c(cc(OC)c(OC)c3)c1)cc(OC)c(OC)c2)Cc1ccccc1 | 57.6 | [24] |
| 21 |  | O=C(OC)CCCCC[N@](Cc1c2c(c3c(cc(OC)c(OC)c3)c1)cc(OC)c(OC)c2)Cc1ccc(N(C)C)cc1 | 55.3 | [24] |
| 22 | 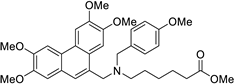 | O=C(OC)CCCCC[N@](Cc1c2c(c3c(cc(OC)c(OC)c3)c1)cc(OC)c(OC)c2)Cc1ccc(OC)cc1 | 50.0 | [24] |
| 23 | 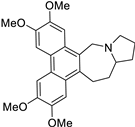 | O(C)c1c(OC)cc2c(c3c(c4c2C[N@@]2[C@@H](CC4)CCC2)cc(OC)c(OC)c3)c1 | 43.8 | [9] |
| 24 | 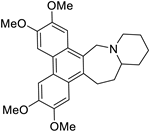 | O(C)c1c(OC)cc2c(c3c(c4c2C[N@@]2[C@@H](CC4)CCCC2)cc(OC)c(OC)c3)c1 | 54.7 | [9] |
| 25 | 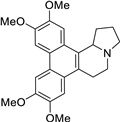 | O(C)c1c(OC)cc2c(c3c(c4c2[C@@H]2[N@](CC4)CCC2)cc(OC)c(OC)c3)c1 | 60.0 | [9] |
| 26 | 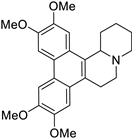 | O(C)c1c(OC)cc2c(c3c(c4c2[C@H]2[N@](CC4)CCCC2)cc(OC)c(OC)c3)c1 | 37.7 | [9] |
| 27 | 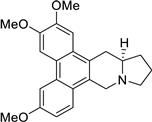 | O(C)c1c(OC)cc2c(c3c(c4c2C[C@@H]2[N@@](C4)CCC2)ccc(OC)c3)c1 | 65.8 | [25] |
| 28 | 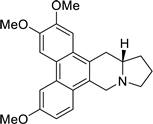 | O(C)c1c(OC)cc2c(c3c(c4c2C[C@H]2[N@@](C4)CCC2)ccc(OC)c3)c1 | 60.2 | [25] |
| 29 | 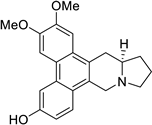 | O(C)c1c(OC)cc2c(c3c(c4c2C[C@@H]2[N@@](C4)CCC2)ccc(O)c3)c1 | 70.5 | [25] |
| 30 | 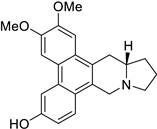 | O(C)c1c(OC)cc2c(c3c(c4c2C[C@H]2[N@@](C4)CCC2)ccc(O)c3)c1 | 52.2 | [25] |
| 31 | 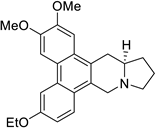 | O(CC)c1cc2c3c(c4c(c2cc1)C[N@@]1[C@@H](C4)CCC1)cc(OC)c(OC)c3 | 50.0 | [25] |
| 32 | 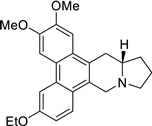 | O(CC)c1cc2c3c(c4c(c2cc1)C[N@@]1[C@H](C4)CCC1)cc(OC)c(OC)c3 | 56.2 | [25] |
| 33 | 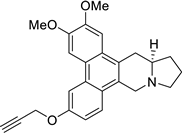 | O(CC#C)c1cc2c3c(c4c(c2cc1)C[N@@]1[C@@H](C4)CCC1)cc(OC)c(OC)c3 | 61.2 | [25] |
| 34 | 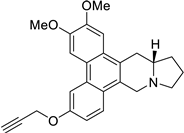 | O(CC#C)c1cc2c3c(c4c(c2cc1)C[N@@]1[C@H](C4)CCC1)cc(OC)c(OC)c3 | 75.0 | [25] |
| 35 | 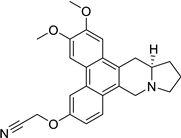 | O(CC#N)c1cc2c3c(c4c(c2cc1)C[N@@]1[C@@H](C4)CCC1)cc(OC)c(OC)c3 | 79.3 | [25] |
| 36 | 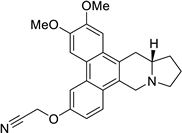 | O(CC#N)c1cc2c3c(c4c(c2cc1)C[N@@]1[C@H](C4)CCC1)cc(OC)c(OC)c3 | 60.0 | [25] |
| 37 | 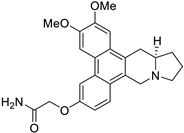 | O=C(N)COc1cc2c3c(c4c(c2cc1)C[N@@]1[C@@H](C4)CCC1)cc(OC)c(OC)c3 | 78.5 | [25] |
| 38 | 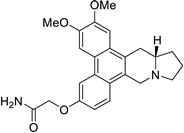 | O=C(N)COc1cc2c3c(c4c(c2cc1)C[N@@]1[C@H](C4)CCC1)cc(OC)c(OC)c3 | 52.3 | [25] |
| 39 | 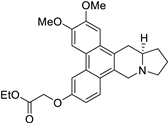 | O=C(OCC)COc1cc2c3c(c4c(c2cc1)C[N@@]1[C@@H](C4)CCC1)cc(OC)c(OC)c3 | 74.6 | [25] |
| 40 | 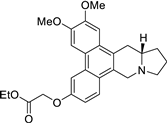 | O=C(OCC)COc1cc2c3c(c4c(c2cc1)C[N@@]1[C@H](C4)CCC1)cc(OC)c(OC)c3 | 50.4 | [25] |
| 41 | 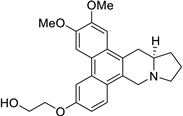 | O(CCO)c1cc2c3c(c4c(c2cc1)C[N@@]1[C@@H](C4)CCC1)cc(OC)c(OC)c3 | 78.2 | [25] |
| 42 |  | O(CCO)c1cc2c3c(c4c(c2cc1)C[N@@]1[C@H](C4)CCC1)cc(OC)c(OC)c3 | 43.9 | [25] |
| 43 | 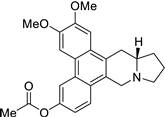 | O=C(Oc1cc2c3c(c4c(c2cc1)C[N@@]1[C@H](C4)CCC1)cc(OC)c(OC)c3)C | 68.7 | [25] |
| TMV-CP | 3D Structure of TMV-CP | |
|---|---|---|
| Code | 2OM3 |  |
| Method | Electron Microscopy | |
| Resolution | 4.40 Å | |
| Number of Residues | 158 |
| Compd | HOMO (eV) | LUMO (eV) | ΔE (eV) a | ƞ (eV) a | S | µ | ω (eV) | N (eV) |
|---|---|---|---|---|---|---|---|---|
| 1 | −6.38 | −1.63 | 4.75 | 2.37 | 155.96 | −4.00 | 3.38 | 2.28 |
| 2 | −6.96 | −1.29 | 5.66 | 2.83 | 130.71 | −4.12 | 3.00 | 1.70 |
| 3 | −5.23 | −0.72 | 4.51 | 2.25 | 164.20 | −2.97 | 1.96 | 3.43 |
| 4 | −5.31 | −0.86 | 4.45 | 2.23 | 166.34 | −3.08 | 2.14 | 3.35 |
| 5 | −5.27 | −0.81 | 4.46 | 2.23 | 166.05 | −3.04 | 2.07 | 3.38 |
| 6 | −5.04 | −0.93 | 4.11 | 2.06 | 180.08 | −2.99 | 2.17 | 3.61 |
| 7 | −5.21 | −0.67 | 4.54 | 2.27 | 163.26 | −2.94 | 1.91 | 3.45 |
| 8 | −5.38 | −0.88 | 4.50 | 2.25 | 164.66 | −3.13 | 2.17 | 3.28 |
| 9 | −5.50 | −0.96 | 4.54 | 2.27 | 163.25 | −3.23 | 2.30 | 3.16 |
| 10 | −5.17 | −0.78 | 4.40 | 2.20 | 168.38 | −2.98 | 2.01 | 3.49 |
| 11 | −5.10 | −0.84 | 4.26 | 2.13 | 173.79 | −2.97 | 2.07 | 3.55 |
| 12 | −5.17 | −0.71 | 4.46 | 2.23 | 166.14 | −2.94 | 1.94 | 3.49 |
| 13 | −5.32 | −0.85 | 4.46 | 2.23 | 165.86 | −3.08 | 2.13 | 3.34 |
| 14 | −5.16 | −0.75 | 4.41 | 2.21 | 167.83 | −2.95 | 1.97 | 3.50 |
| 15 | −5.10 | −0.87 | 4.24 | 2.12 | 174.80 | −2.99 | 2.10 | 3.55 |
| 16 | −5.21 | −0.79 | 4.42 | 2.21 | 167.42 | −3.00 | 2.04 | 3.44 |
| 17 | −5.55 | −1.11 | 4.44 | 2.22 | 166.64 | −3.33 | 2.50 | 3.10 |
| 18 | −5.37 | −1.03 | 4.34 | 2.17 | 170.47 | −3.20 | 2.36 | 3.28 |
| 19 | −5.52 | −1.51 | 4.02 | 2.01 | 184.41 | −3.51 | 3.08 | 3.13 |
| 20 | −5.52 | −1.02 | 4.50 | 2.25 | 164.46 | −3.27 | 2.37 | 3.14 |
| 21 | −4.94 | −0.87 | 4.06 | 2.03 | 182.20 | −2.90 | 2.07 | 3.72 |
| 22 | −5.55 | −0.96 | 4.60 | 2.30 | 161.10 | −3.25 | 2.30 | 3.10 |
| 23 | −5.34 | −0.92 | 4.42 | 2.21 | 167.58 | −3.13 | 2.22 | 3.32 |
| 24 | −5.39 | −0.93 | 4.45 | 2.23 | 166.23 | −3.16 | 2.24 | 3.27 |
| 25 | −5.50 | −0.93 | 4.56 | 2.28 | 162.22 | −3.22 | 2.27 | 3.16 |
| 26 | −5.30 | −0.94 | 4.36 | 2.18 | 169.81 | −3.12 | 2.23 | 3.36 |
| 27 | −5.27 | −0.84 | 4.43 | 2.21 | 167.17 | −3.06 | 2.11 | 3.39 |
| 28 | −5.37 | −0.82 | 4.54 | 2.27 | 163.00 | −3.09 | 2.11 | 3.29 |
| 29 | −5.39 | −0.87 | 4.52 | 2.26 | 163.96 | −3.13 | 2.17 | 3.27 |
| 30 | −5.42 | −0.90 | 4.53 | 2.26 | 163.59 | −3.16 | 2.21 | 3.23 |
| 31 | −5.25 | −0.82 | 4.42 | 2.21 | 167.35 | −3.03 | 2.08 | 3.41 |
| 32 | −5.29 | −0.84 | 4.45 | 2.22 | 166.48 | −3.06 | 2.11 | 3.37 |
| 33 | −5.34 | −0.91 | 4.43 | 2.21 | 167.17 | −3.13 | 2.21 | 3.31 |
| 34 | −5.38 | −0.93 | 4.45 | 2.22 | 166.43 | −3.16 | 2.24 | 3.28 |
| 35 | −5.55 | −1.13 | 4.42 | 2.21 | 167.47 | −3.34 | 2.53 | 3.10 |
| 36 | −5.57 | −1.15 | 4.42 | 2.21 | 167.52 | −3.36 | 2.55 | 3.09 |
| 37 | −5.51 | −1.07 | 4.44 | 2.22 | 166.74 | −3.29 | 2.44 | 3.15 |
| 38 | −5.53 | −1.09 | 4.44 | 2.22 | 166.72 | −3.31 | 2.46 | 3.13 |
| 39 | −5.29 | −0.83 | 4.46 | 2.23 | 166.03 | −3.06 | 2.10 | 3.37 |
| 40 | −5.38 | −0.93 | 4.45 | 2.22 | 166.43 | −3.16 | 2.24 | 3.28 |
| 41 | −5.34 | −0.90 | 4.44 | 2.22 | 166.69 | −3.12 | 2.20 | 3.31 |
| 42 | −5.42 | −0.88 | 4.55 | 2.27 | 162.85 | −3.15 | 2.18 | 3.23 |
| 43 | −5.65 | −1.26 | 4.39 | 2.20 | 168.54 | −3.45 | 2.71 | 3.01 |
| Compd | Score (kcal/mol) | RMSD (Å) | Compd | Score (kcal/mol) | RMSD (Å) |
|---|---|---|---|---|---|
| 1 | −6.98 | 2.83 | 25 | −6.25 | 1.36 |
| 2 | −5.29 | 2.07 | 26 | −6.39 | 1.70 |
| 3 | −6.30 | 1.21 | 23 | −6.59 | 1.96 |
| 4 | −6.16 | 1.43 | 24 | −6.60 | 1.72 |
| 5 | −6.73 | 1.03 | 27 | −6.41 | 0.71 |
| 6 | −6.41 | 2.05 | 28 | −6.05 | 1.50 |
| 7 | −6.07 | 1.24 | 29 | −6.30 | 1.52 |
| 8 | −6.40 | 1.16 | 30 | −5.86 | 1.89 |
| 9 | −6.24 | 2.04 | 31 | −6.49 | 1.26 |
| 10 | −6.18 | 1.67 | 32 | −6.35 | 1.59 |
| 11 | −6.28 | 2.27 | 33 | −6.72 | 0.71 |
| 12 | −6.26 | 1.34 | 34 | −6.71 | 1.63 |
| 13 | −6.08 | 2.43 | 35 | −6.49 | 1.59 |
| 14 | −6.28 | 1.22 | 36 | −6.59 | 1.01 |
| 15 | −6.65 | 1.66 | 37 | −6.70 | 1.08 |
| 16 | −6.39 | 1.41 | 38 | −6.58 | 1.91 |
| 17 | −6.37 | 1.90 | 39 | −7.07 | 1.66 |
| 18 | −6.72 | 1.64 | 40 | −6.72 | 2.78 |
| 19 | −6.66 | 1.51 | 41 | −6.40 | 1.22 |
| 20 | −6.73 | 2.30 | 42 | −6.32 | 1.67 |
| 21 | −7.93 | 2.37 | 43 | −6.23 | 1.52 |
| 22 | −7.25 | 2.31 |
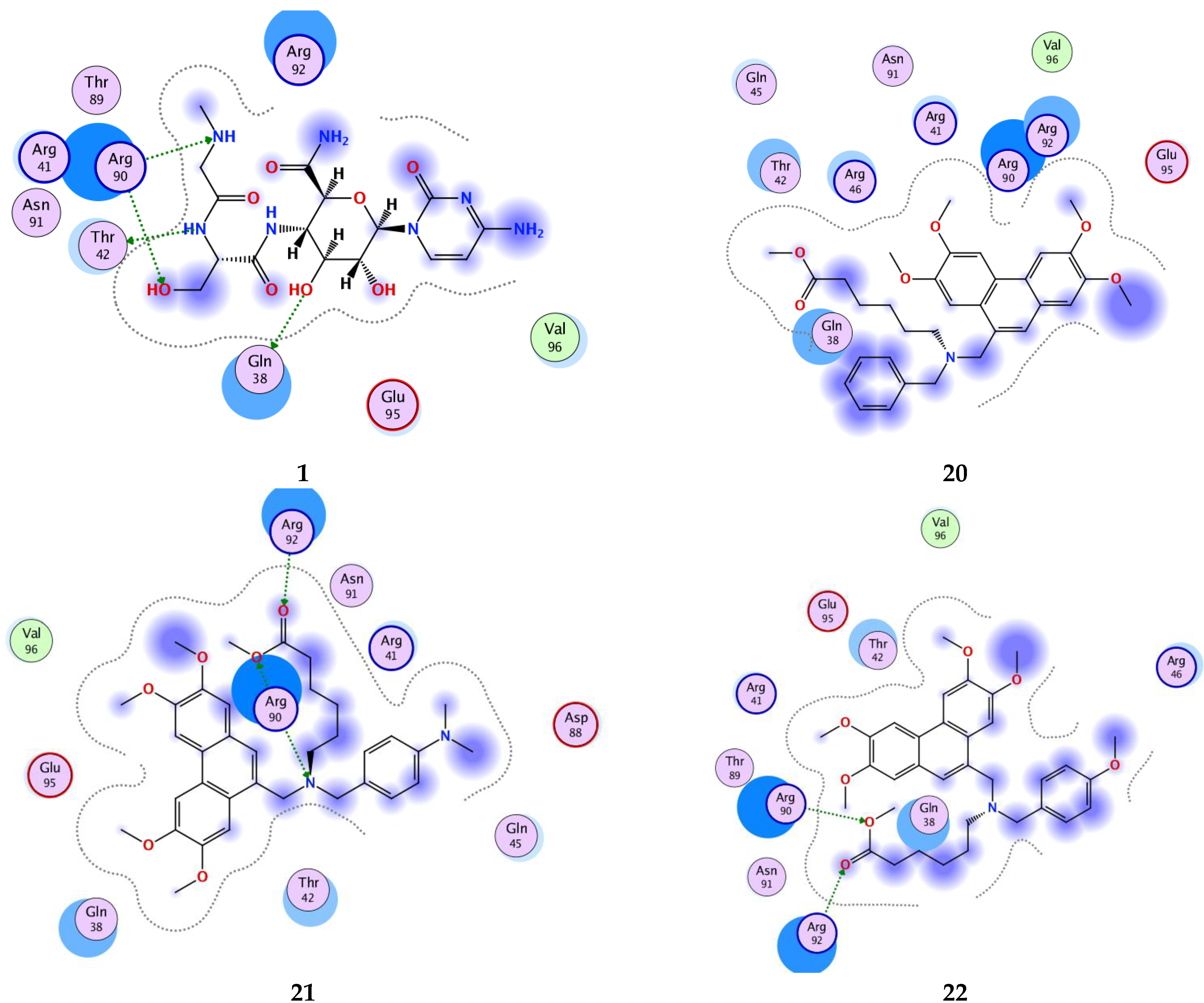
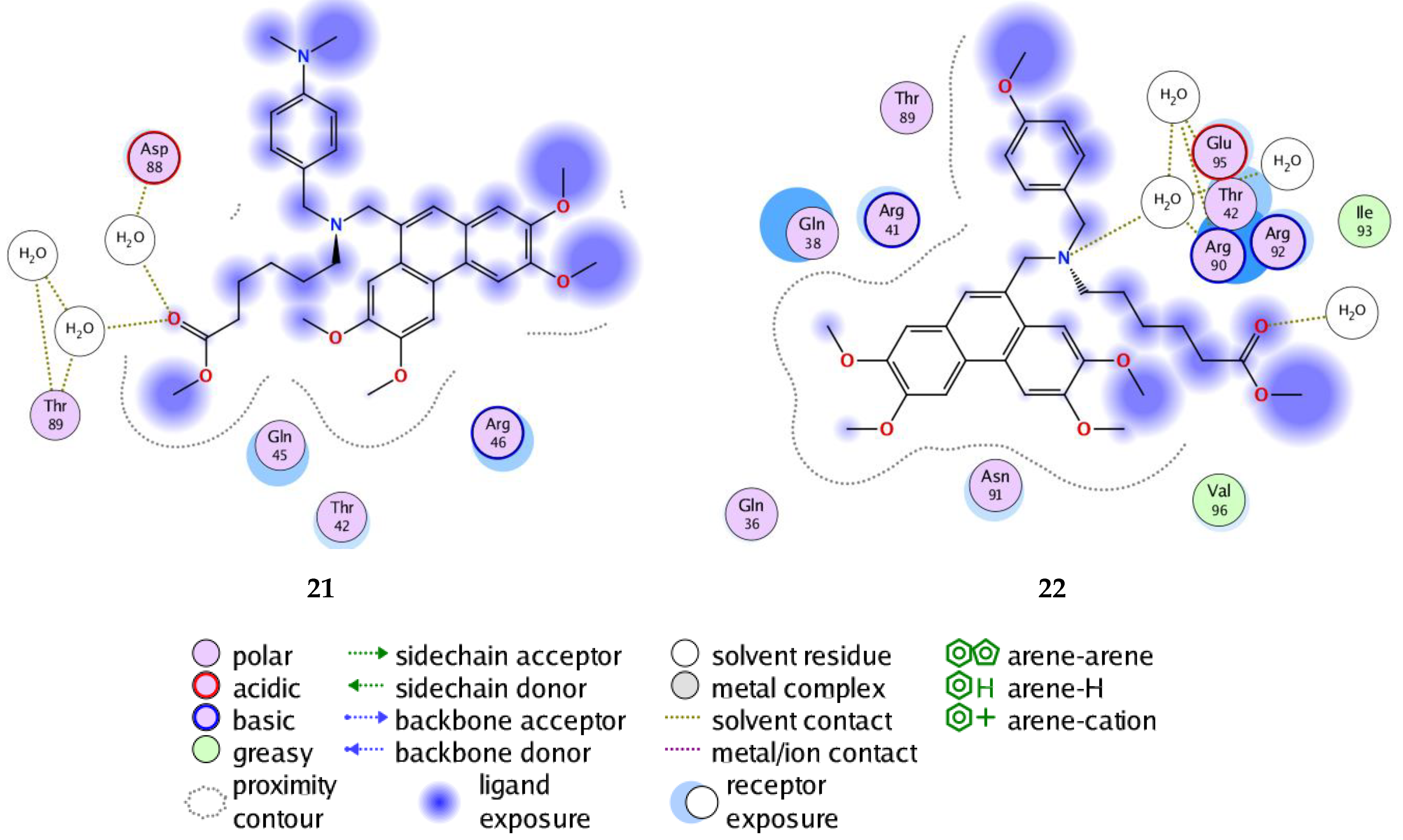
| Compd | Score (kcal/mol) | RMSD (Å) | Bonds between Atoms of Compounds and Residues of Active Site of 2OM3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compd Atoms | Receptor Atoms | Receptor residues | Interaction | d (Å) | E (kcal/mol) | Total E (kcal/mol) | |||
| 1 | −6.98 | 2.83 | N | OG1 | Thr42 | H—D | 3.01 | −0.8 | −37.59 |
| O | OE1 | Gln38 | H—D | 2.80 | −2.1 | ||||
| O | NH2 | Arg90 | H—A | 3.08 | −2.7 | ||||
| N | NH1 | Arg90 | H—A | 3.00 | −2.3 | ||||
| 2 | −5.29 | 2.07 | O | OE1 | Gln38 | H—D | 2.86 | −2.3 | −26.92 |
| O | N | Asn91 | H—A | 3.16 | −1.9 | ||||
| 3 | −6.30 | 1.21 | ― | ― | ― | ― | ― | ― | −31.46 |
| 4 | −6.16 | 1.43 | ― | ― | ― | ― | ― | ― | −31.92 |
| 6 | −6.41 | 2.05 | 6-ring | NH1 | Arg92 | π-cation | 3.52 | −1.1 | −33.66 |
| 17 | −6.37 | 1.90 | O | N | Asn91 | H—A | 3.11 | −1.3 | −32.72 |
| 18 | −6.72 | 1.64 | N | NH2 | Arg90 | H—A | 3.15 | −5.4 | −36.16 |
| 19 | −6.66 | 1.51 | N | OE1 | Gln38 | H—D | 3.26 | −1.0 | −34.24 |
| O | N | Asn91 | H—A | 3.19 | −1.0 | ||||
| 20 | −6.73 | 2.30 | ― | ― | ― | ― | ― | ― | −32.50 |
| 21 | −7.93 | 2.37 | O | NE | Arg92 | H—A | 3.14 | −3.0 | −44.72 |
| O | NH2 | Arg92 | H—A | 3.01 | −2.3 | ||||
| O | NH2 | Arg90 | H—A | 2.92 | −1.2 | ||||
| N | NH2 | Arg90 | H—A | 3.31 | −2.3 | ||||
| 22 | −7.25 | 2.32 | O | NE | Arg92 | H—A | 3.00 | −3.7 | −39.90 |
| O | NH2 | Arg92 | H—A | 3.20 | −0.8 | ||||
| O | NH2 | Arg90 | H—A | 3.02 | −1.3 | ||||
| 23 | −6.59 | 1.96 | N | NH2 | Arg90 | H—A | 3.26 | −2.0 | −35.28 |
| 24 | −6.60 | 1.72 | O | N | Asn91 | H—A | 3.13 | −1.3 | −35.19 |
| 38 | −6.58 | 1.91 | N | OD1 | Asn91 | H—D | 2.87 | −4.5 | −33.62 |
| Compd | MM-GBSA |
|---|---|
| 1 | −42.14 |
| 20 | −19.95 |
| 21 | −33.71 |
| 22 | −49.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulhassan, H.A.; Saleh, B.A.; Harkati, D.; Khelfaoui, H.; Hewitt, N.L.; El-Hiti, G.A. In Silico Pesticide Discovery for New Anti-Tobacco Mosaic Virus Agents: Reactivity, Molecular Docking, and Molecular Dynamics Simulations. Appl. Sci. 2022, 12, 2818. https://doi.org/10.3390/app12062818
Abdulhassan HA, Saleh BA, Harkati D, Khelfaoui H, Hewitt NL, El-Hiti GA. In Silico Pesticide Discovery for New Anti-Tobacco Mosaic Virus Agents: Reactivity, Molecular Docking, and Molecular Dynamics Simulations. Applied Sciences. 2022; 12(6):2818. https://doi.org/10.3390/app12062818
Chicago/Turabian StyleAbdulhassan, Hala A., Basil A. Saleh, Dalal Harkati, Hadjer Khelfaoui, Natalie L. Hewitt, and Gamal A. El-Hiti. 2022. "In Silico Pesticide Discovery for New Anti-Tobacco Mosaic Virus Agents: Reactivity, Molecular Docking, and Molecular Dynamics Simulations" Applied Sciences 12, no. 6: 2818. https://doi.org/10.3390/app12062818
APA StyleAbdulhassan, H. A., Saleh, B. A., Harkati, D., Khelfaoui, H., Hewitt, N. L., & El-Hiti, G. A. (2022). In Silico Pesticide Discovery for New Anti-Tobacco Mosaic Virus Agents: Reactivity, Molecular Docking, and Molecular Dynamics Simulations. Applied Sciences, 12(6), 2818. https://doi.org/10.3390/app12062818









