The Effect of Electrolytes and Urea on the Ethyl Lauroyl Arginate and Cellulose Nanocrystals Foam Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Foaming
2.2. Particle Characterization
2.3. Surface Tension
3. Results and Discussion
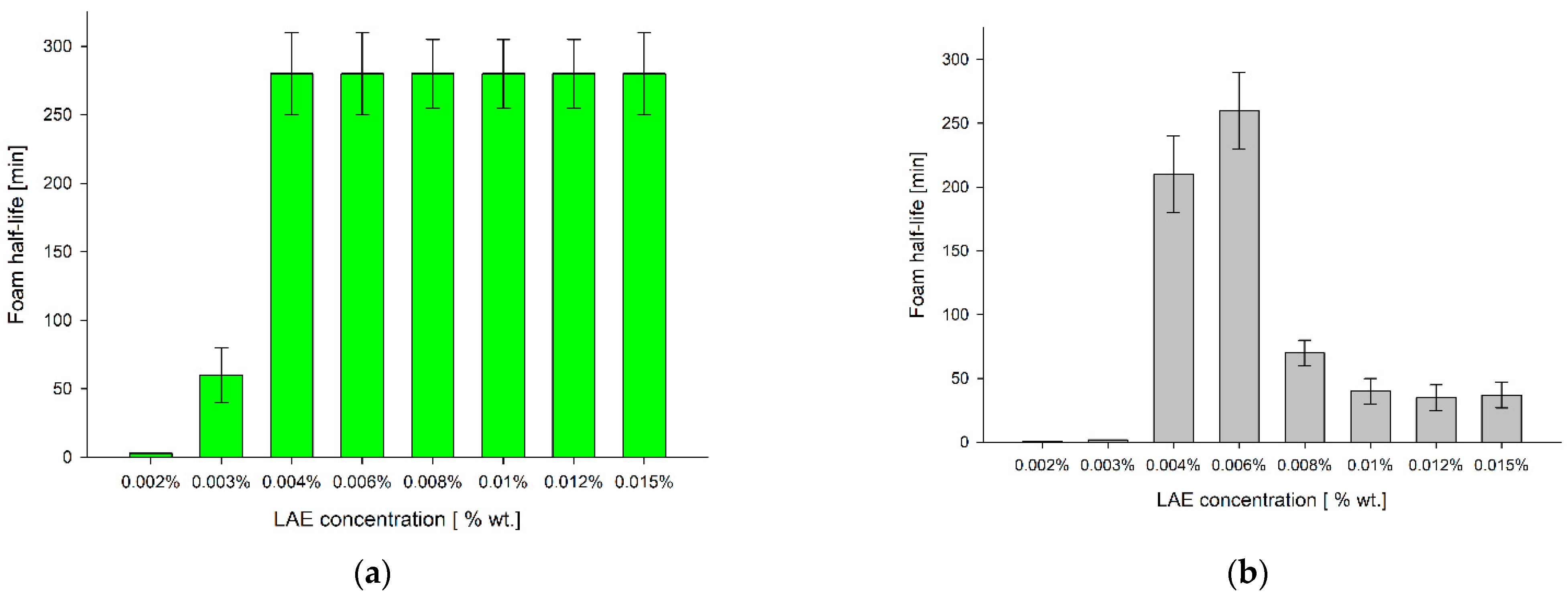
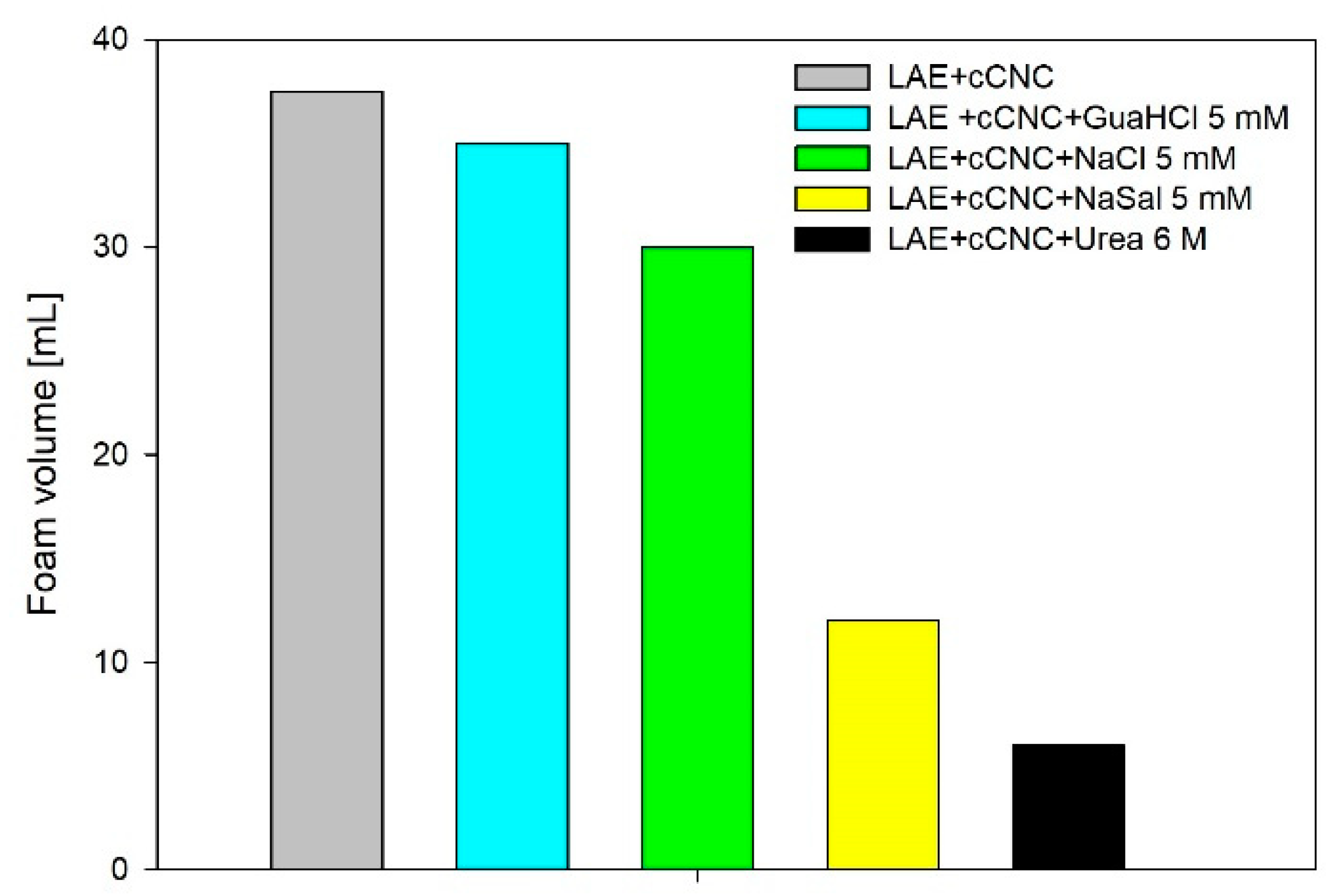
3.1. Foaming
3.2. Zeta Potential and Hydrodynamic Diameter
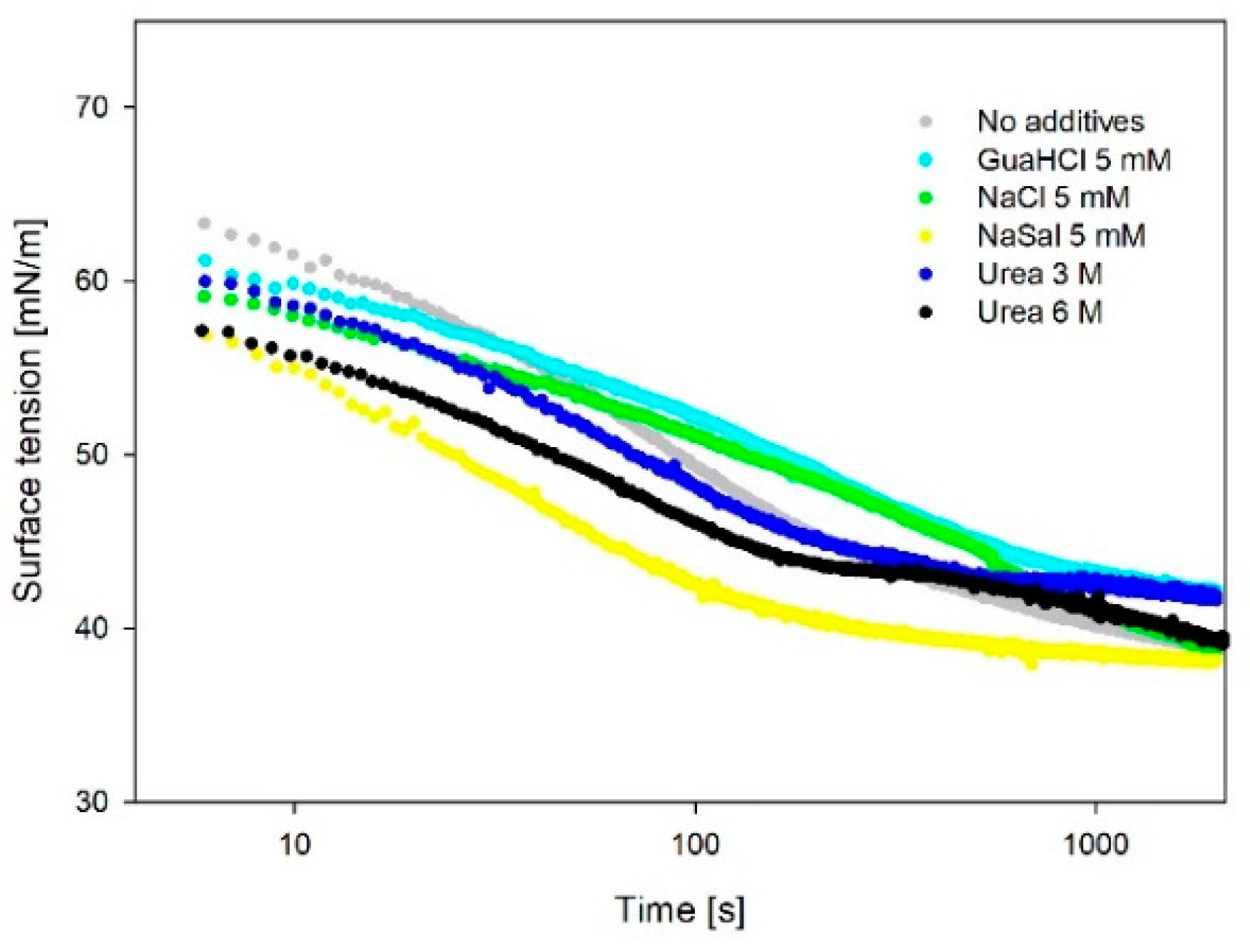
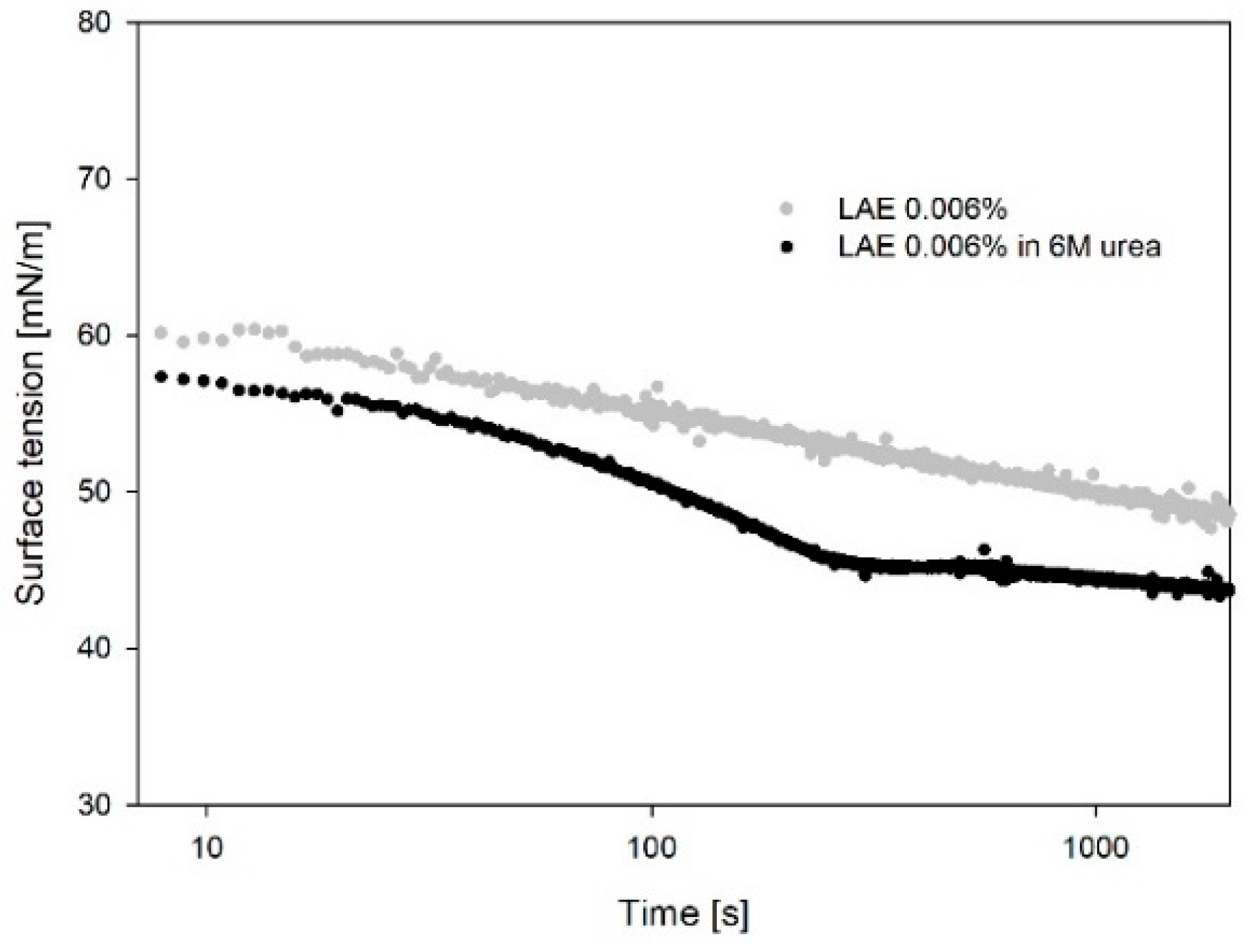
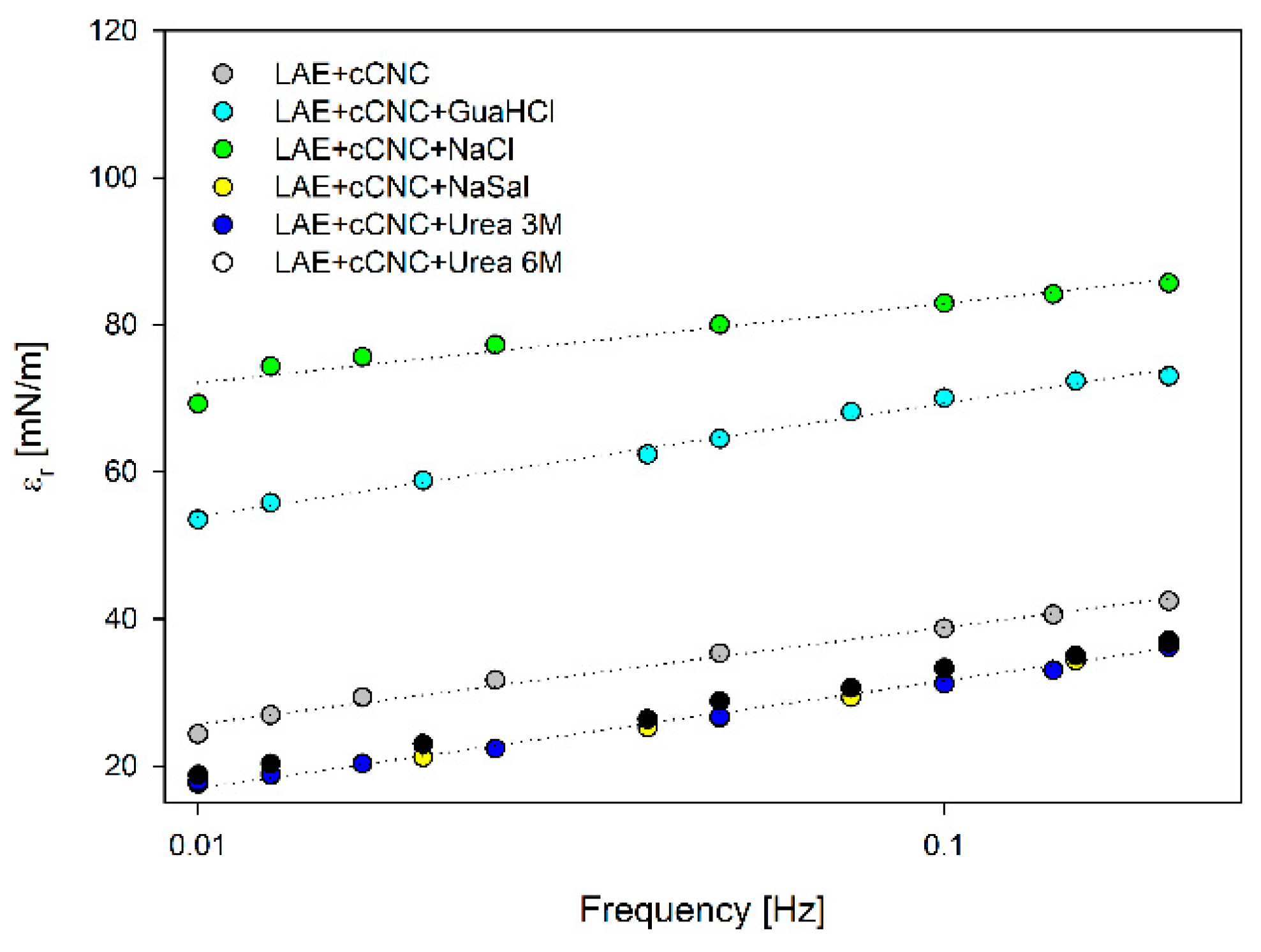
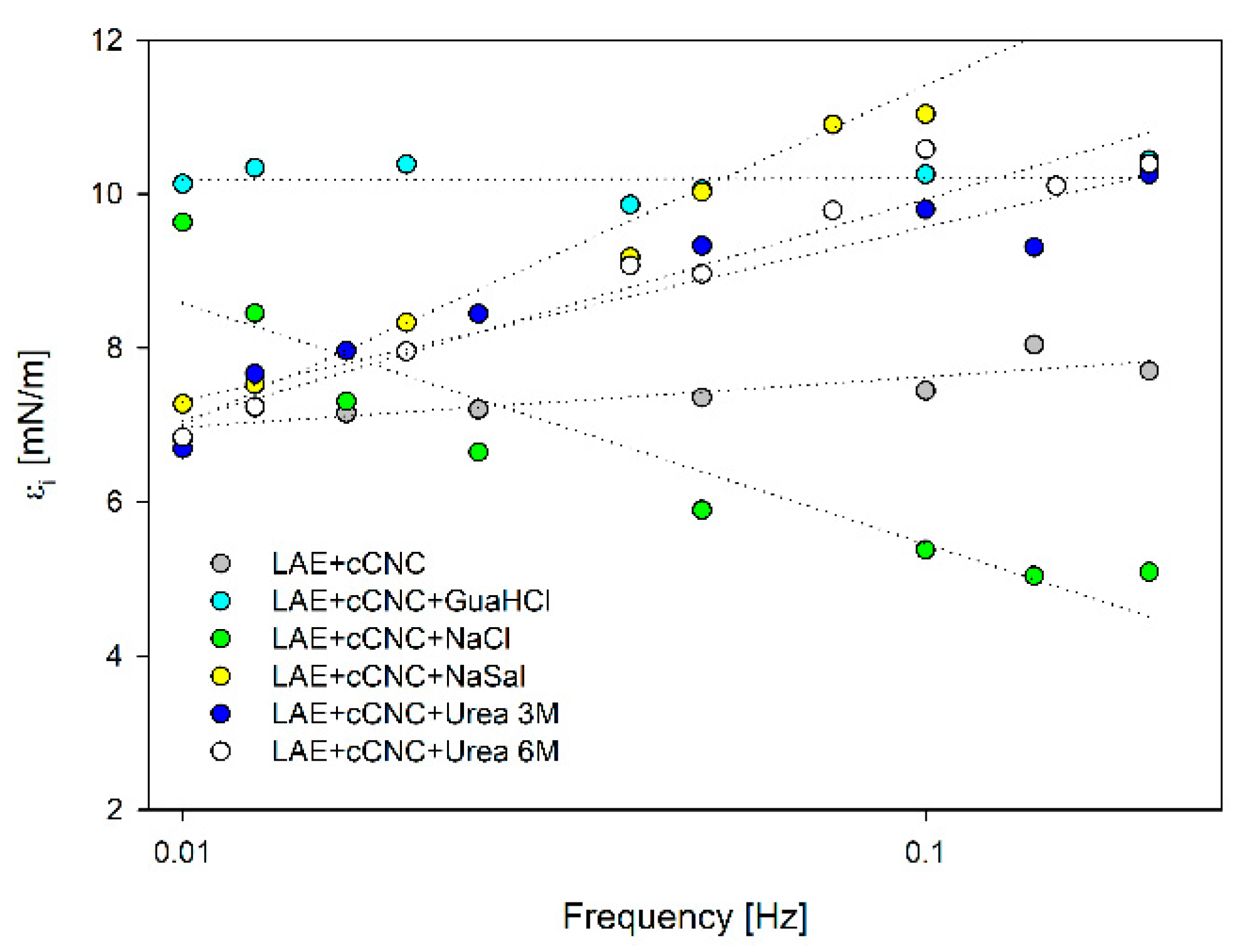
3.3. Surface Tension
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Materialstoday 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa; Gröschel, A.H.; Orlando, J.; Rojas, O.J.; Ikkala, O. Advanced materials through assembly of nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Carlos, C.; Zhang, Z.; Li, J.; Long, Y.; Yang, F.; Dong, Y.; Qiu, X. Piezoelectric nanocellulose thin film with large-scale vertical crystal alignment. ACS Appl. Mater. Interfaces 2020, 12, 26399–26404. [Google Scholar] [CrossRef]
- Droguet, B.E.; Liang, H.-L.; Frka-Petesic, B.; Parker, R.M.; De Volder, M.F.L.; Baumberg, J.J.; Vignolini, S. Large-scale fabrication of structurally coloured cellulose nanocrystal films and effect pigments. Nat. Mater. 2021, 21, 352–358. [Google Scholar] [CrossRef]
- Koshani, R.; Tavakolian, M.; van de Ven, T.G.M. Cellulose-based dispersants and flocculants. J. Mater. Chem. B 2020, 8, 10502–10526. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, R.A.; Nuruddin, Md.; Clarkson, C.; Montes, F.; Howarter, J.; Youngblood, J.P. Cellulose nanocrystal (CNC) coatings with controlled anisotropy as high-performance gas barrier films. ACS Appl. Mater. Interfaces 2019, 11, 1376–1383. [Google Scholar] [CrossRef]
- Gicquel, E.; Bras, J.; Rey, C.; Putaux, J.-L.; Pignon, F.; Jean, B.; Martin, C. Impact of sonication on the rheological and colloidal properties of highly concentrated cellulose nanocrystal suspensions. Cellulose 2019, 26, 7619–7634. [Google Scholar] [CrossRef]
- Chakraborty, A.; Nonappa; Mondal, B.; Chaudhari, K.; Rekola, H.; Hynninen, V.; Kostiainen, M.A.; Ras, R.H.A.; Pradeep, T.J. Near-infrared chiral plasmonic microwires through precision assembly of gold nanorods on soft biotemplates. Phys. Chem. C 2021, 125, 3256–3267. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.; Velikov, L.P.; Velev, O.D. Pickering stabilization of foams and emulsions with particles of biological origin. Curr. Opin. Colloid Interface Sci. 2014, 19, 490–500. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144. [Google Scholar] [CrossRef]
- Andrews, M.P.; Morse, T. Method for Producing Functionalized Nanocrystalline Cellulose and Functionalized Nanocrystalline Cellulose Thereby Produced. U.S. Patent 20,170,260,298 A1, 14 September 2017. [Google Scholar]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding nanocellulose chirality and structure–properties relationship at the single fibril level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef] [Green Version]
- Surov, O.V.; Voronova, M.I.; Zakharov, A.G. Functional materials based on nanocrystalline cellulose. Russ. Chem. Rev. 2017, 86, 907–933. [Google Scholar] [CrossRef]
- Kang, K.; Eremin, A. Solvent-dependent morphology and anisotropic microscopic dynamics of cellulose nanocrystals under electric fields. Phys. Rev. E 2021, 103, 032606. [Google Scholar] [CrossRef] [PubMed]
- Czakaj, A.; Jarek, E.; Krzan, M.; Warszyński, P. Ethyl lauroyl arginate, an inherently multicomponent surfactant system. Molecules 2021, 26, 5894. [Google Scholar] [CrossRef]
- Drenckhan, W.; Saint-Jalmes, A. The science of foaming. Adv. Colloid Interface Sci. 2015, 222, 228–259. [Google Scholar] [CrossRef]
- Briceño-Ahumada, Z.; Drenckhan, W.; Langevin, D. Coalescence in draining foams made of very small bubbles. Phys. Rev. Lett. 2016, 116, 128302-1-5. [Google Scholar] [CrossRef]
- Czakaj, A.; Kannan, A.; Wiśniewska, A.; Grześ, G.; Krzan, M.; Warszyński, P.; Fuller, G.G. Viscoelastic interfaces comprising of cellulose nanocrystals and lauroyl ethyl arginate for enhanced foam stability. Soft Matter 2020, 16, 3981–3990. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathalaa, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Cervin, N.T.; Johansson, E.; Benjamins, J.-W.; Wågberg, L. Mechanisms behind the stabilizing action of cellulose nanofibrils in wet-stable cellulose foams. Biomacromolecules 2015, 16, 3, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Fazilati, M.; Ingelsten, S.; Wojno, S.; Nypelö, T.; Kádár, R. Thixotropy of cellulose nanocrystal suspensions. J. Rheol. 2021, 65, 1035. [Google Scholar] [CrossRef]
- Bertsch, P.; Diener, M.; Adamcik, J.; Scheuble, N.; Geue, T.; Mezzenga, R.; Fischer, P. Adsorption and interfacial layer structure of unmodified nanocrystalline cellulose at air/water interfaces. Langmuir 2018, 34, 15195–15202. [Google Scholar] [CrossRef]
- Delepierre, G.; Eyley, S.; Thielemans, W.; Weder, C.; Cranston, E.D.; Zoppe, J.O. Patience is a virtue: Self-assembly and physico-chemical properties of cellulose nanocrystal allomorphs. Nanoscale 2020, 12, 17480–17493. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, R.; Cranston, E.D.; Pelton, R.H. Stable aqueous foams from cellulose nanocrystals and methyl cellulose. Biomacromolecules 2016, 17, 4095–4099. [Google Scholar] [CrossRef]
- Xiang, W.; Preisig, N.; Ketola, A.; Tardy, B.L.; Bai, L.; Ketoja, J.A.; Stubenrauch, C.; Rojas, O.J. Surface activity and foaming capacity of aggregates formed between an anionic surfactant and non-cellulosics leached from wood fibers. Biomacromolecules 2019, 20, 6, 2286–2294. [Google Scholar] [CrossRef]
- Para, G.; Jarek, E.; Warszyński, P.; Adamczyk, Z. Effect of electrolytes on surface tension of ionic surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 2003, 222, 213–222. [Google Scholar] [CrossRef]
- Sharma, V.K.; Srinivasan, H.; Mitra, S.; Garcia-Sakai, V.; Mukhopadhyay, R.J. Effects of hydrotropic salt on the nanoscopic dynamics of DTAB micelles. Phys. Chem. B 2017, 121, 5562–5572. [Google Scholar] [CrossRef]
- Ogawa, T.; Matsumura, Y. Revealing 3D structure of gluten in wheat dough by optical clearing imaging. Nat. Commun. 2021, 12, 1708. [Google Scholar] [CrossRef] [PubMed]
- Tschoegl, N.W.; Alexander, A.E. The surface chemistry of wheat gluten II. Measurements of surface viscoelasticity. J. Colloid Sci. 1960, 15, 168–182. [Google Scholar] [CrossRef]
- Ruiz, C.C. Micelle formation and microenvironmental properties of sodium dodecyl sulfate in aqueous urea solutions. Colloids Surf. A Physicochem. Eng. Asp. 1999, 147, 349–357. [Google Scholar] [CrossRef]
- Kumari, S.; Sonu, K.; Halder, S.; Aggrawal, R.; Sundar, G.; Saha, S.K. Effect of urea on solvation dynamics and rotational relaxation of coumarin 480 in aqueous micelles of cationic gemini surfactants with different spacer groups. ACS Omega 2018, 3, 3079–3095. [Google Scholar] [CrossRef]
- Kancharla, S.; Dong, D.; Bedrov, D.; Tsianou, M.; Alexandridis, P. Structure and interactions in perfluorooctanoate micellar solutions revealed by small-angle neutron scattering and molecular dynamics simulations studies: Effect of urea. Langmuir 2021, 37, 17, 5339–5347. [Google Scholar] [CrossRef]
- Moll, C.J.; Versluis, J.; Bakker, H.B. Direct observation of the orientation of urea molecules at charged interfaces. J. Phys. Chem. Lett. 2021, 12, 10823–10828. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, P.; Hu, K.; Zhang, L.; Cheng, G. Dissolution of cellulose in aqueous NaOH/urea solution: Role of urea. Cellulose 2014, 21, 1183–1192. [Google Scholar] [CrossRef]
- Heyda, J.; Okur, H.I.; Hladilkova, J.; Rembert, K.B.; Hunn, W.; Yang, T.; Dzubiella, J. Guanidinium can both cause and prevent the hydrophobic collapse of biomacromolecules. J. Am. Chem. Soc. 2017, 139, 863–870. [Google Scholar] [CrossRef]
- Delepierre, G.; Vanderfleet, O.M.; Niinivaara, E.; Zakani, B.; Cranston, E.D. Benchmarking cellulose nanocrystals Part II: New industrially produced materials. Langmuir 2021, 37, 8393–8409. [Google Scholar] [CrossRef]
- Bhattacharjee, S.J. In relation to the following article “DLS and zeta potential—What they are and what they are not?”. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Javadi, A.; Mucic, N.; Karbaschi, M.; Won, J.Y.; Fainerman, V.B.; Sharipova, A.; Aksenenko, E.V.; Kovalchuk, V.I.; Kovalchuk, N.M.; Makievski, A.; et al. Interfacial Dynamics Methods. In Encyclopedia of Colloid and Interface Science; Tadros, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Loglio, G.; Pandolfini, P.; Miller, R.; Makievski, A.V.; Ravera, F.; Ferrari, M.; Liggieri, L. Drop and bubble shape analysis as tool for dilational rheology studies of interfacial layers. In Novel Methods to Study Interfacial Layers; Möbius, D., Miller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 439–483. [Google Scholar]
- Ravera, F.; Ferrari, M.; Santini, E.; Liggieri, L. Influence of surface processes on the dilational visco-elasticity of surfactant solutions. Adv. Colloid Interface Sci. 2005, 117, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, M.; Kovalchuk, V.I.; Loglio, G.; Miller, R. Salt effects on the dilational viscoelasticity of surfactant adsorption layers. Curr. Opin. Colloid Interface Sci. 2022, 57, 101538. [Google Scholar] [CrossRef]
- Amani, P.; Karakashev, S.I.; Grozev, N.A.; Simeonova, S.S.; Miller, R.; Rudolph, V.; Firouzi, M. Effect of selected monovalent salts on surfactant stabilized foams. Adv. Colloid Interface Sci. 2021, 295, 102490. [Google Scholar] [CrossRef] [PubMed]
- Briceño-Ahumada, Z.; Maldonado, A.; Impéror-Clerc, M.; Langevin, D. On the stability of foams made with surfactant bilayer phases. Soft Matter 2016, 12, 1459–1467. [Google Scholar] [CrossRef]
- Lim, W.K.; Rösgen, J.; Englander, S.W. Urea, but not guanidinium, destabilizes proteins by forming hydrogen bonds to the peptide group. Proc. Natl. Acad. Sci. USA 2009, 106, 2595–2600. [Google Scholar] [CrossRef] [Green Version]
- Tikhonov, M.M.; Akentiev, A.V.; Noskov, B.A. Influence of guanidine hydrochloride and urea on the dynamic surface properties of lysozyme solutions. Mendeleev Commun. 2015, 25, 288–289. [Google Scholar] [CrossRef]
- Rullier, B.; Axelos, M.A.V.; Langevin, D.; Novales, B. β-Lactoglobulin aggregates in foam films: Effect of the concentration and size of the protein aggregate. J. Colloid Interface Sci. 2010, 343, 330–337. [Google Scholar] [CrossRef]

| LAE-sCNC | LAE-cCNC | |
|---|---|---|
| Foam volume [mL] | 19 ± 1 | 37 ± 1 |
| Foam half-life [min] | 260 ± 20 | >280 |
| cCNC 0.3 wt.% with | Hydrodynamic Diameter [nm] (Polydispersity Index) |
|---|---|
| 0.008% LAE | 87 (0.38) |
| 0.01% LAE | 88 (0.30) |
| 0.015% LAE | 112 (0.44) |
| 0.02% LAE | 206 (0.78) |
| 0.05% LAE | ~7000 (1.00) |
| LAE 0.006 wt.%—cCNC 0.3 wt.% with: | Zeta Potential [mV] | Hydrodynamic Diameter [nm] (Polydispersity Index) |
|---|---|---|
| No additives | −35 ± 5 | 77 (0.44) |
| NaCl 5 mmol/L | −30 ± 5 | 197 (0.53) |
| Urea 6 mol/L | −26 ± 4 | 101 (0.28) |
| Sodium salicylate 5 mmol/L | −31 ± 4 | 108 (0.43) |
| Guanidine hydrochloride chloride 5 mmol/L | −26 ± 3 | 209 (0.52) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czakaj, A.; Krzan, M.; Warszyński, P. The Effect of Electrolytes and Urea on the Ethyl Lauroyl Arginate and Cellulose Nanocrystals Foam Stability. Appl. Sci. 2022, 12, 2797. https://doi.org/10.3390/app12062797
Czakaj A, Krzan M, Warszyński P. The Effect of Electrolytes and Urea on the Ethyl Lauroyl Arginate and Cellulose Nanocrystals Foam Stability. Applied Sciences. 2022; 12(6):2797. https://doi.org/10.3390/app12062797
Chicago/Turabian StyleCzakaj, Agnieszka, Marcel Krzan, and Piotr Warszyński. 2022. "The Effect of Electrolytes and Urea on the Ethyl Lauroyl Arginate and Cellulose Nanocrystals Foam Stability" Applied Sciences 12, no. 6: 2797. https://doi.org/10.3390/app12062797
APA StyleCzakaj, A., Krzan, M., & Warszyński, P. (2022). The Effect of Electrolytes and Urea on the Ethyl Lauroyl Arginate and Cellulose Nanocrystals Foam Stability. Applied Sciences, 12(6), 2797. https://doi.org/10.3390/app12062797







