Abstract
The campaign “No action today, no cure tomorrow” against antimicrobial resistance proposed by the World Health Organization (WHO) has not only propeled people to take action to prevent antimicrobial resistance, but has also encouraged researchers to develop new antimicrobial agents. 4(3H)-quinazolinone and its derivatives belong to a group of compounds with many potential applications; this study was conducted to find new derivatives of heterocyclic 4(3H)-quinazolinone with biological effects, contributing to research on antibacterial and antifungal compounds. Using the closed-loop method between anthranilic acid and acetic anhydride, followed by reaction with aniline derivatives, a substituted product of position 3 of 4(3H)-quinazolinone was obtained, along with bromizing to replace the hydrogen of the methyl group in position 2 with dibromo. Heterocyclic derivatives such as imidazole, triazole, and thiazole were replaced from this dibromo product to obtain 19 derivatives. The structures of these derivatives were checked by modern methods such as IR, 1H-NMR, and MS. The results indicated that all of the structures were as expected, so the process of creating new derivatives from 4(3H)-quinazolinone was achieved in this study. Fourteen of the derivatives, namely 3d, 3e, 3f, 3g, 3h, 3i, 3j, 3k, 3m, 3o, 3p, 3q, 3r, and 3s, had antibacterial or antifungal effects. Among these, there were five potential derivatives: Antifungal activity was observed on A. niger by 3j and 3f (MIC: 32 μg/mL) and 3s (MIC: 64 μg/mL), and on C. albicans by 3f (MIC: 8 μg/mL); antibacterial activity was observed on S. aureus by 3p (MIC: 16 μg/mL) and 3f and 3r (MIC: 32 μg/mL), on MRSA by 3f and 3r (MIC: 32 μg/mL), and on E. coli by 3f (MIC: 32 μg/mL).
1. Introduction
While the discovery of new antimicrobials is decreasing all over the world, the level of antimicrobial resistance (AMR) is considered one of the biggest global health challenges. This situation occurs when microorganisms such as microbes, viruses, fungi, and parasites change in a way that disables or reduces the effects of drugs used in the treatment of infections. It is estimated that, by 2050, antimicrobial resistance may cause 10 million deaths globally. On World Health Day on 7 April 2011, the World Health Organization proposed actions against drug resistance via the “No action today, no cure tomorrow” campaign. Besides actions for preventing antimicrobial resistance, the development of new antimicrobial compounds is considered a high priority for researchers [1,2,3].
Among the studies of biological substances, 4(3H)-quinazolinone derivatives have shown abundant biological activities, such as inhibitory effects on the central nervous system, inhibition of thromboxane A2 synthesis and platelet aggregation, reduction in blood pressure, and sedative, antispasmodic, anticonvulsant, diuretic, hypothermic, analgesic, antiulcer, antituberculosis, antibacterial, antiparasitic, antiviral, and anticancer properties [4,5]. Thus, they have attracted the attention of many scientists around the world, and the development of new antimicrobial compounds has been one of the key areas of study.
4(3H)-quinazolinone is a heterocyclic compound containing two nitrogen atoms, first synthesized by the Russian chemist Niementowski in 1895 via the cyclization reaction between anthranilic acid and formamide [6,7]. Since then, more and more studies have focused on understanding the mechanism by which the Niementowski reaction can be improved, and have found a variety of methods to synthesize heterocyclic compounds containing a nucleus of 4(3H)-quinazolinone [8,9]. Indian scientists have been pioneers in the study of 4(3H)-quinazolinone derivatives and have made great contributions to the history of this compound [10].
4(3H)-quinazolinone and its derivatives are recognized as a group of compounds with many potential applications. Meanwhile, antibiotic resistance is currently being investigated as a major public health risk in light of the fact that we may no longer have antibiotics to fight against infectious diseases. Thus, this study was conducted in order to find new derivatives of heterocyclic 4(3H)-quinazolinone with biological effects, contributing to research on antibacterial and antifungal compounds, serving the pharmaceutical industry in the production of antibiotics in the future.
2. Materials and Methods
2.1. Materials
Determination of melting points was conducted using a Gallenkamp Melting Point Apparatus Aar 3235 (Gallenkamp, Cambridge, UK). Infrared spectra (IR) were measured by IRTracer-100 (Shimazu, Japan). 1H nuclear magnetic resonance spectrometry (1H-NMR) was implemented on a Bruker AV-500 NMR Spectrometer (Bruker, Germany). Mass spectrometry (MS) was measured on a Waters Quattro Micro LC/MS–MS System (Waters Alliance, MA, USA). Anthranilic acid, acetic anhydride, acetic acid, polyphosphoric acid, N-bromosuccinimide (NBS), pyridine, chloroform, sodium sulfate, silica gel for column, thin-layer chromatography (TLC) (silica gel 60 F254), imidazole, triazole, 2-aminothiazol, ethanol, metal sodium, chloroform, potassium carbonate, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (Burlington, MA, USA). C. albicans ATCC 10231, A. niger ATCC 16404, E. coli ATCC 25923, P. aeruginosa ATCC 27853, B. subtilis PY 79, S. faecalis ATCC 29212, S. aureus ATCC 25922, and methicillin-resistant S. aureus (MRSA) ATCC 43300 were obtained from American Type Culture Collection (Manassas, VA 20110, USA).
2.2. Methods
2.2.1. Synthesis of 2-methyl-3-p-substitutedphenyl-4(3H)-quinazolinone (1a–d)
Synthesis of 2,3 position derivatives of 4(3H)-quinazolinone via N-acetylanthranilic acid with aniline derivatives or 2-methyl-4H-3,1-benzoxazin-4-on [11] was carried out.
N-acetylanthranilic acid and 2-methyl-4H-3,1-benzoxazin-4-on were both synthesized by the reaction between anthranilic acid and anhydric acid. However, closed-loop products were easily hygroscopic to open rings, so the substituted 2,3 of 4(3H)-quinazolinone should be prepared using the in situ 2-methyl-4(3H)-quinazolinone method. After removal, the residual solvent was then further reacted with aniline derivatives. The reaction process can be summarized as follows (Scheme 1):
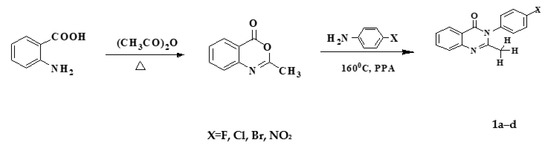
Scheme 1.
Synthesis of 2-methyl-3-p-substitutedphenyl-4(3H)-quinazolinone (1a–d).
The experiments were carried out in two-neck flasks. First of all, anthranilic acid was placed into a two-neck flask. Then, acetic anhydride (Ac2O) was added, heated under reflux, and then stirred. After eliminating acetic anhydride and acetic acid (AcOH) in a reduced-pressure, the mixture was transferred into another two-neck flask, then aniline and polyphosphoric acid (PPA) derivatives were added to the combination before the mixture was heated at approximately 130–140 °C. This was followed by a reaction process via thin-layer chromatography.
After completing the reaction, the combination vessel was left to cool down; then, the mixture was added to the 10% alcohol solvent and heated slowly. Next, the mixture was filtered to obtain the precipitate. The precipitate was re-crystallized in 10% alcohol and bleached with activated carbon and then dried at 60 °C.
2.2.2. Synthesis of 2-dibromomethyl-3-p-substitutedphenyl-4(3H)-quinazolinone (2a–d)
2-dibromomethyl-3-p-substitutedphenyl-4(3H)-quinazolinone was brominated into a methyl group (–CH3) using NBS (Br2 can also be used). NBS was chosen because it is less toxic and easier to manipulate than Br2. The reaction process can be summarized as follows (Scheme 2) [12]:
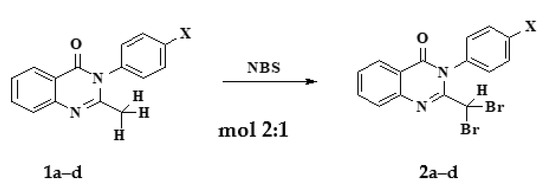
Scheme 2.
Synthesis of 2-dibromomethyl-3-p-substitutedphenyl-4(3H)-quinazolinone (2a–d).
The 2-methyl-4(3H)-quinazolinone derivative was placed into a two-neck flask containing anhydrous chloroform, and the mixture was stirred. After this, NBS and a few drops of pyridine were added to the mixture, which was stirred continuously. The mixture was heated and stirred at 55 °C, then the reaction process was checked with TLC. The mixture after reaction was filtered to remove excess NBS, shaken with Na2S2O5 solution to react with excess NBS (if any), then continued to the anhydrous stage with sodium sulfate under reduced pressure. The products were purified by column chromatography with an appropriate solvent system before being dried at 60 °C.
2.2.3. Synthesis of 2-disubstitutedmethyl-3-p-substitutedphenyl-4(3H)-quinazolinone (3a–s)
In order to replace bromine with imidazole or triazole, sodium hydride, butyl lithium, or sodium ethylate can be utilized. In this study, sodium ethylate was used because it is possible to prepare it in situ from absolute ethanol and metal sodium [13]. The process can be summarized as follows (Scheme 3):
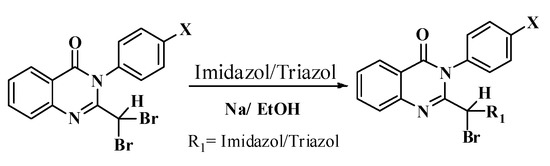
Scheme 3.
Replacing Br with imidazole or triazole.
Sodium was added into a two-neck flask containing absolute ethanol (there was some delay time for the sodium to dissolve) before being stirred for 30 min. Then, imidazole/triazole was added into the mixture and stirred for 60 min. In the next step, the quinazolinone derivative was added, stirred at room temperature, and refluxed. Then, the reaction process was checked by TLC.
After refrigerating the mixture, water was added to break down the excess sodium ethylate, and the products in the mixture were extracted with chloroform. Then, the chloroform was evaporated to obtain raw products. After this, the products were purified by column chromatography with an appropriate solvent system and dried at 60 °C.
When substituting 2-aminothiazol bromine, potassium carbonate, potassium hydroxide, or sodium hydride can be used; in this study, we used potassium carbonate. The process can be summarized as follows (Scheme 4):
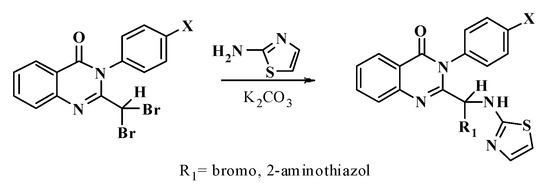
Scheme 4.
Replacing Br with 2-aminothiazol.
Quinazolinone derivatives, 2-aminothiazol, and potassium carbonate were added into a two-neck flask containing ethanol. Then, the mixture was stirred and refluxed. The reaction was checked by TLC.
The solvent was removed from the reaction mixture; the sediment was dissolved with chloroform and shaken with distilled water several times to remove inorganic salts in the sediment, and then an anhydrous chloroform solution was achieved with sodium sulfate before removing the chloroform to obtain the raw products.
The products were purified by column chromatography with an appropriate solvent system and then dried at 60 °C.
This study aimed to generate potential compounds for the development of antibacterial and antifungal agents, not to create a completely novel structural compound for the data system of basic chemistry. Therefore, we used three kinds of spectra—IR, MS, and 1H-NMR—to confirm whether the 4(3H)-quinazolinone derivatives created from this synthesis process matched the predictions.
2.2.4. Testing of the Antifungal and Antibacterial Effects
Antifungal effect: The diffusion method in agar was used in this study. First, the quinazolinone derivative was dissolved in DMSO to obtain a mixture with a concentration of 2048 μg/mL. Next, a volume of approximately 10 μL of the mixture was added into a hole on the agar plates with a 3 mm diameter, which were cultured with C. albicans or A. niger. Then, the agar plates were incubated at 35–37 °C for C. albicans and at 30 °C for A. niger for 24–48 h. Reading the results: For the control hole containing DMSO, it was recorded that it did not inhibit the growth of fungi. In contrast, the derivative was recorded as being resistant to fungi with the appearance of antifungal rings [14].
Antibacterial effect: The procedure was similar to that of testing the antifungal effects, except for incubation time, which was set at 16–18 h (24 h if the bacterium was MRSA) [15,16,17].
2.2.5. Determination of the MIC of Those Derivatives with Antifungal Antibacterial Effects
The derivatives that revealed antifungal or antibacterial effects were further tested for their minimal inhibitory concentration (MIC). The MIC was determined by the semi-quantitative dilution test. The results are expressed as the minimum concentration of the test substance (µg/mL) that inhibited the growth of fungi or bacteria [14,16,18].
The fungi suspension was spread onto agar medium plates to reach a fungi density of 104 and 103 CFU/mL for A. niger and C. albicans, respectively. After this, the agar plates were incubated at 37 °C for C. albicans and at 30 °C for A. niger for 24–48 h, and then holes were made on the agar plates.
The bacteria suspension was spread onto agar medium plates to achieve a bacteria density of 104 CFU/mL for each species—E. coli, P. aeruginosa, B. subtilis, S. faecalis, S. aureus, methicillin-resistant S. aureus (MRSA). Similarly to the procedure of the antifungal test, the agar plates were incubated at 37 °C for 16–18 h (24 h if the bacterium was MRSA), and then holes were made on the agar plates.
The derivative was dissolved in DMSO and diluted by DMSO at concentrations of 1024, 512, 256, 128, 64, 32, 16, 8, 4, and 2 µg/mL. Then, each concentration solution was added into every separated hole on the agar plates as above.
Reading the results: The hole with the lowest concentration was the MIC.
3. Results
3.1. Synthesis of 4(3H)-quinazolinone
3.1.1. Synthesis of 2-methyl-3-p-substitutedphenyl-4(3H)-quinazolinone (1a–d)
A total of four 2-methyl-3-p-substitutedphenyl-4(3H)-quinazolinone derivatives were successfully synthesized, purified, and their properties demonstrated, which were named 1a–d (Figure 1, Table 1).
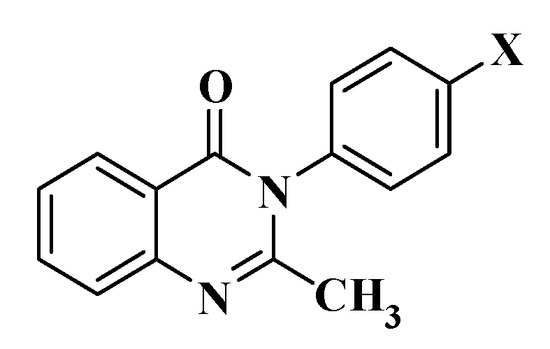
Figure 1.
2-methyl-3-p-substitutedphenyl-4(3H)-quinazolinone derivatives (1a–d) (X = F, Cl, Br, NO2).

Table 1.
Properties of the synthesized 2-methyl-3-p-substitutedphenyl-4(3H)-quinazolinone derivatives (1a–d).
3.1.2. Synthesis of 2-dibromomethyl-3-p-substitutedphenyl-4(3H)-quinazolinone (2a–d)
A total of four 2-dibromomethyl-3-p-substitutedphenyl-4(3H)-quinazolinone derivatives were successfully synthesized, purified, and their properties demonstrated, which were named 2a–d (Figure 2, Table 2).
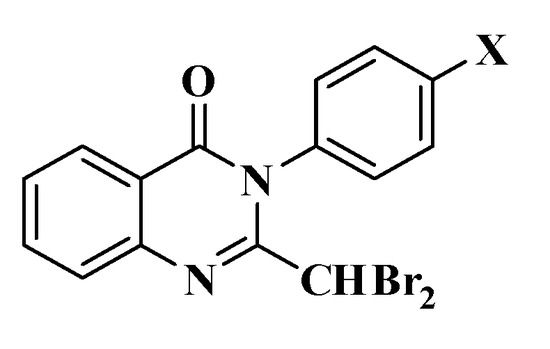
Figure 2.
2-dibromomethyl-3-p-substitutedphenyl-4(3H)-quinazolinone derivatives (2a–d) (X = F, Cl, Br, NO2).

Table 2.
Properties of the synthesized 2-dibromomethyl-3-p-substitutedphenyl-4(3H)-quinazolinone derivatives (2a–d).
3.1.3. Synthesis of 2-disubstitutedmethyl-3-p-substitutedphenyl-4(3H)-quinazolinone (3a–s)
A total of 19 2-disubstitutedmethyl-3-p-substitutedphenyl-4(3H)-quinazolinone derivatives were successfully synthesized, purified (purity ≥95%), and their properties demonstrated, which were named as 3a–s (Figure 3, Table 3).
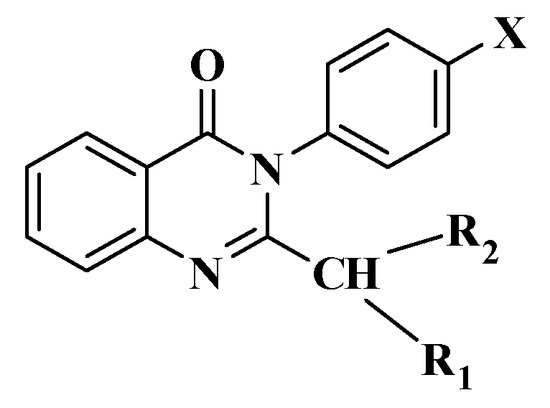
Figure 3.
2-disubstitutedmethyl-3-p-substitutedphenyl-4(3H)-quinazolinone derivatives (3a–s).

Table 3.
Properties of the synthesized 2-disubstitutedmethyl-3-p-substitutedphenyl-4(3H)-quinazolinone derivatives (3a–s).
3.2. Antifungal and Antibacterial Tests of the Derivatives
Upon testing 19 derivatives, three revealed antifungal activity with two strains and 14 (including the three derivatives mentioned) revealed antibacterial activity with five strains. None of the derivatives were recorded as having antibacterial activity against B. subtilis PY79 (Table 4).

Table 4.
Results of the antifungal and antibacterial effects of the derivatives.
3.3. MIC of Derivatives with Antifungal and Antibacterial Effects
Antifungal: On A. niger at a MIC of 32 μg/mL (3f and 3j) and at 64 μg/mL (3s). On C. albicans, only 3f at 8 μg/mL (Table 5); compared to the MIC on Candida spp. For flucytosine (an antifungal agent), the MIC was found to be at a sensitive level [14].

Table 5.
MIC of the antifungal and antibacterial effects of derivatives 3d, 3e, 3f, 3g, 3h, 3i, 3j, 3k, 3m, 3o, 3p, 3q, 3r, and 3s, (μg/mL).
Antibacterial: On S. aureus at a MIC of 16 μg/mL (3p) and at 32 μg/mL (3f and 3r), and on MRSA at 32 μg/mL (3f and 3r) (Table 5); compared to the MIC on Staphylococcus spp. for some antibiotics from cephem (cefazoline, cefotaxime, ceftazidime, ceftriaxone, and cefaclor) and the glycopeptide group (vancomycin), this MIC was at an intermediate level, but, compared to the others from the cephem group (cefmetazole, cefoperazone, and cefotetan) and the aminoglycoside group (amikacin, kanamycin), the MIC was found to be at a sensitive level [16]. Furthermore, 3f on E. coli at a MIC of 32 μg/mL; compared to the MIC on Enterobacteriaceae for penicillin antibiotics (piperacillin and carbenicillin) and the cephem group (cefoperazone, cefotaxim, and ceftriaxone), this MIC was found to be at a sensitive level [16].
4. Discussion
From the results of this research, it was found that, although the 13C NMR spectrum has not been measured yet, the results of the three kinds of spectra of IR, MS, and 1H-NMR were strong enough for the authors to confirm that the substances created from this synthesis process matched the predictions. Therefore, other researchers can utilize this process to synthesize the 4(3H)-quinazolinone derivatives mentioned in the title on a larger scale.
In particular, in line with the goals of this study, 14 substances were revealed to possess antibacterial and antifungal effects, in which there were five potential derivatives with good MICs. Hence, other researchers could expand our pilot study to other bacterial or fungal strains to obtain more data. By the results of this study, the derivatives 3a–s showed different effects on bacteria and fungi because of the main difference of R1 or R2 in the 2-disubstitutedmethyl functional group of 4(3H)-quinazolinone derivatives, in which we should focus on five potential derivatives (3f, 3j, 3p, 3r, 3s). Among them, there were four derivatives containing R1 = R2 (3f: R1 = R2 = Thi; 3j: R1 = R2 = Tri; 3p: R1 = R2 = Tri; 3s: R1 = R2 = Imi), which means the C in the 2-disubstitutedmethyl functional group is not the C* (asymmetric carbon atom), and there is no C* in these compounds, so we except the chirality effects to five potential derivatives in this study.
Additionally, there are some main ways that the antibiotics act on bacteria: They inhibit DNA synthesis, RNA synthesis, cell wall synthesis, and protein synthesis [19], meaning that the antibiotics must cross the bacterial cell membrane to achieve such inhibition. The boundary of the cell membranes behaves as a lipid-like barrier against the passage of many foreign organic compounds. Numerous drugs diffuse across the cell boundary due to the fact that they are lipid soluble, and the rates of transfer are, in general, related to the relative lipid/water partition coefficients of the molecules and the almost lipid-insoluble or ionized forms of drugs that can difficultly diffuse across these boundaries [20]. In addition, some studies have confirmed the correlation of bacterial cell membranes with antimicrobial agents, in which the lipid composition of the bacterial membrane could affect the effect of antibacterial agents, indicating the importance of the lipid composition of bacterial membranes in determining the susceptibility of an organism to the action of certain antimicrobial agents [21]. Based on the results of this study, most R1 = R2 = heterocyclic compounds (3f: R1 = R2 = Thi; 3j: R1 = R2 = Tri; 3p: R1 = R2 = Tri; 3s: R1 = R2 = Imi) had better antibacterial effects than R1 = halogen compounds. Because R1 = R2 = heterocyclic compounds were well dissolved in the lipid medium, their permeability through cell membranes was better than that of R1 = halogen compounds. This important property helps active substances penetrate fungal and bacterial cells, leading to the enhancement of their antibacterial and antifungal effects, in which the difference between 3f and other substances is the thiazole heterocycle, consisting of 1 N and 1 S. Thus, it can be preliminarily recognized that different heterocyclic elements are able to increase the capacity of cell membrane permeability. Therefore, the antibacterial and antifungal effects can be optimized in further research by focusing on substance 3f or by increasing the solubility of potential substances in lipids by using other heterocycles as agent replacements, with particular attention paid to heterocycles containing N and S.
5. Conclusions
Using the closed-loop method between anthranilic acid and acetic anhydride, followed by a reaction with aniline derivatives, a substituted product of position 3 of 4(3H)-quinazolinone was obtained, along with bromizing to replace the hydrogen of the methyl group in position 2 with dibromo. Heterocyclic derivatives such as imidazole, triazole, and thiazole were replaced by this dibromo product to obtain 19 derivatives. The structures of these derivatives were checked using modern methods such as IR, 1H-NMR, and MS, the results of which indicated that all of the structures were as expected, so the process of creating new derivatives from 4(3H)-quinazolinone was achieved in this study.
In terms of antifungal activity, 3j and 3s revealed such activity on A. niger at a MIC of 32 μg/mL (3j) and 64 μg/mL (3s), while 3f was active on both A. niger and C. albicans at a MIC of 32 μg/mL and 8 μg/mL.
Regarding antibacterial activity, there were 14 active derivatives, namely 3d, 3e, 3f, 3g, 3h, 3i, 3j, 3k, 3m, 3o, 3p, 3q, 3r, and 3s. Among them, 3f, 3p, and 3r revealed such activity on S. aureus at a MIC of 16 μg/mL (3p) and 32 μg/mL (3f and 3r), on MRSA at a MIC of 32 μg/mL (3f and 3r), and on E. coli at a MIC of 32 μg/mL (3f).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12052710/s1, Figures S1–S19: 1H-NMR, IR, and MS spectra of 3a–s.
Author Contributions
Conceptualization, H.T.T. and O.N.T.V.; data curation, H.T.T., O.N.T.V., T.N.T.L. and N.N.V.; formal analysis, H.T.T. and T.N.T.L.; investigation, O.N.T.V., T.N.T.L. and N.N.V.; methodology, H.T.T. and O.N.T.V.; project administration, H.T.T.; resources, H.T.T. and B.D.C.N.; software, O.N.T.V., B.D.C.N. and N.N.V.; supervision, H.T.T., O.N.T.V. and B.D.C.N.; validation, H.T.T.; visualization, H.T.T. and B.D.C.N.; writing—original draft, O.N.T.V., T.N.T.L. and N.N.V.; writing—review and editing, H.T.T., O.N.T.V. and N.N.V. All authors have read and agreed to the published version of this manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the support of time and facilities from the Center for Standardization and Quality Control in the Medical Lab of Ho Chi Minh City, Pham Ngoc Thach University of Medicine, University of Medicine and Pharmacy at Ho Chi Minh City.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
1H-NMR (DMSO, 500 MHz) of the synthesized quinazolinone derivatives.
Table A1.
1H-NMR (DMSO, 500 MHz) of the synthesized quinazolinone derivatives.
| Synthesized Quinazolinone Derivatives | 1H-NMR (DMSO, 500 MHz) |
|---|---|
| 1a | 8.09 (dd, J = 1.5 Hz, 8 Hz, 1H, H5); 7.85–7.81 (m, 1H, H7); 7.65 (d, J = 1 Hz, 8.5 Hz, 1H, H8); 7.53–7.49 (m, 3H, H6, H2′, H6′); 7.41–7.37 (m, 2H, H3′, H5′); 2.13 (s, 3H, –CH3) |
| 1b | 8.09 (dd, J = 1.5 Hz, 7.75 Hz, 1H, H5); 7.86–7.82 (m, 1H, H7); 7.66–7.62 (m, 3H, H8, H3′, H5′); 7.53–7.50 (m, 3H, H6, H2′, H6′); 2.13 (s, 3H, –CH3) |
| 1c | 8.09 (dd, J = 1.5 Hz, 8 Hz, 1H, H5); 7.86–7.82 (m, 1H, H7); 7.78–7.75 (m, 2H, H2′, H6′); 7.66 (d, J = 7.5 Hz, 1H, H8); 7.53–7.50 (m, 1H, H6); 7.46–7.43 (m, 2H, H3′, H5′; 2.13 (s, 3H, –CH3) |
| 1d | 8.42–8.40 (m, 2H, H3′, H5′); 8.11 (dd, J = 1.5 Hz, 7.75 Hz, 1H, H5); 7.87–7.84 (m, 1H, H7); 7.66–7.80 (m, 2H, H2′, H6′); 7.68 (d, J = 8 Hz, 1H, H8); 7.55–7.21 (m, 1H, H6); 2.14 (s, 3H, –CH3) |
| 2a | 8.29–8.28 (d, J = 8 Hz, 1H, H5); 7.90–7.83 (m, 2H, H-aromatic); 7.58–7.55 (m, 1H, H7); 7.37–7.35 (m, 2H, H6, H8); 7.31–7.27 (m, 2H, H-aromatic); 6.07 (s, 1H, HCHBr2) |
| 2b | 8.29–8.27 (dd, J = 1.5 Hz; 7.75 Hz, 1H, H5); 7.91–7.83 (m, 2H, H-aromatic); 7.58–7.54 (m, 1H, H7); 7.37–7.35 (m, 2H, H6, H8); 7.31–7.28 (2H, H-aromatic); 6.07 (s, 1H, HCHBr2) |
| 2c | 8.29–8.28 (dd, J = 8 Hz, 1H, H5); 7.90–7.85 (m, 2H, H6, H8); 7.75–7.73 (d, J = 8.5 Hz, 2H, H-aromatic); 7.58–7.55 (m, 1H, H7); 7.25–7.24 (m, 1H, H-aromatic); 6.06 (s, 1H, HCHBr2) |
| 2d | 8.49–8.48 (m, 2H, H-aromatic); 8.30–8.29 (dd, J = 1 Hz, 7.75 Hz, 1H, H5); 7.93–7.87 (m, 2H, H-aromatic); 7.63–7.59 (m, 3H, H6, H7, H8); 6.01 (s, 1H, HCHBr2) |
| 3a | 8.28–8.22 (m, 2H, H2′, H6′); 7.85 (d, J = 2 Hz, 1H, H5); 7.39 (d, J = 1 Hz, 1H, H2″); 7.32–7.27 (m, 3H,H6, H7, H8); 7.06–7.03 (m, 2H, H3′, H5′); 6.94 (d, J = 3.5 Hz, 1H, H5″); 6.45 (d, J = 3.5 Hz, 1H, H4″); 5.79 (s, 1H, CH) |
| 3b | 7.76–7.70 (m, 2H, H2′, H6′); 7.50 (d, J = 1.5 Hz, 1H, H5); 7.35 (d, J = 1 Hz, 2H, H2″); 7.29–7.24 (m, 3H,H6, H7, H8); 7.06–7.03 (m, 2H, H3′, H5′); 6.91 (d, J = 3.5 Hz, 1H, H5″); 6.40 (d, J = 3.5 Hz, 1H, H4″); 5.49 (s, 1H, CH) |
| 3c | 8.02–7.96 (m, 2H, H2′, H6′); 7.85 (d, J = 4 Hz, 1H, H5″); 7.29 (d, J = 1 Hz, 1H, H5); 7.21 (d, J = 4 Hz, 1H, H3″); 7.09–7.04 (m, 3H, H6, H7, H8); 6.95–6.92 (m, 2H, H3′, H5′); 5.21 (s, 1H, CH) |
| 3d | 7.57–7.51 (m, 2H, H2′, H6′); 7.45 (d, J = 4 Hz, 1H, H5″); 7.25 (d, J = 1.5 Hz, 1H, H5); 7.24 (d, J = 4 Hz, 1H, H3″); 6.80–6.75 (m, 3H, H6, H7, H8); 6.62–6.59 (m, 2H, H3′, H5′); 5.29 (s, 1H, CH) |
| 3e | 8.25–8.22 (m, 2H, H2′, H6′); 7.85 (d, J = 1 Hz, 1H, H5); 7.30–7.27 (m, 3H, H6, H7, H8); 7.26–7.24 (m, 2H, H3′, H5′); 7.07 (d, J = 3.5 Hz, 1H, H5″); 6.53 (d, J = 3.5 Hz, 1H, H4″); 5.87 (d, J = 3 Hz, 1H, CH); 5.06 (d, J = 3.5 Hz, 1H, NH) |
| 3f | 8.01–7.95 (m, 2H, H2′, H6′); 7.52 (d, J = 1.5 Hz, 1H, H5); 7.06–7.01 (m, 3H, H6, H7, H8); 6.89–6.85 (m, 2H, H3′, H5′); 6.59 (d, J = 4 Hz, 1H, H5″); 6.02 (d, J = 4 Hz, 1H, H4″); 5.31 (d, J = 2 Hz, 2H, NH); 4.87 (t, 1H, CH) |
| 3g | 8.69 (d, J = 5 Hz, 1H, H5′); 7.99 (d, J = 1 Hz, 1H, H2″); 7.61–7.56 (m, 3H, H6, H7, H8); 7.36–7.30 (m, 2H, H2′, H6′); 7.28–7.25 (m, 2H, H3′, H5′); 6.92 (d, J = 3.5 Hz, 1H, H5″); 6.70 (d, J = 3.5 Hz, 1H, H4″); 4.91 (s, 1H, CH) |
| 3h | 8.10 (d, J = 5 Hz, 1H, H5′); 7.70 (d, J = 1 Hz, 1H, H2″); 7.49–7.45 (m, 3H, H6, H7, H8); 7.44–7.29 (m, 2H, H2′, H6′); 7.27–7.24 (m, 2H, H3′, H5′); 7.04 (d, J = 3.5 Hz, 1H, H5″); 6.71 (d, J= 3.5 Hz, 1H, H4″); 4.90 (s, 1H, CH) |
| 3i | 7.97 (d, J = 3.5 Hz, 1H, H5″); 7.54 (d, J = 5 Hz, 1H, H5); 7.46 (d, J = 3.5 Hz, 1H, H3″); 7.29–7.24 (m, 3H, H6, H7, H8); 6.95–6.89 (m, 2H, H2′, H6′); 6.62–6.59 (m, 2H, H3′, H5′); 4.91 (s, 1H, CH) |
| 3j | 7.99 (d, J = 4 Hz, 1H, H5″); 7.66 (d, J = 5.5 Hz, 1H, H5); 7.47 (d, J = 4 Hz, 1H, H3″); 7.30–7.25 (m, 3H, H6, H7, H8); 6.86–6.80 (m, 2H, H2′, H6′); 6.60–6.57 (m, 2H, H3′, H5′); 4.91 (s, 1H, CH) |
| 3k | 7.82 (d, J = 4 Hz, 1H, H5″); 7.51 (d, J = 5 Hz, 1H, H5); 7.44 (d, J = 4 Hz, 1H, H3″); 7.27–7.22 (m, 3H, H6, H7, H8); 6.86–6.80 (m, 2H, H2′, H6′); 6.60–6.57 (m, 2H, H3′, H5′); 4.09 (s, 1H, CH) |
| 3l | 8.68 (d, J = 5 Hz, 1H, H5); 8.05 (d, J = 1 Hz, 2H, H2″); 7.63–7.58 (m, 3H, H6, H7, H8); 7.41–7.37 (m, 2H, H2′, H6′); 7.35–7.25 (m, 2H, H3′, H5′); 6.95 (d, J = 3.5 Hz, 1H, H5″); 6.74 (d, J = 3.5 Hz, 1H, H4″); 4.07 (s, 1H, CH) |
| 3n | 8.26–8.56 (m, 2H, H2′, H6′); 7.97 (d, J = 1.5 Hz, 1H, H5); 7.61 (d, J = 1 Hz, 1H, H2″); 7.39–7.34 (m, 3H, H6, H7, H8); 7.24–7.21 (m, 2H, H3′, H5′); 6.95 (d, J = 3.5 Hz, 1H, H5″); 6.46 (d, J = 3.5 Hz, 1H, H4″); 5.70 (s, 1H, CH) |
| 3m | 8.16 (d, J = 1.5 Hz, 1H, H5); 7.87 (d, J = 1 Hz, 2H, H2″); 7.36–7.31 (m, 3H, H6, H7, H8); 7.19–7.10 (m, 4H, H2′, H6′, H3′, H5′); 6.91 (d, J = 3.5 Hz, 1H, H5″); 6.70 (d, J = 3.5 Hz, 1H, H4″); 5.27 (s, 1H, CH) |
| 3o | 8.31–8. 25 (m, 2H, H2′, H6′); 7.82 (d, J = 4.5 Hz, 1H, H5″); 7.40 (d, J = 1.5 Hz, 1H, H5); 7.38 (d, J = 4.5 Hz, 1H, H3″); 7.26–7.21 (m, 3H, H6, H7, H8); 7.19–7.16 (m, 2H, H3′, H5′); 4.93 (s, 1H, CH) |
| 3p | 8.03 (d, J = 4.5 Hz, 2H, H5″); 8.02–7.96 (m, 2H, H2′, H6′); 7.30 (d, J = 1.5 Hz, 1H, H5); 7.26 (d, J = 4.5 Hz, 2H, H3″); 7.17–7.12 (m, 3H, H6, H7, H8); 7.04–7.01 (m, 2H, H3′, H5′); 5.21 (s, 1H, CH) |
| 3q | 8.25–8.19 (m, 2H, H2′, H6′); 7.84 (d, J = 1 Hz, 1H, H5); 7.30–7.25 (m, 3H, H6, H7, H8); 7.17–7.14 (m, 2H, H3′, H5′); 7.02 (d, J = 3.5 Hz, 1H, H5″); 6.49 (d, J = 3.5 Hz, 1H, H4″); 5.78 (d, J = 4 Hz, 1H, CH); 4.92 (d, J = 4.5 Hz, 1H, NH) |
| 3r | 8.05–8.02 (m, 2H, H3′, H5′); 7.90 (d, J = 1.5 Hz, 1H, H5); 7.64 (d, J = 1 Hz, 1H, H2″); 7.63–7.57 (m, 2H, H2′, H6′); 7.56–7.51 (m, 3H, H6, H7, H8); 6.96 (d, J = 3.5 Hz, 1H, H5″); 6.48 (d, J = 3.5 Hz, 1H, H4″); 5.32 (s, 1H, CH) |
| 3s | 8.04–8.01 (m, 2H, H3′, H5′); 7.89 (d, J = 1.5 Hz, 1H, H5); 7.63 (d, J = 1 Hz, 1H, H2″); 7.60–7.54 (m, 2H, H2′, H6′); 7.53–7.48 (m, 3H, H6, H7, H8); 6.92 (d, J = 3.5 Hz, 1H, H5″); 6.45 (d, J = 3.5 Hz, 1H, H4″); 5.38 (s, 1H, CH) |
References
- WHO. Antimicrobial Resistance, 17 November 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 4 January 2022).
- WHO. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis, 29 April 2019. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 4 January 2022).
- WHO. Antimicrobial Resistance: No Action Today, No Cure Tomorrow, 7 April 2011. Available online: https://www.who.int/director-general/speeches/detail/antimicrobial-resistance-no-action-today-no-cure-tomorrow (accessed on 4 January 2022).
- Ingle, R.G.; Magar, D.D. Heterocyclic chemistry of benzimidazoles and potential activities of derivatives. Int. J. Drug Res. Technol. 2011, 1, 26–32. [Google Scholar]
- Mahato, A.K.; Srivastava, B.; Nithya, S. Chemistry, structure activity relationship and biological activity of quinazoline-4 (3H)-one derivatives. Inventig. Rapid. Med. Chem. 2011, 2, 13–19. [Google Scholar]
- Meyer, J.F.; Wagner, E.C. The Niementowski reaction. The use of methyl anthranilate or isatoic anhydride with substituted amides or amidines in the formation of 3-substituted-4-keto-3, 4-dihydroquinazolines. The course of the reaction. J. Org. Chem. 1943, 8, 239–252. [Google Scholar] [CrossRef]
- Philipova, I.; Dobrikov, G.; Krumova, K.; Kaneti, J. Convenient synthesis of some 2-substituted 4(3H)-quinazolinone derivatives. J. Heterocycl. Chem. 2006, 43, 1057–1063. [Google Scholar] [CrossRef]
- Kidwai, M.; Rastogi, S.; Mohan, R. A Novel Route to the Niementowski Reaction. Croat. Chem. Acta 2003, 76, 365–369. [Google Scholar]
- Cheng, C.; Yan, S. The Friedländer Synthesis of Quinolines. Org. React. 2004, 28, 37–201. [Google Scholar]
- Kacker, I.K.; Zaheer, S.H. Synthesis of substituted 4-quinazolones. J. Indian Chem. Soc. 1951, 28, 344–346. [Google Scholar]
- Katritzky, A.R.; Rees, C.W.; Scriven, E.F. Comprehensive Heterocyclic Chemistry II; Pergamon: Oxford, UK, 1996. [Google Scholar]
- Furniss, B.S. Vogel's Textbook of Practical Organic Chemistry; Pearson Education India: London, UK, 1989. [Google Scholar]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Rodriguez-Tudela, J.L.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Verweij, P.E. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2003, 9, i–viii. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.M. The development of the BSAC standardized method of disc diffusion testing. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 29–42. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. (Eds.) Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- MacGowan, A.P.; Wise, R. Establishing MIC breakpoints and the interpretation of in vitro susceptibility tests. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C. Antibiotics: Actions, Origins, Resistance; American Society for Microbiology (ASM) Press: Washington, DC, USA, 2003. [Google Scholar]
- Schanker, L.S. Mechanisms of drug absorption and distribution. Annu. Rev. Pharmacol. 1961, 1, 29–45. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F. Bacterial membrane lipids in the action of antimicrobial agents. J. Pept. Sci. 2011, 17, 298–305. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).