Mn(II) Complexes of Enlarged Scorpiand-Type Azamacrocycles as Mimetics of MnSOD Enzyme
Abstract
:1. Introduction
2. Materials and Methods
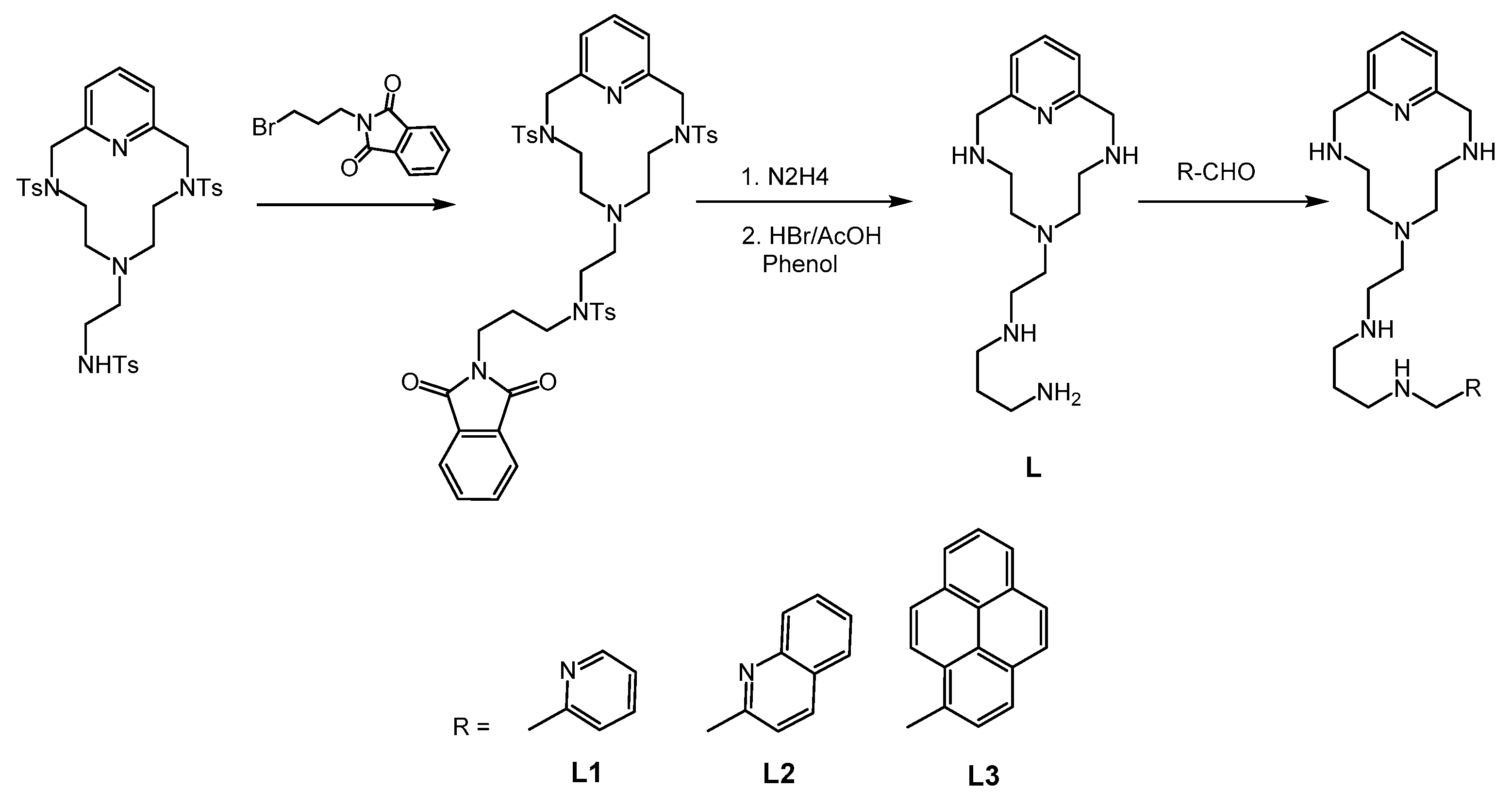
2.1. Synthesis
2.2. Electromotive Force Measurements (emf)
2.3. Electrochemistry
2.4. NMR Measurements
2.5. In Vitro Evaluation of the SOD Activity
3. Results
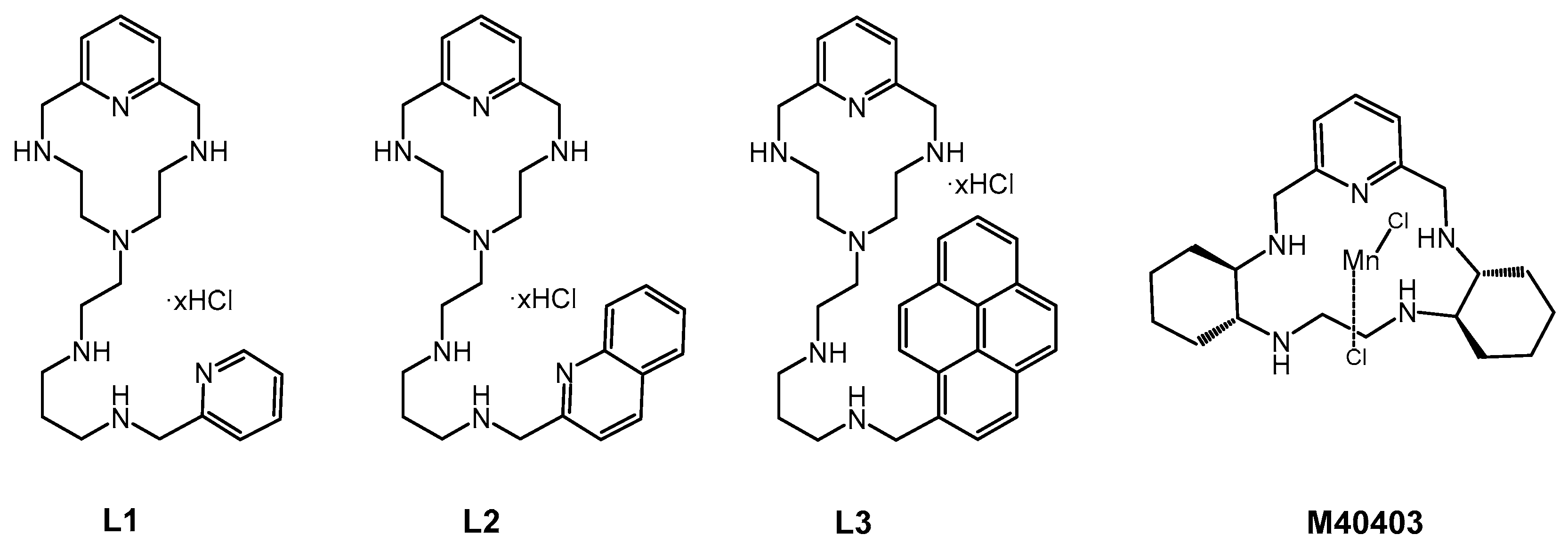
3.1. Basicity of the Ligands and Mn(II) Coordination
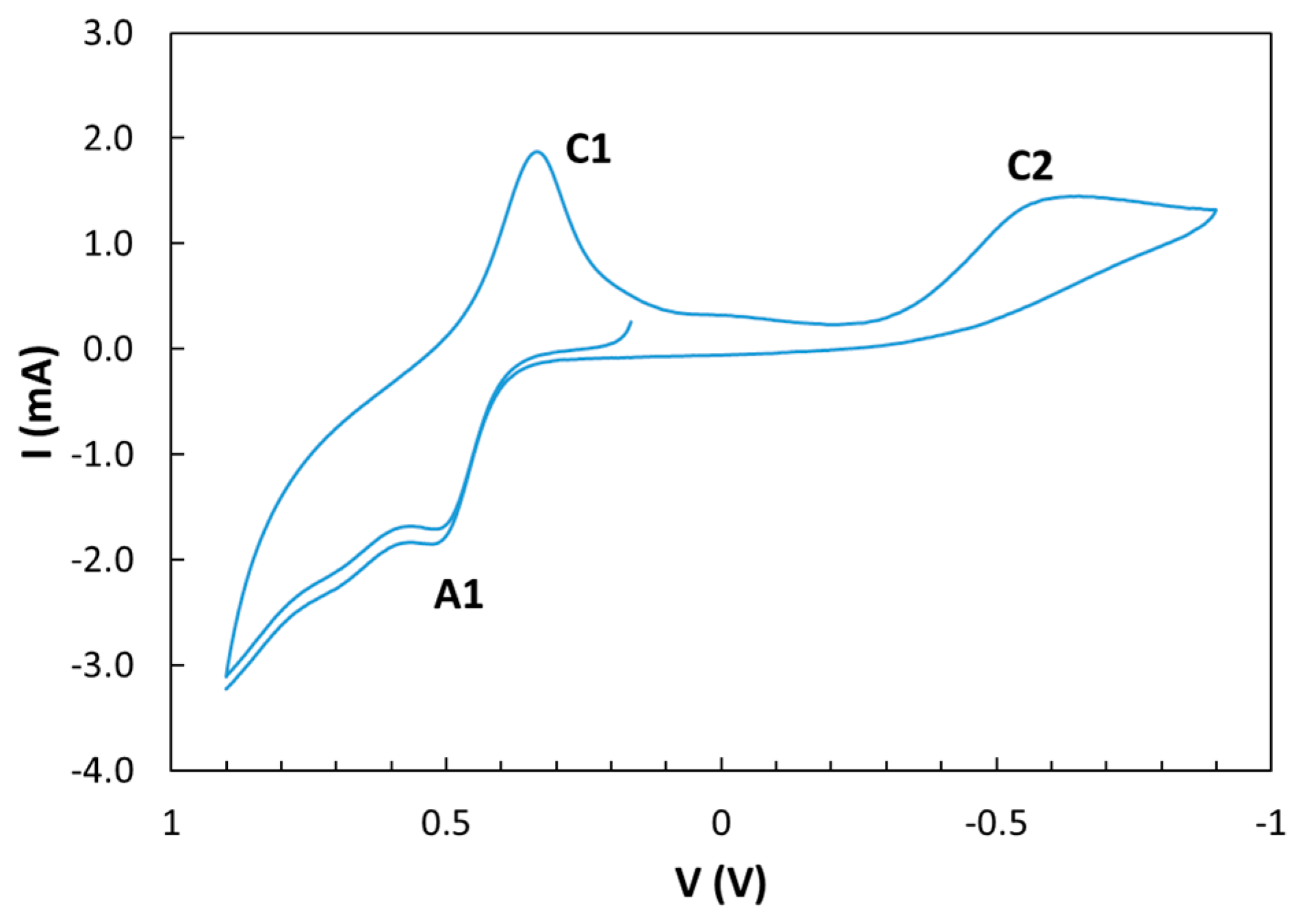
3.2. Electrochemical Behaviour
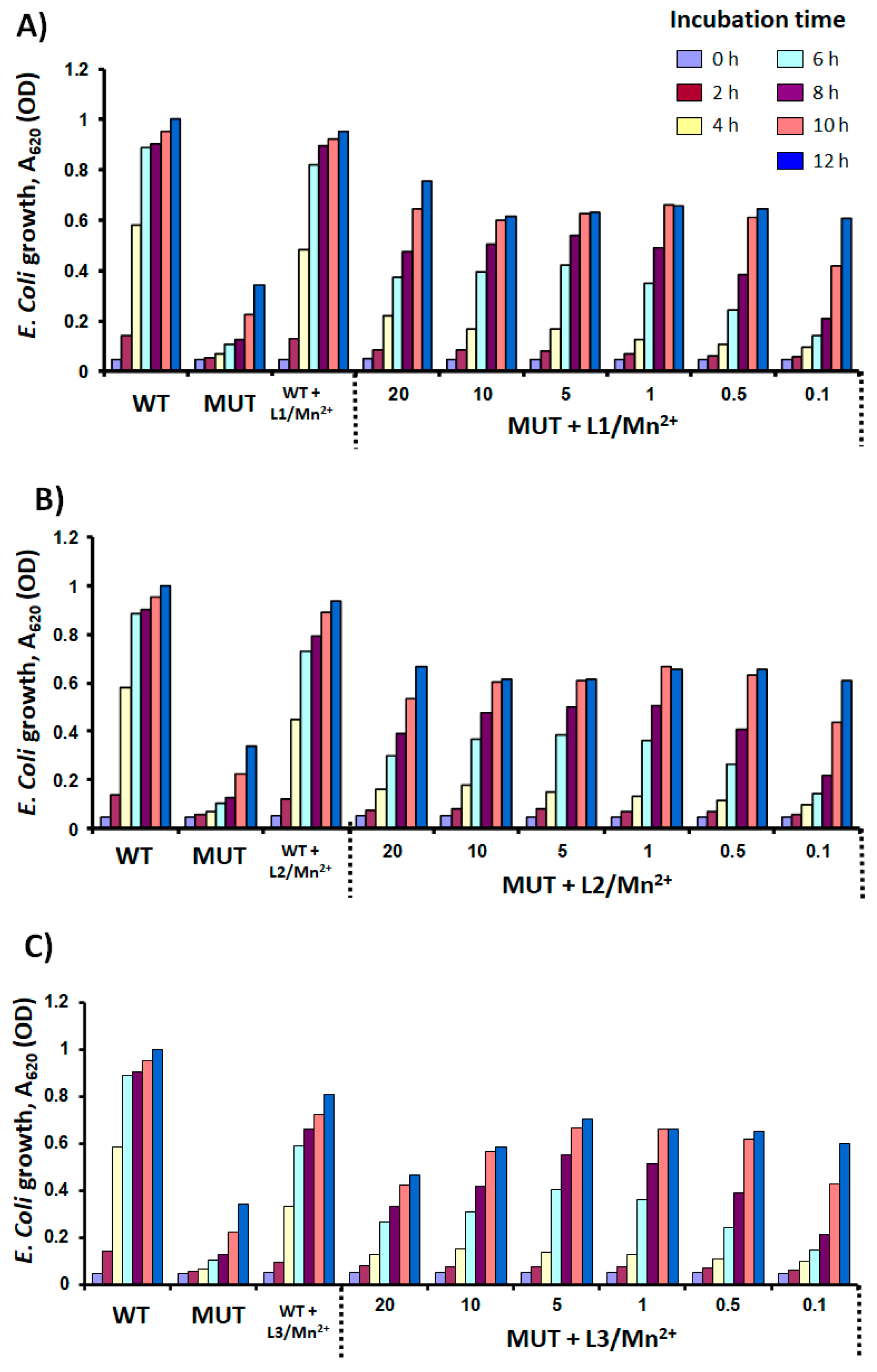
3.3. SOD Mimetic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brawn, K.; Fridovich, I. Superoxide Radical and Superoxide Dismutases: Threat and Defense. In Autoxidation in Food and Biological Systems; Simic, M.G., Karel, M., Eds.; Springer: Boston, MA, USA, 1980; pp. 429–446. [Google Scholar]
- Fridovich, I. Superoxide anion radical (O2−), superoxide dismutases, and related matters. J. Biol. Chem. 1997, 272, 18515–18517. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.; Giulivi, C. Superoxide dismutases and their impact upon human health. Mol. Asp. Med. 2005, 26, 340–352. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuuchi, T.; Doh-ura, K.; Yoshihara, S.; Ohta, S. Metal complexes with superoxide dismutase-like activity as candidates for anti-prion drug. Bioorg. Med. Chem. Lett. 2006, 16, 5982–5987. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.P.; Schall, O.F. Structure-Activity Studies and the Design of Synthetic Superoxide Dismutase (SOD) Mimetics as Therapeutics. Adv. Inorg. Chem. 2006, 59, 233–263. [Google Scholar]
- Iranzo, O. Manganese complexes displaying superoxide dismutase activity: A balance between different factors. Bioorg. Chem. 2011, 39, 73–87. [Google Scholar] [CrossRef]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; St. Clair, D.; Batinic-Haberle, I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta-Mol. Basis Dis. 2012, 1822, 794–814. [Google Scholar] [CrossRef] [Green Version]
- Ivanović-Burmazović, I.; Filipović, M.R. Chapter 3—Reactivity of manganese superoxide dismutase mimics toward superoxide and nitric oxide. Selectivity versus cross-reactivity. Adv. Inorg. Chem. 2012, 64, 53–95. [Google Scholar]
- DeFreitas-Silva, G.; Rebouças, J.S.; Spasojević, I.; Benov, L.; Idemori, Y.M.; Batinić-Haberle, I. SOD-like activity of Mn(II) β-octabromo-meso-tetrakis(N-methylpyridinium-3-yl)porphyrin equals that of the enzyme itself. Arch. Biochem. Biophys. 2008, 477, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Doctrow, R.; Huffman, K.; Marcus, C.B.; Musleh, W.; Bruce, A.; Baudry, M.; Malfroy, B. Salen-Manganese Complexes: Combined Superoxide Dismutase/Catalase Mimics with Broad Pharmacological Efficacy. Adv. Pharmacol. 1996, 247–269. [Google Scholar]
- Salvemini, D.; Wang, Z.Q.; Zweier, J.L.; Samouilov, A.; Macarthur, H.; Misko, T.P.; Currie, M.G.; Cuzzocrea, S.; Sikorski, J.A.; Riley, D.P. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science 1999, 286, 304–306. [Google Scholar] [CrossRef]
- Clares, M.P.; Blasco, S.; Inclán, M.; del Castillo Agudo, L.; Verdejo, B.; Soriano, C.; Doménech, A.; Latorre, J.; García-España, E. Manganese(II) complexes of scorpiand-like azamacrocycles as MnSOD mimics. Chem. Commun. 2011, 47, 5988–5990. [Google Scholar] [CrossRef]
- Clares, M.P.; Serena, C.; Blasco, S.; Nebot, A.; del Castillo, L.; Soriano, C.; Domènech, A.; Sánchez-Sánchez, A.V.; Soler-Calero, L.; Mullor, J.L.; et al. Mn(II) complexes of scorpiand-like ligands. A model for the MnSOD active centre with high in vitro and in vivo activity. J. Inorg. Biochem. 2015, 143, 1–8. [Google Scholar] [CrossRef]
- Guijarro, L.; Inclán, M.; Pitarch-Jarque, J.; Doménech-Carbó, A.; Ugarte Chicote, J.; Trefler, S.; García-España, E.; García-España, A.; Verdejo, B. Homo- and Heterobinuclear Cu2+ and Zn2+ Complexes of Ditopic Aza Scorpiand Ligands as Superoxide Dismutase Mimics. Inorg. Chem. 2017, 56, 13748–13758. [Google Scholar] [CrossRef]
- Lotz, T.J.; Kaden, T.A. pH-Induced co-ordination geometry change in a macrocyclic nickel(II) complex. J. Chem. Soc. Chem. Commun. 1977, 15–16. [Google Scholar] [CrossRef]
- Pallavicini, P.S.; Perotti, A.; Poggi, A.; Seghi, B.; Fabbrizzi, L. N-(aminoethyl)cyclam: A tetraaza macrocycle with a coordinating tail (scorpiand). Acidity controlled coordination of the side chain to nickel(II) and nickel(III) cations. J. Am. Chem. Soc. 1987, 109, 5139–5144. [Google Scholar] [CrossRef]
- Verdejo, B.; Ferrer, A.; Blasco, S.; Castillo, C.E.; González, J.; Latorre, J.; Máñez, M.A.; Basallote, M.G.; Soriano, C.; García-España, E. Hydrogen and Copper Ion-Induced Molecular Reorganizations in Scorpionand-like Ligands. A Potentiometric, Mechanistic, and Solid-State Study. Inorg. Chem. 2007, 46, 5707–5719. [Google Scholar] [CrossRef] [PubMed]
- Inclán, M.; Albelda, M.T.; Frías, J.C.; Blasco, S.; Verdejo, B.; Serena, C.; Salat-Canela, C.; Díaz, M.L.; García-España, A.; García-España, E. Modulation of DNA binding by reversible metal-controlled molecular reorganizations of scorpiand-like ligands. J. Am. Chem. Soc. 2012, 134, 9644–9656. [Google Scholar] [CrossRef] [PubMed]
- Inclán, M.; Albelda, M.T.; Carbonell, E.; Blasco, S.; Bauzá, A.; Frontera, A.; García-España, E. Molecular recognition of nucleotides in water by scorpiand-type receptors based on nucleobase discrimination. Chem. Eur. J. 2014, 20, 3730–3741. [Google Scholar] [CrossRef] [PubMed]
- Olmo, F.; Clares, M.P.; Marín, C.; González, J.; Inclán, M.; Soriano, C.; Urbanová, K.; Tejero, R.; Rosales, M.J.; Krauth-Siegel, R.L.; et al. Synthetic single and double aza-scorpiand macrocycles acting as inhibitors of the antioxidant enzymes iron superoxide dismutase and trypanothione reductase in Trypanosoma cruzi with promising results in a murine model. RSC Adv. 2014, 4, 65108–65120. [Google Scholar] [CrossRef]
- García-España, E.; Ballester, M.-J.; Lloret, F.; Moratal, J.M.; Faus, J.; Bianchi, A. Low-spin six-co-ordinate cobalt(II) complexes. A solution study of tris(violurato)cobaltate(II) ions. J. Chem. Soc. Dalton Trans. 1988, 101–104. [Google Scholar] [CrossRef]
- Fontanelli, M.; Micheloni, M. Proceedings of the I Spanish–Italian Congress on Thermodynamics of Metal Complexes; Diputación de Castellón: Castellón, Spain, 1990.
- Savastano, M.; Fiaschi, M.; Ferraro, G.; Gratteri, P.; Mariani, P.; Bianchi, A.; Bazzicalupi, C. Sensing Zn2+ in Aqueous Solution with a Fluorescent Scorpiand Macrocyclic Ligand Decorated with an Anthracene Bearing Tail. Molecules 2020, 25, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gran, G. Determination of the Equivalence Point in Potentiometric Titrations. Part II. Analyst 1952, 77, 661–671. [Google Scholar] [CrossRef]
- Rossotti, F.J.C.; Rossoti, H. Potentiometric titrations using Gran plots: A textbook omission. J. Chem. Educ. 1965, 42, 375–378. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Bencini, A.; Bianchi, A.; García-España, E.; Micheloni, M.; Ramírez, J.A. Proton coordination by polyamine compounds in aqueous solution. Coord. Chem. Rev. 1999, 188, 97–156. [Google Scholar] [CrossRef]
- Barnese, K.; Gralla, E.B.; Cabelli, D.E.; Valentine, J.S. Manganous phosphate acts as a superoxide dismutase. J. Am. Chem. Soc. 2008, 130, 4604–4606. [Google Scholar] [CrossRef] [Green Version]
- Muscoli, C.; Cuzzocrea, S.; Ridley, D.P.; Zweier, J.L.; Thiemermann, C.; Wang, Z.-Q.; Salvemini, D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br. J. Pharmacol. 2003, 140, 445–460. [Google Scholar] [CrossRef] [Green Version]
- Imlay, J.A.; Linn, S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J. Bacteriol. 1987, 169, 2967–2976. [Google Scholar] [CrossRef] [Green Version]
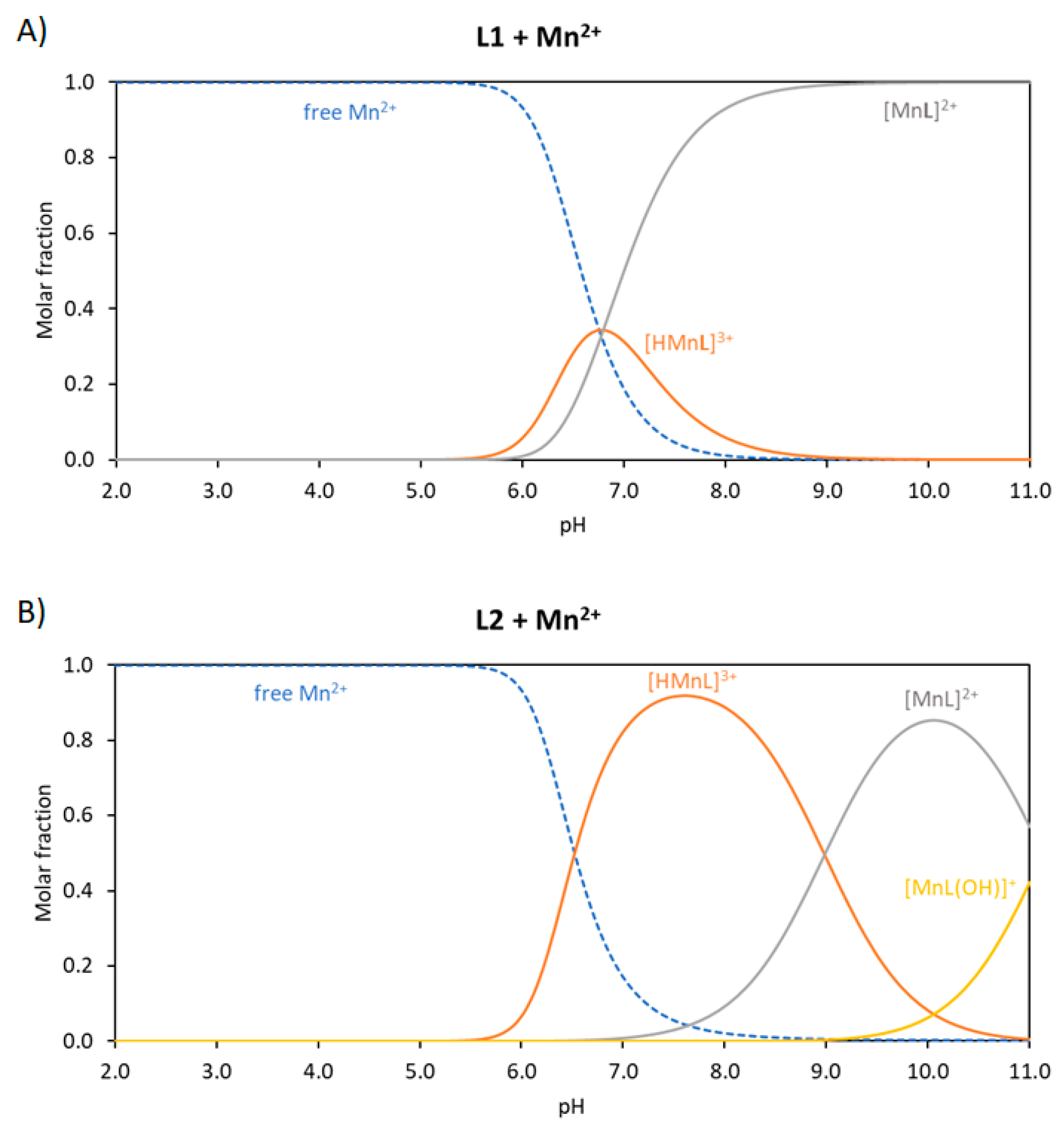
| Reaction (a) | L1 | L2 | L3 (c) | |
|---|---|---|---|---|
| L + H ⇄ HL (b) | KH1L | 9.49 (1) | 9.71 (2) | 9.43 (2) |
| LH + H ⇄ H2L | KH2L | 8.91 (1) | 9.03 (1) | 9.18 (2) |
| H2L + H ⇄ H3L | KH3L | 7.85 (1) | 8.00 (2) | 7.88 (3) |
| H3L + H ⇄ H4L | KH4L | 6.66 (2) | 6.70 (2) | 6.92 (3) |
| H4L + H ⇄ H5L | KH5L | 2.25 (8) | ||
| log β | 32.92 (2) | 35.69 (2) | 33.41 (3) |
| Reaction (a) | L1 | L2 | L3 |
|---|---|---|---|
| L + Mn ⇄ MnL (b) | 9.67 (3) | 8.42 (3) | N.D. |
| MnL + H ⇄ MnHL | 6.80 (4) | 8.97 (3) | N.D. |
| MnL + H2O ⇄ MnL(OH) + H | - | −11.1 (1) | N.D. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inclán, M.; Albelda, M.T.; Blasco, S.; Serena, C.; Chicote, J.U.; García-España, A.; García-España, E. Mn(II) Complexes of Enlarged Scorpiand-Type Azamacrocycles as Mimetics of MnSOD Enzyme. Appl. Sci. 2022, 12, 2447. https://doi.org/10.3390/app12052447
Inclán M, Albelda MT, Blasco S, Serena C, Chicote JU, García-España A, García-España E. Mn(II) Complexes of Enlarged Scorpiand-Type Azamacrocycles as Mimetics of MnSOD Enzyme. Applied Sciences. 2022; 12(5):2447. https://doi.org/10.3390/app12052447
Chicago/Turabian StyleInclán, Mario, María Teresa Albelda, Salvador Blasco, Carolina Serena, Javier Ugarte Chicote, Antonio García-España, and Enrique García-España. 2022. "Mn(II) Complexes of Enlarged Scorpiand-Type Azamacrocycles as Mimetics of MnSOD Enzyme" Applied Sciences 12, no. 5: 2447. https://doi.org/10.3390/app12052447
APA StyleInclán, M., Albelda, M. T., Blasco, S., Serena, C., Chicote, J. U., García-España, A., & García-España, E. (2022). Mn(II) Complexes of Enlarged Scorpiand-Type Azamacrocycles as Mimetics of MnSOD Enzyme. Applied Sciences, 12(5), 2447. https://doi.org/10.3390/app12052447