Impact of Waste Tea Litter on NH3 and CO2 Emissions during Broiler Rearing
Abstract
:1. Introduction
2. Materials and Methods
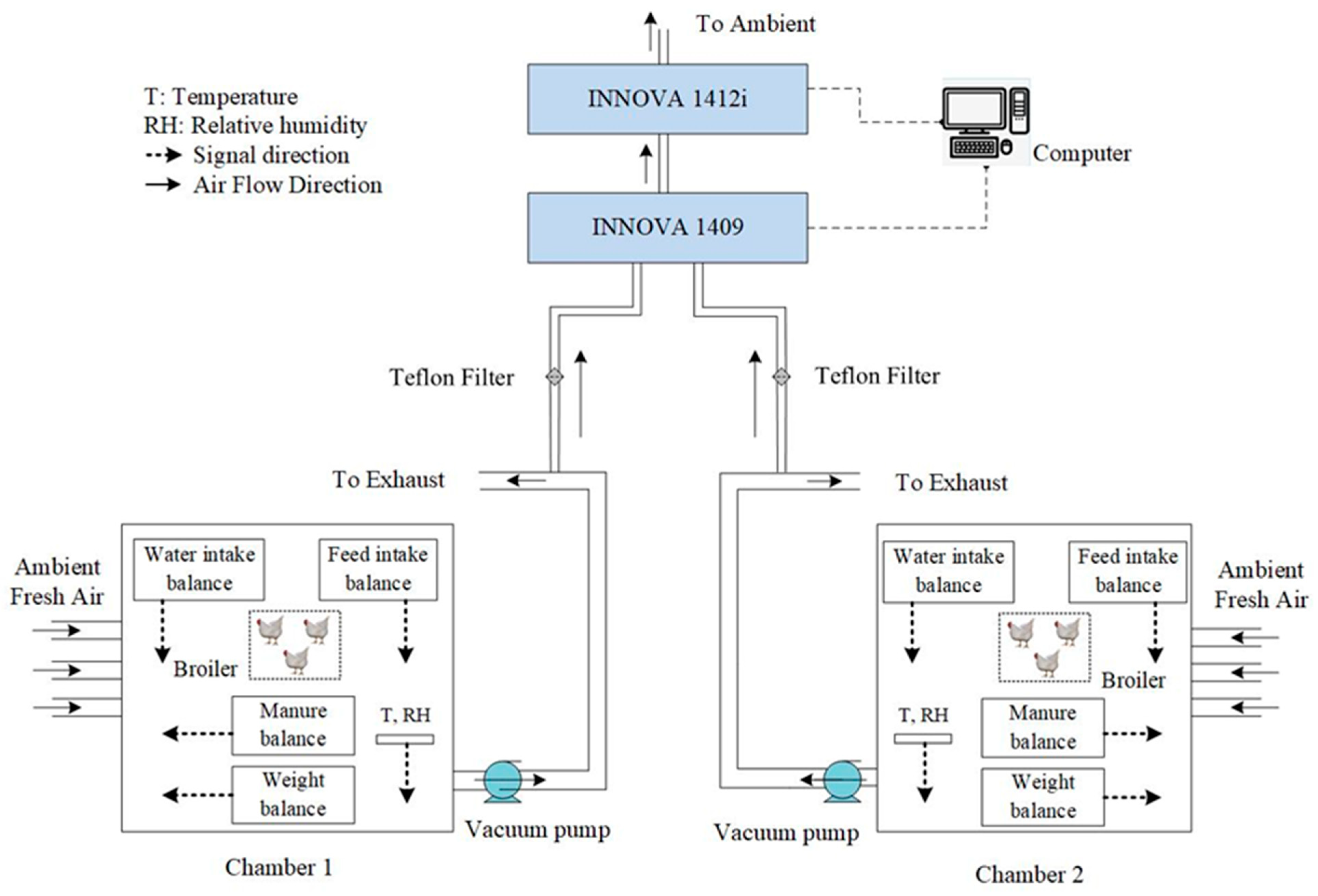
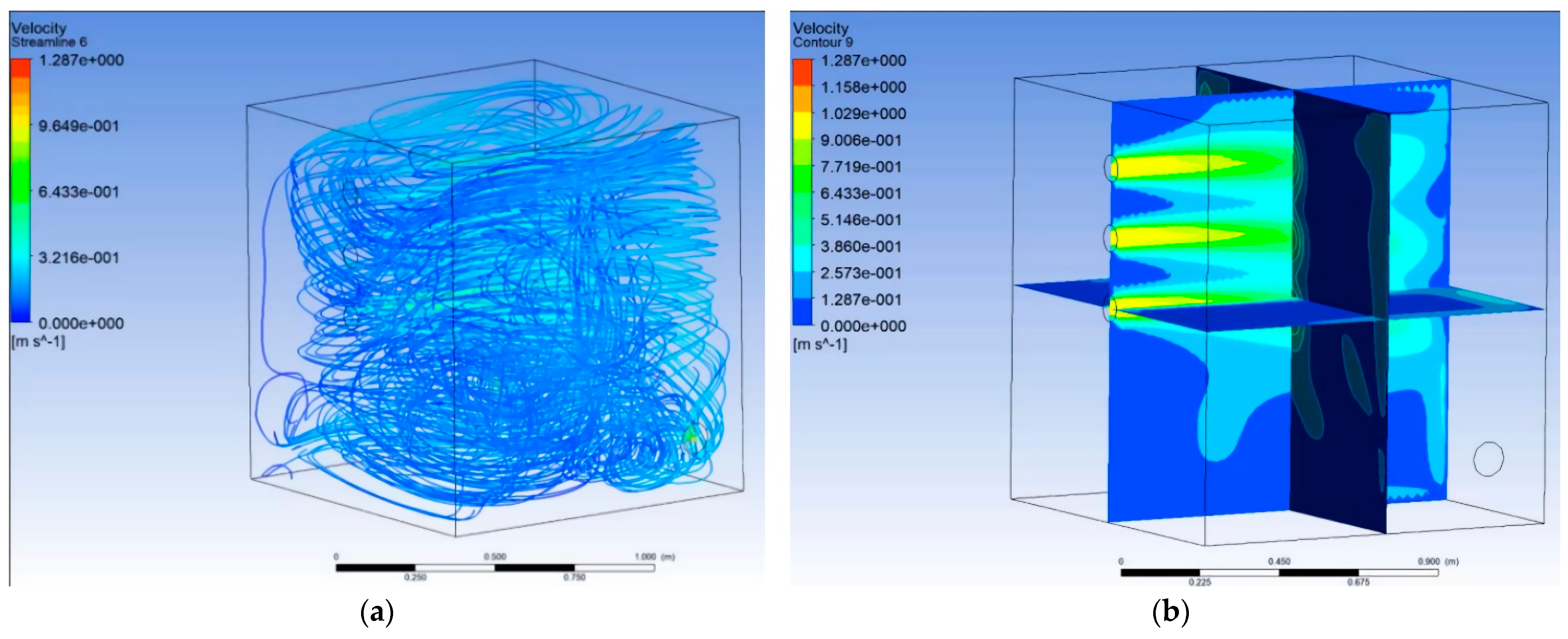
2.1. Digital Rearing Chamber Inspection System
2.2. Digital Rearing Chamber Inspection System
2.3. Experiment Process
2.4. Measurement Index and Method
3. Results and Discussions
3.1. Growth Performance Indexes
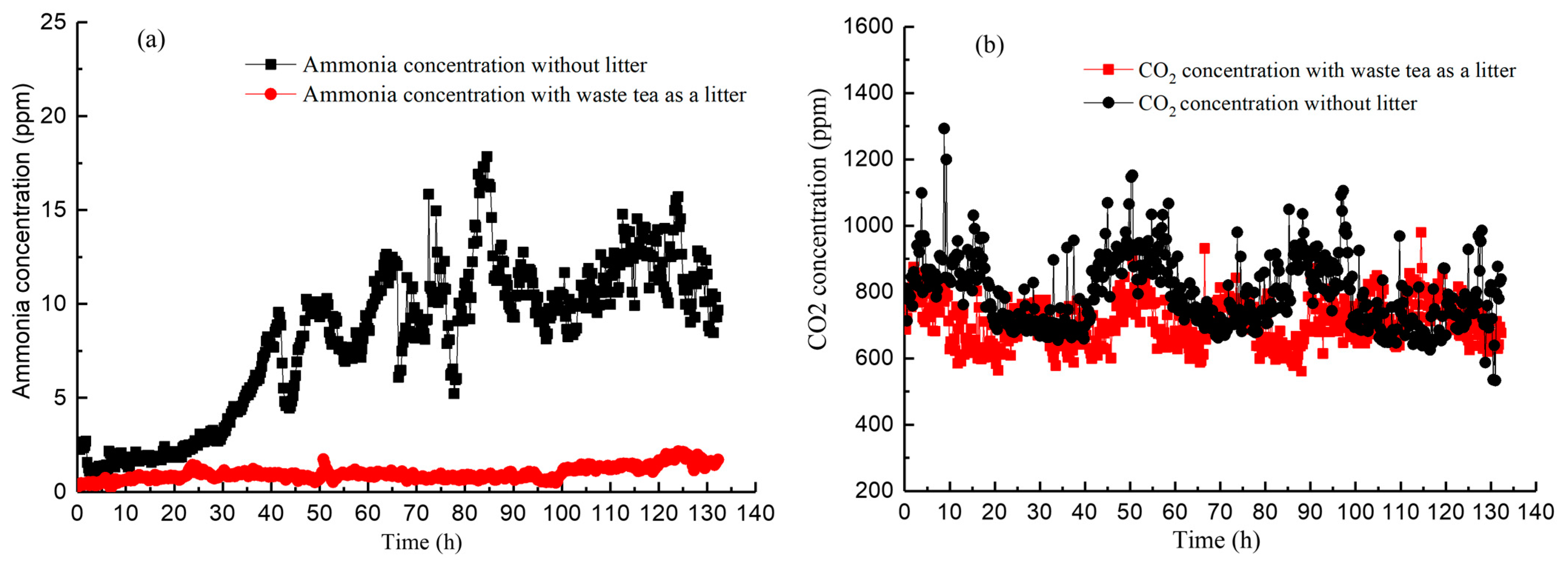
3.2. Measurement of NH3 and CO2 Levels
3.3. Assessment of Litter Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Li, C.; Tang, X.; Lu, Q.; Sa, R.; Zhang, H. High Concentrations of Atmospheric Ammonia Induce Alterations in the Hepatic Proteome of Broilers (Gallus gallus): An iTRAQ-Based Quantitative Proteomic Analysis. PLoS ONE 2015, 10, e0123596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.J.; Zhu, J. Effectiveness of short-term aeration in treating swine finishing manure to reduce odour generation potential. Agric. Ecosyst. Environ. 2005, 105, 115–125. [Google Scholar] [CrossRef]
- Groenestein, C.M.; Hutchings, N.J.; Haenel, H.D.; Amon, B.; Menzi, H.; Mikkelsen, M.H.; Misselbrook, T.H.; van Bruggen, C.; Kupper, T.; Webb, J. Comparison of ammonia emissions related to nitrogen use efficiency of livestock production in Europe. J. Clean. Prod. 2019, 211, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Yang, Z.; Li, H.; Buser, M.D.; Wanjura, J.D.; Downey, P.M.; Zhang, C.; Craige, C.; Torrents, A.; McConnell, L.L.; et al. Assessment of particulate matter and ammonia emission concentrations and respective plume profiles from a commercial poultry house. Environ. Pollut. 2018, 238, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, H.; Zhu, Z.; Gerber, P.J.; Xin, H.; Smith, P.; Opio, C.; Steinfeld, H.; Chadwick, D. Mitigating Greenhouse Gas and Ammonia Emissions from Swine Manure Management: A System Analysis. Environ. Sci. Technol. 2017, 51, 4503–4511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, X.; Yang, J.; Tian, Z.; Dong, H. Mitigating Greenhouse Gas and Ammonia Emissions from Beef Cattle Feedlot Production: A System Meta-Analysis. Environ. Sci. Technol. 2018, 52, 11232–11242. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Hristov, A.N.; Arogo, J.; Sheffield, R.E. A review of ammonia emission mitigation techniques for concentrated animal feeding operations. Biosyst. Eng. 2008, 100, 453–469. [Google Scholar] [CrossRef]
- Philippe, F.; Cabaraux, J.F.; Nicks, B. Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Tullo, E.; Finzi, A.; Guarino, M. Review: Environmental impact of livestock farming and Precision Livestock Farming as a mitigation strategy. Sci. Total Environ. 2019, 650, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.W.; Blackall, P.J.; Stuetz, R.M. Odour emissions from poultry litter—A review litter properties, odour formation and odorant emissions from porous materials. J. Environ. Manag. 2016, 177, 306–319. [Google Scholar] [CrossRef] [Green Version]
- Tasistro, A.S. Manipulating Bedding Materials and Sodium Bisulfate to Reduce Ammonia Emissions from Broiler Litter. Bioresour. Technol. 2008, 99, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, F.A.; Chambers, B.J.; Walker, A.W. Ammonia Emissions from Broiler Litter and Laying Hen Manure Management Systems. Biosyst. Eng. 2004, 89, 175–185. [Google Scholar] [CrossRef]
- Toghyani, M.; Gheisari, A.; Modaresi, M.; Tabeidian, S.A.; Toghyani, M. Effect of different litter material on performance and behavior of broiler chickens. Appl. Anim. Behav. Sci. 2010, 122, 48–52. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gayatri, S.L. Activated Tea Waste as a Potential Low-Cost Adsorbent for the Removal of p-Nitrophenol from Wastewater. J. Chem. Eng. Data 2010, 55, 4614–4623. [Google Scholar] [CrossRef]
- Fan, D.M.; Fan, K.; Cui-Ping, Y.U.; Ya-Ting, L.U.; Wang, X.C. Tea polyphenols dominate the short-term tea(Camellia sinensis) leaf litter decomposition. J. Zhejiang Univ. Sci. B 2017, 18, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Aso, Y.; Kasai, W.; Kondo, T. Synergetic deodorant effect and antibacterial activity of composite paper containing waste tea leaves. J. Wood Sci. 2011, 57, 308–316. [Google Scholar] [CrossRef]
- Patil, C.S.; Gunjal, D.B.; Naik, V.M.; Harale, N.S.; Jagadale, S.D.; Kadam, A.N.; Patil, P.S.; Kolekar, G.B.; Gore, A.H. Waste tea residue as a low cost adsorbent for removal of hydralazine hydrochloride pharmaceutical pollutant from aqueous media: An environmental remediation. J. Clean. Prod. 2019, 206, 407–418. [Google Scholar] [CrossRef]
- Machado, F.S.; Tomich, T.R.; Ferreira, A.L.; Cavalcanti, L.F.L.; Campos, M.M.; Paiva, C.A.V.; Ribas, M.N.; Pereira, L.G.R. Technical note: A facility for respiration measurements in cattle. J. Dairy Sci. 2016, 99, 4899–4906. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.Y.; Cao, Z.; Liao, X.D.; Wu, Y.B.; Liang, J.B.; Yu, B. Methane production of growing and finishing pigs in southern China. Anim. Feed Sci. Technol. 2011, 166–167, 430–435. [Google Scholar] [CrossRef]
- Sales, G.T.; Green, A.R.; Gates, R.S. Commissioning an animal preference chamber for behavioral studies with laying hens exposed to atmospheric ammonia. Comput. Electron. Agric. 2013, 95, 48–57. [Google Scholar] [CrossRef]
- Yi, B.; Chen, L.; Sa, R.; Zhong, R.; Zhang, H. Transcriptome Profile Analysis of Breast Muscle Tissues from High or Low Levels of Atmospheric Ammonia Exposed Broilers (Gallus gallus). PLoS ONE 2016, 11, e0162631. [Google Scholar] [CrossRef] [PubMed]
- Sa, R.N.; Xing, H.; Luan, S.J.; Sun, Y.B.; Sun, C.Y.; Zhang, H.F. Atmospheric ammonia alters lipid metabolism-related genes in the livers of broilers (Gallus gallus). J. Anim. Physiol. Anim. Nutr. 2018, 102, e941–e947. [Google Scholar] [CrossRef] [PubMed]
- Koerkamp, P.W.G.G. Review on Emissions of Ammonia from Housing Systems for Laying Hens in Relation to Sources, Processes, Building Design and Manure Handling. J. Agric. Eng. Res. 1994, 59, 73–87. [Google Scholar] [CrossRef]
- Chepete, H.J.; Xin, H.; Li, H. Effect of partially covering the turkey litter surface on ammonia emission. J. Appl. Poult. Res. 2012, 21, 513–521. [Google Scholar] [CrossRef]
- Chepete, J.H.; Xin, H.; Hong, L. Ammonia Emissions of Laying-Hen Manure as Affected by Accumulation Time. J. Poult. Sci. 2011, 48, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Webb, J.; Menzi, H.; Pain, B.F.; Misselbrook, T.H.; Dämmgen, U.; Hendriks, H.; Döhler, H. Managing ammonia emissions from livestock production in Europe. Environ. Pollut. 2005, 135, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, E.; Norring, M.; Valros, A. Effect of litter quality on foot pad dermatitis, hock burns and breast blisters in broiler breeders during the production period. Avian Pathol. 2016, 45, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Blunden, J.; Aneja, V.P. Characterizing ammonia and hydrogen sulfide emissions from a swine waste treatment lagoon in North Carolina. Atmos. Environ. 2008, 42, 3277–3290. [Google Scholar] [CrossRef]
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. Res. 2013, 20, 8092–8131. [Google Scholar] [CrossRef]
- Singh, A.; Casey, K.D.; King, W.D.; Pescatore, A.J.; Gates, R.S.; Ford, M.J. Efficacy of urease inhibitor to reduce ammonia emission from poultry houses. J. Appl. Poult. Res. 2009, 18, 34–42. [Google Scholar] [CrossRef]
- Kai, P.; Pedersen, P.; Jensen, J.E.; Hansen, M.N.; Sommer, S.G. A whole-farm assessment of the efficacy of slurry acidification in reducing ammonia emissions. Eur. J. Agron. 2008, 28, 148–154. [Google Scholar] [CrossRef]
- Wang, K.; Huang, D.; Ying, H.; Luo, H.; Biosystemseng, J.; Slurry, D.P.; Effects, R. Effects of acidification during storage on emissions of methane, ammonia, and hydrogen sulfide from digested pig slurry. Biosyst. Eng. 2014, 122, 23–30. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.W.; Moss, A.F.; Groves, P.J.; Wilkinson, S.J.; Stuetz, R.M.; Selle, P.H. The multidimensional causal factors of ‘wet litter’ in chicken-meat production. Sci. Total Environ. 2016, 562, 766–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misselbrook, T.H.; Powell, J.M. Influence of Bedding Material on Ammonia Emissions from Cattle Excreta. J. Dairy Sci. 2005, 88, 4304–4312. [Google Scholar] [CrossRef]
- Miles, D.M.; Rowe, D.E.; Cathcart, T.C. High litter moisture content suppresses litter ammonia volatilization. Poult. Sci. 2011, 90, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B.; Zaborska, W. The effect of phosphate buffer in the range of pH 5.80–8.07 on jack bean urease activity. J. Mol. Catal. B Enzym. 1999, 6, 75–81. [Google Scholar] [CrossRef]
- Kim, W.K.; Patterson, P.H. Effect of minerals on activity of microbial uricase to reduce ammonia volatilization in poultry manure. Poult. Sci. 2003, 82, 223–231. [Google Scholar] [CrossRef]
- Fernando, V.; Roberts, G.R. The partial inhibition of soil urease by naturally occurring polyphenols. Plant Soil 1976, 44, 81–86. [Google Scholar] [CrossRef]
- Manunza, B.; Deiana, S.; Pintore, M.; Gessa, C. The binding mechanism of urea, hydroxamic acid and N-(N-butyl)-phosphoric triamide to the urease active site. A comparative molecular dynamics study. Soil Biol. Biochem. 1999, 31, 789–796. [Google Scholar] [CrossRef]
- Dalton, D.A.; Evans, H.J.; Hanus, F.J. Stimulation by nickel of soil microbial urease activity and urease and hydrogenase activities in soybeans grown in a low-nickel soil. Plant Soil 1985, 88, 245–258. [Google Scholar] [CrossRef]
- Zhang-Wei, L.I. Investigation and Evaluation of Heavy Metals in Tea of FengHuang Mountain Tea Gardens in East Guangdong Province. Insect Sci. 2010, 17, 553–556. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y. Investigations of nickel(II) removal from aqueous solutions using tea factory waste. J. Hazard. Mater. 2005, 127, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Morad, N.; Tan, K.A. Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel(II) from aqueous solution. J. Hazard. Mater. 2011, 186, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Nan, Z.; Wu, X.; Qian, Y.; Xuan, C.; Raza, W.; Li, X. Effect of pruned material, extracts, and polyphenols of tea on enzyme activities and microbial community structure in soil. Soil Sci. Plant Nutr. 2017, 63, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Sommer, S.G.; Zhang, G.Q.; Bannink, A.; Chadwick, D.; Misselbrook, T.; Harrison, R.; Hutchings, N.J.; Menzi, H.; Monteny, G.J.; Ni, J.Q. Algorithms Determining Ammonia Emission from Buildings Housing Cattle and Pigs and from Manure Stores. Adv. Agron. 2006, 89, 261–335. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Tam, N.F.Y. Characterization and composting of poultry litter in forced-aeration piles. Process Biochem. 2002, 37, 869–880. [Google Scholar] [CrossRef]
- Andersson, M. Performance of Bedding Materials in Affecting Ammonia Emissions from Pig Manure. J. Agric. Eng. Res. 1996, 65, 213–222. [Google Scholar] [CrossRef]
| Days | Growth Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Average Daily Weight/g | Average Daily Excretion/g | Average Daily Feed Intake/g | Average Daily Water Intake/g | |||||
| Non-Bedding Materials | Waste Tea Litter | Non-Bedding Materials | Waste Tea Litter | Non-Bedding Materials | Waste Tea Litter | Non-Bedding Materials | Waste Tea Litter | |
| 1 | 1861 | 1964 | 78 | 181 | 113 | 118 | 239 | 229 |
| 2 | 1958 | 1945 | 184 | 125 | 119 | 118 | 237 | 238 |
| 3 | 1963 | 1944 | 172 | 138 | 121 | 120 | 260 | 238 |
| 4 | 1964 | 1992 | 115 | 112 | 121 | 118 | 254 | 238 |
| 5 | 1955 | 1946 | 110 | 209 | 126 | 121 | 238 | 239 |
| 6 | 1980 | 1962 | 128 | 114 | 121 | 120 | 240 | 238 |
| 7 | 1991 | 1949 | 46 | 111 | 122 | 120 | 246 | 239 |
| Notability analysis | NS (p = 0.815) | NS (p = 0.356) | NS (p = 0.446) | NS (p = 0.056) | ||||
| Type | Physicochemical Indices | |||||
|---|---|---|---|---|---|---|
| TC (g/kg) | TN (g/kg) | C/N | Moisture Content (%) | pH | Notability Analysis | |
| Waste tea | 529 ± 24 | 25.5 ± 4.1 | 20.1 ± 2.7 | 25.5 ± 4.1 | 5.8 ± 0.1 | |
| Waste tea litter | 331 ± 20 | 25.2 ± 4.3 | 13.3 ± 2.0 | 25.2 ± 4.3 | 6.4 ± 0.2 | NS (p = 0.866) |
| Non-bedding materials | 254 ± 23 | 19.9 ± 2.1 | 12.8 ± 1.4 | 19.9 ± 2.1 | 7.8 ± 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jie, D.; Zhang, Z.; He, J.; Zhou, Y.; Zhu, G. Impact of Waste Tea Litter on NH3 and CO2 Emissions during Broiler Rearing. Appl. Sci. 2022, 12, 2559. https://doi.org/10.3390/app12052559
Jie D, Zhang Z, He J, Zhou Y, Zhu G. Impact of Waste Tea Litter on NH3 and CO2 Emissions during Broiler Rearing. Applied Sciences. 2022; 12(5):2559. https://doi.org/10.3390/app12052559
Chicago/Turabian StyleJie, Dengfei, Zhanxiang Zhang, Jincheng He, Yafang Zhou, and Guangyou Zhu. 2022. "Impact of Waste Tea Litter on NH3 and CO2 Emissions during Broiler Rearing" Applied Sciences 12, no. 5: 2559. https://doi.org/10.3390/app12052559
APA StyleJie, D., Zhang, Z., He, J., Zhou, Y., & Zhu, G. (2022). Impact of Waste Tea Litter on NH3 and CO2 Emissions during Broiler Rearing. Applied Sciences, 12(5), 2559. https://doi.org/10.3390/app12052559






