Performance Evaluation of Silane in Concrete Bridge Decks Using Transmission X-ray Microscopy
Abstract
:1. Introduction
2. Materials and Methods
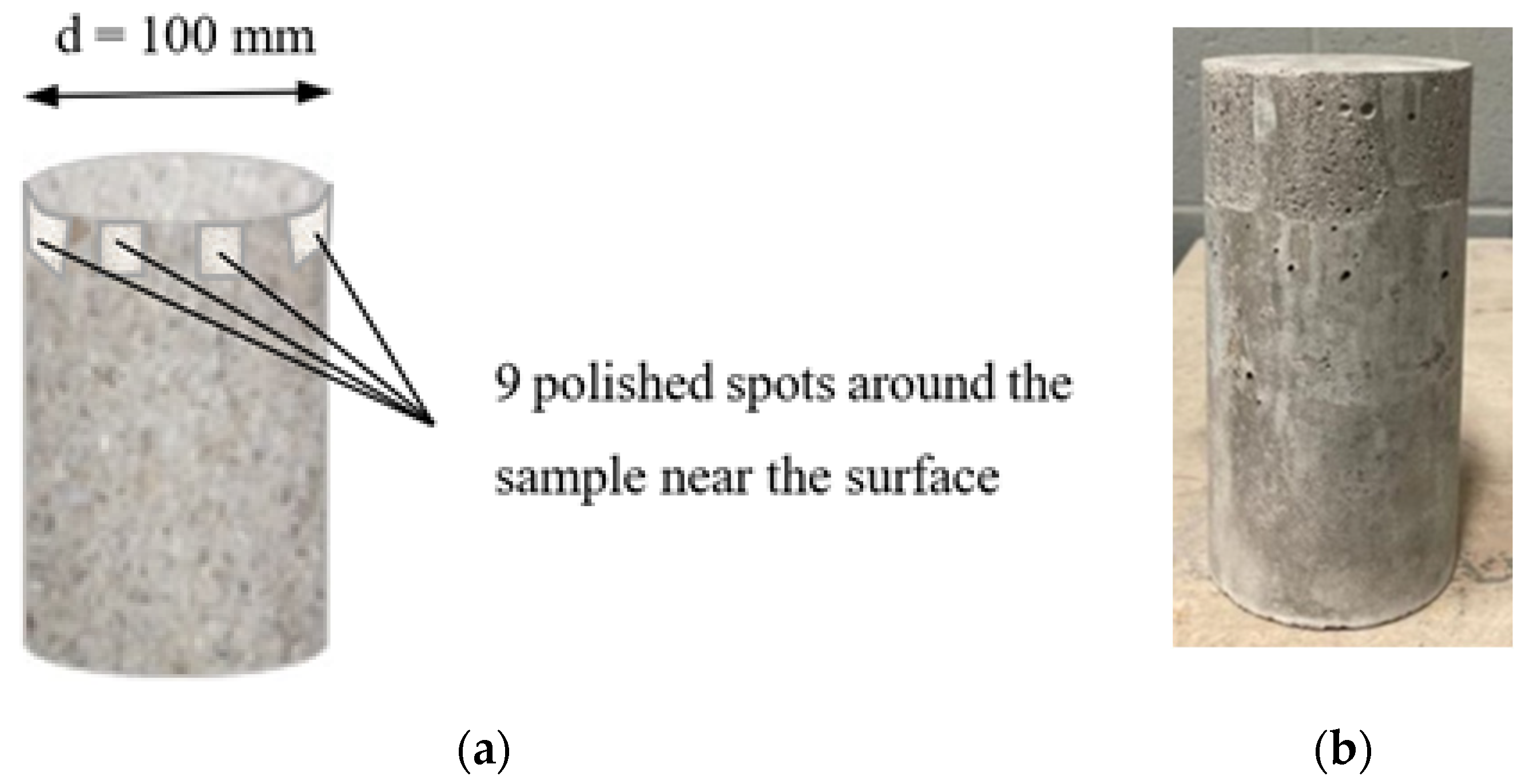
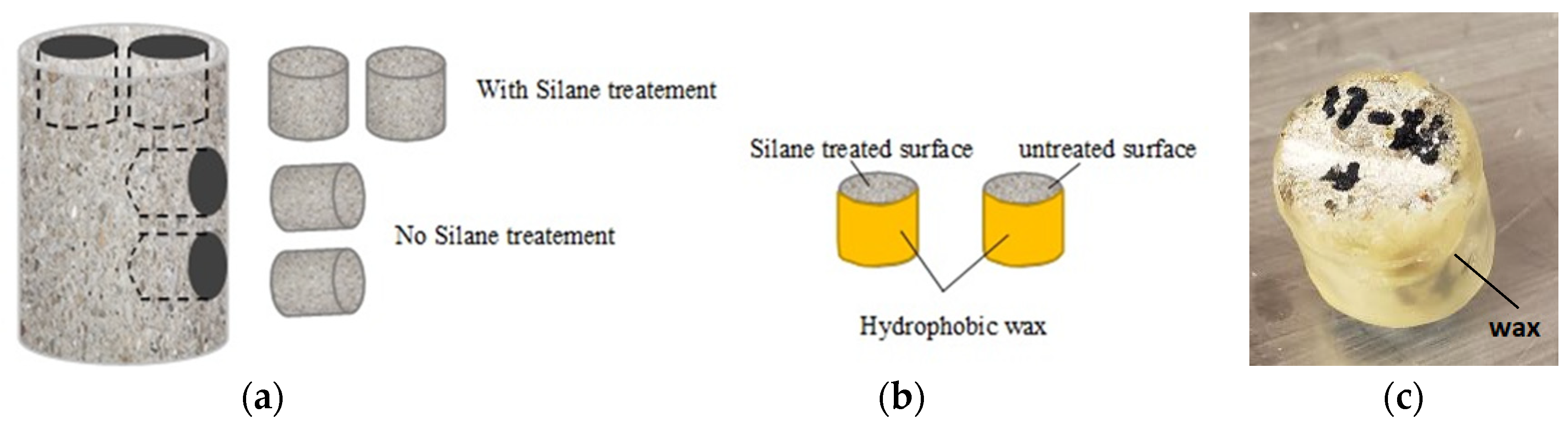
2.1. Sample Acquisition
2.2. Depth of Silane Penetration
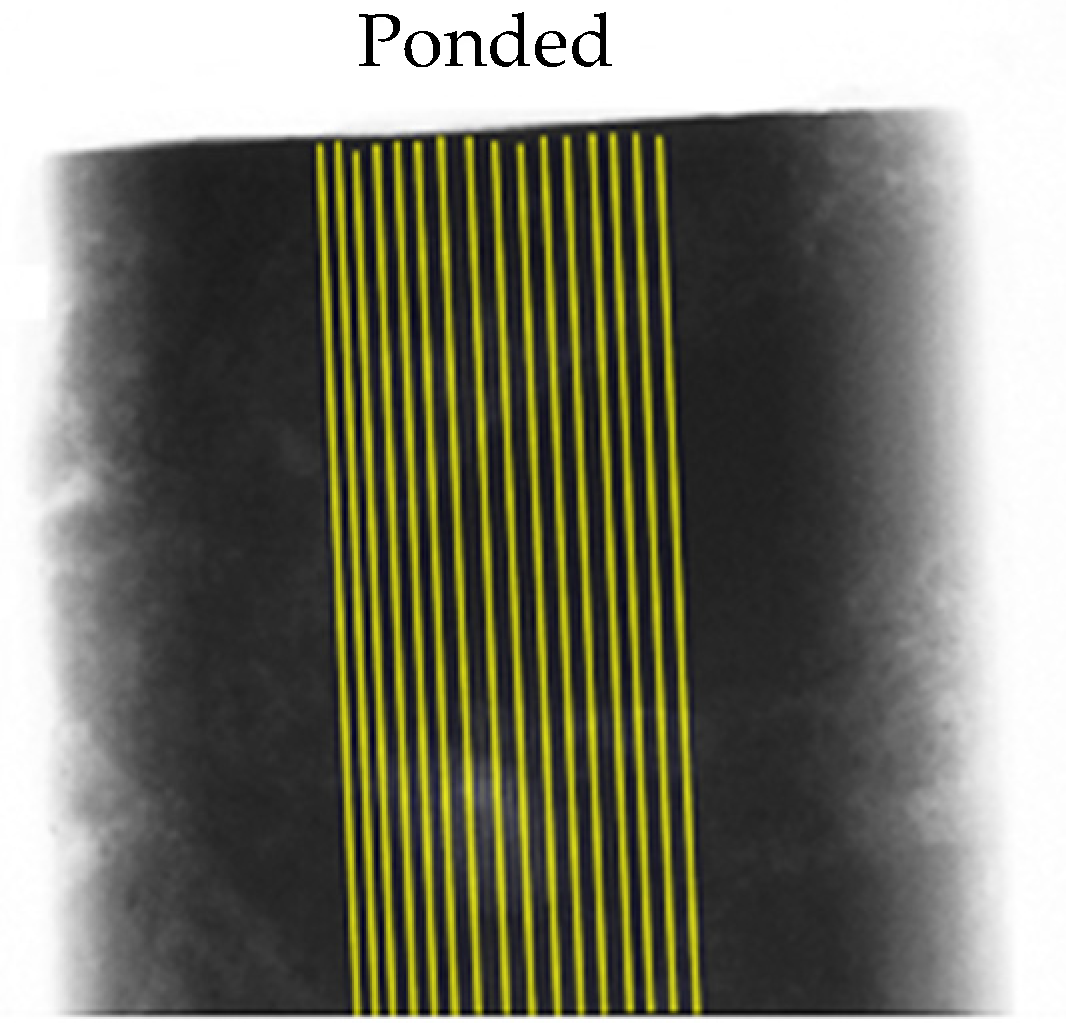
2.3. Diffusion Test
2.4. X-ray Computed Microtomography (XCT)
3. Results and Discussion
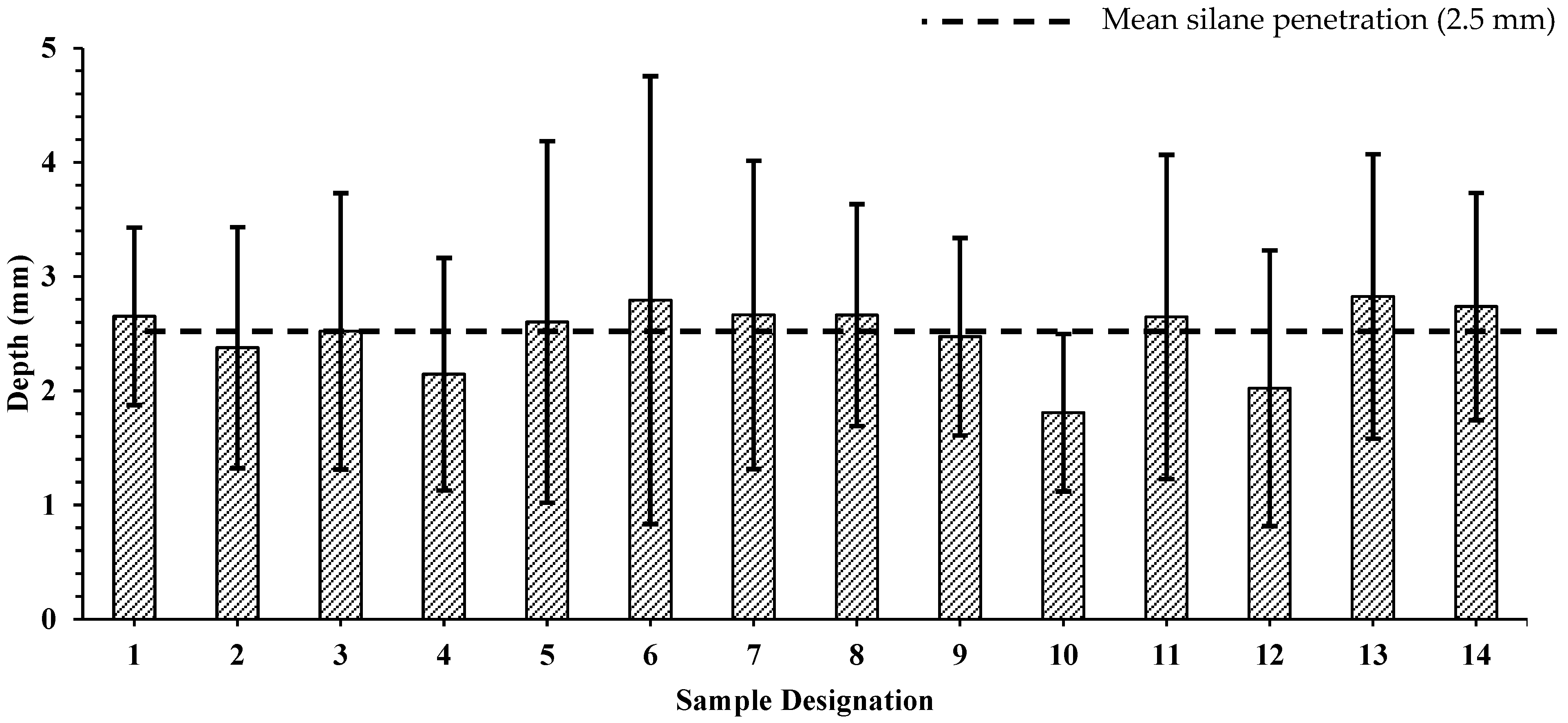
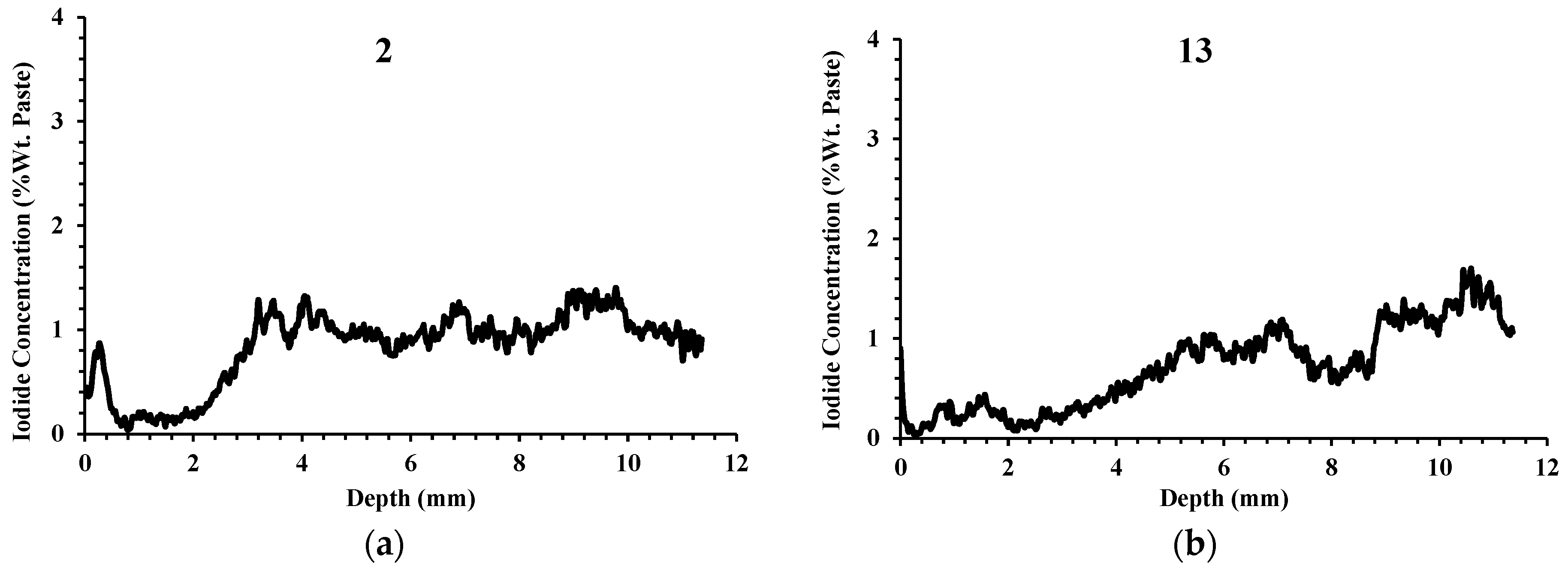
3.1. Depth of Silane Penetration
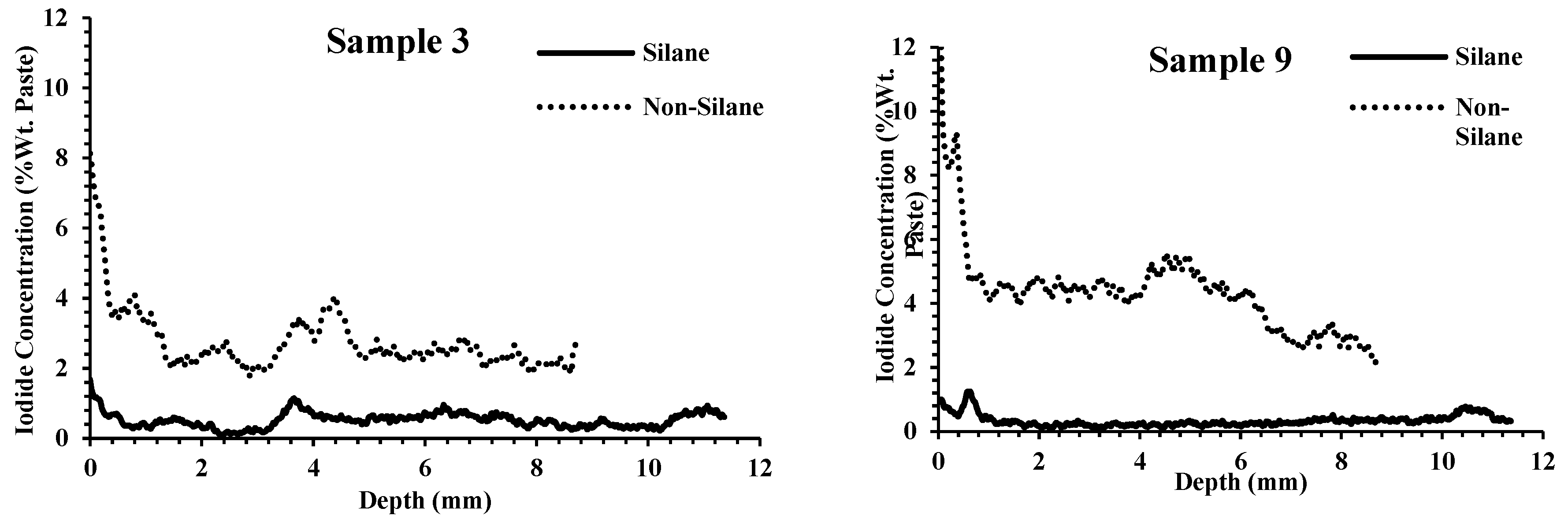
3.2. Resistance of Outside Chemical Penetration
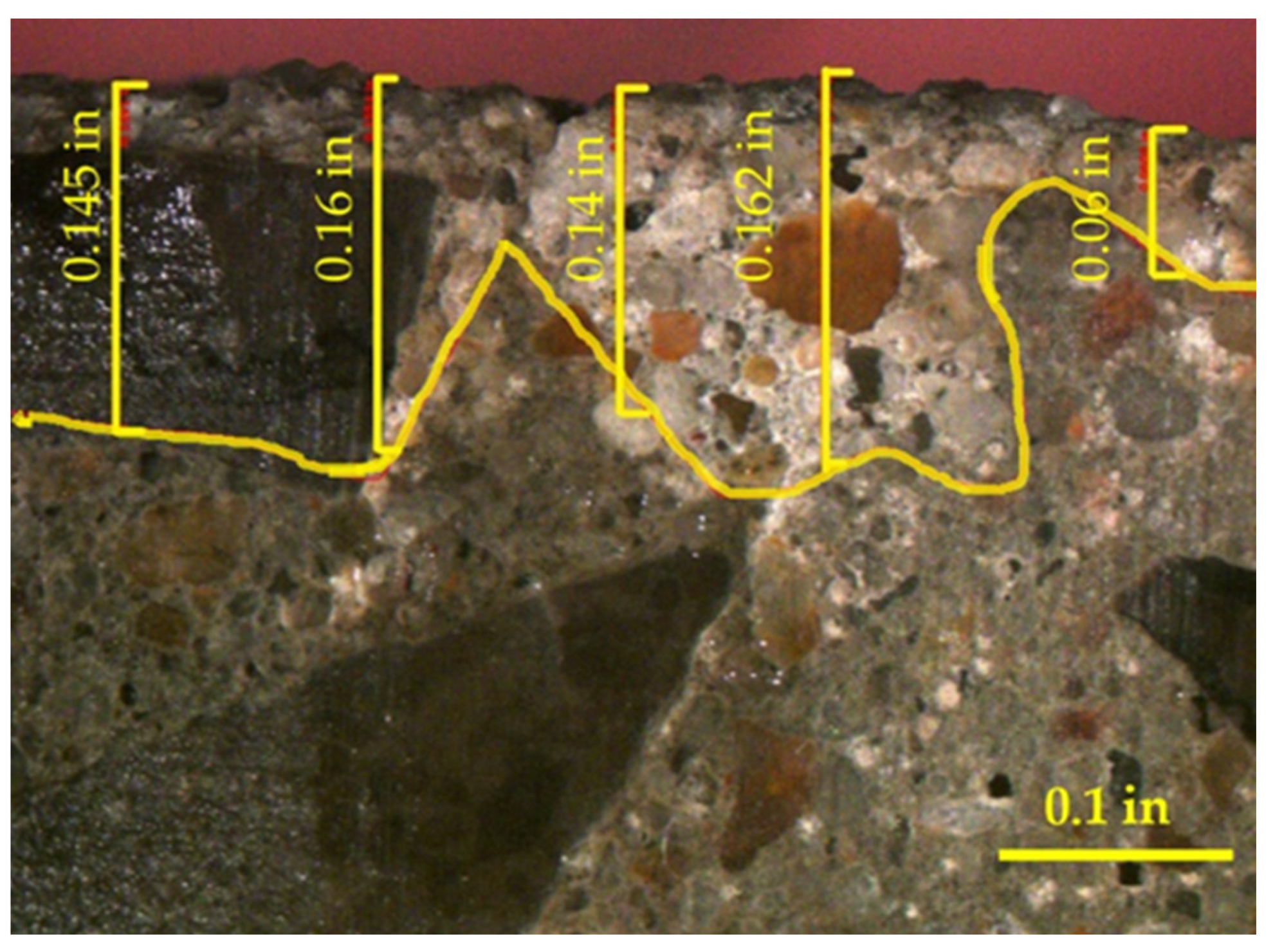
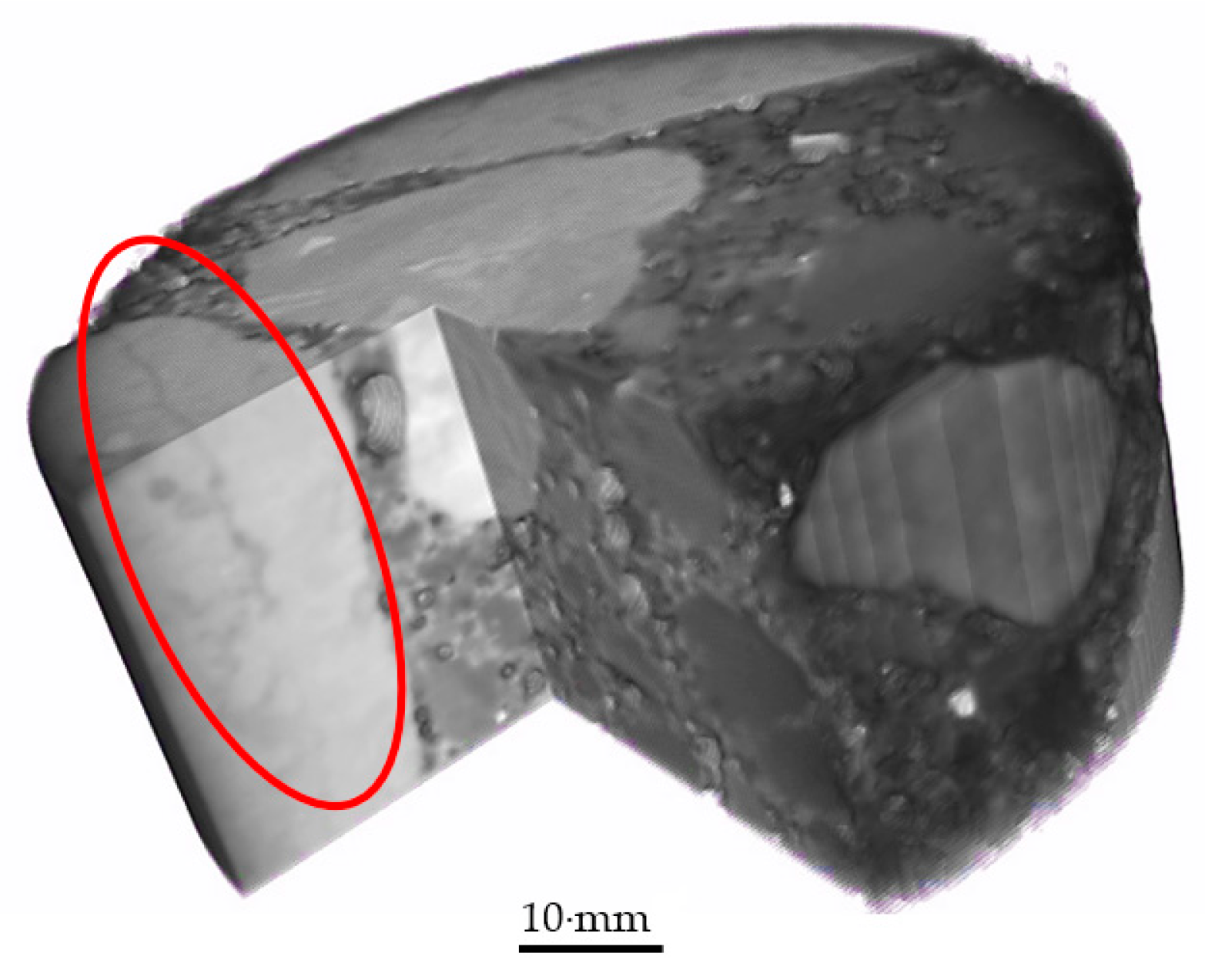
3.3. Cracks
4. Conclusions
- ▪
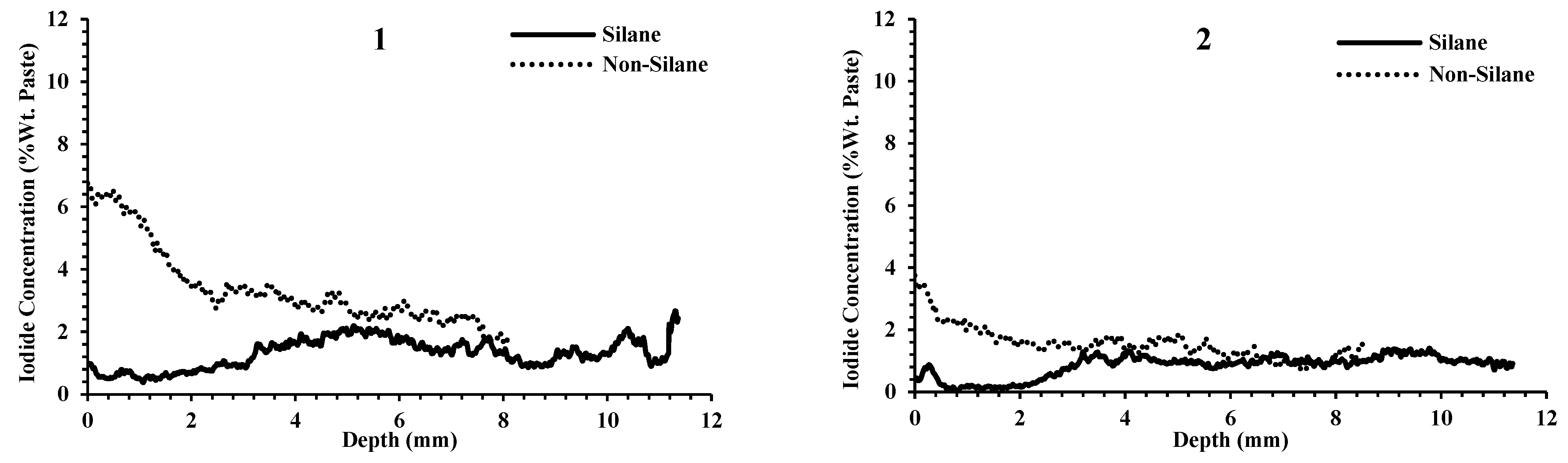
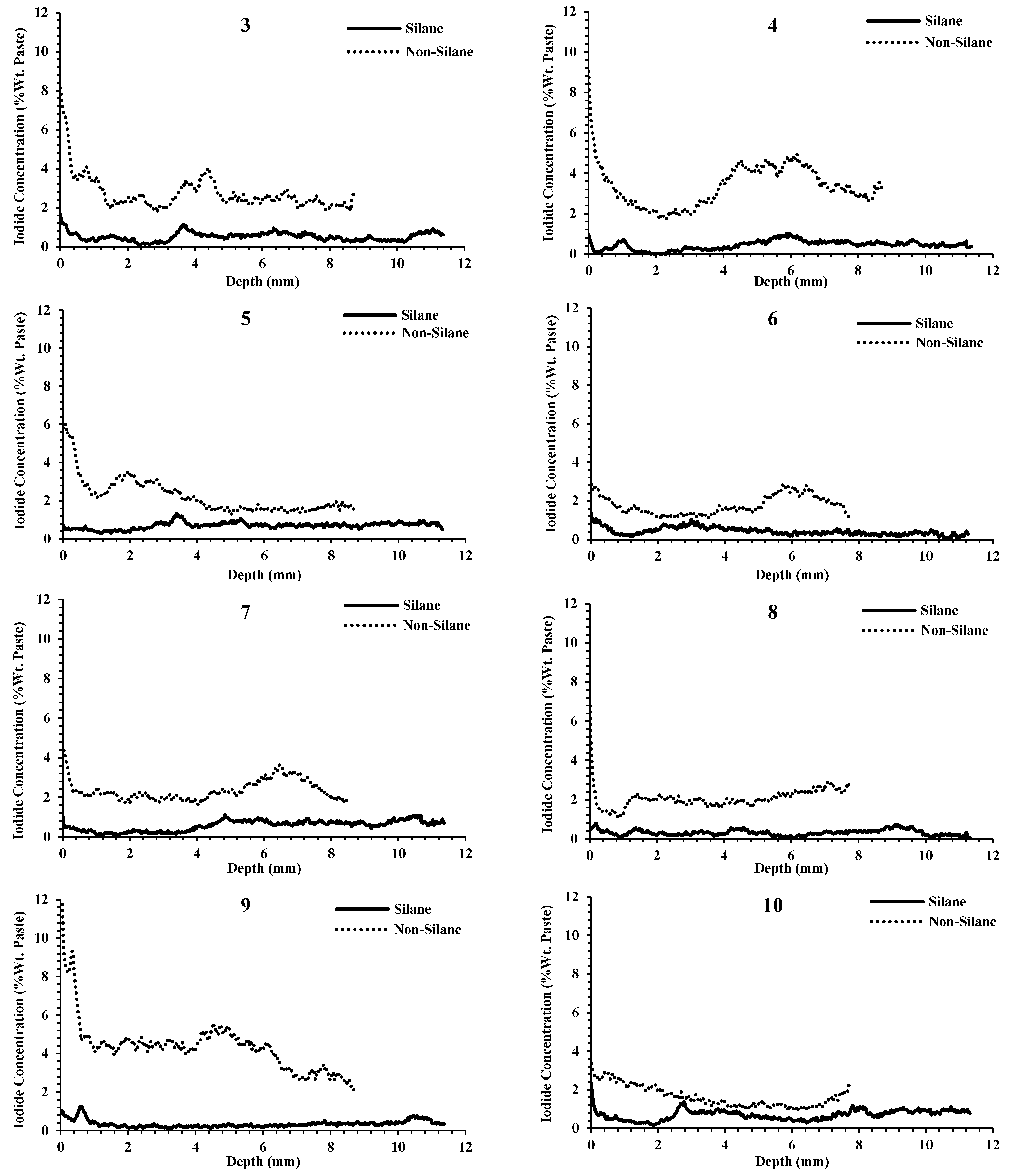
- The average thickness of the silane coating for the 14 projects was 2.5 mm, and the silane reduced the penetration of outside chemicals on average 9× more than samples that were not treated with silane.
- ▪
- For the samples not coated with silane, there was large variability among the samples. At 2 mm in depth, some samples had iodide concentrations as low as 1% by weight and some had concentrations greater than 4%. Despite this high variability, the same concrete that was treated with silane showed low iodide concentration at 2 mm, varying between 0.03% and 0.66%.
- ▪
- Following the application of silane, a high reduction in chemical penetration occurred regardless of the ability of the nontreated concrete to resist chemical penetration. This shows the effectiveness of silane to resist chemical penetration for concretes with a wide range of qualities.
- ▪
- Outside chemicals were observed to penetrate past silane coatings when surface cracking was observed. This shows that cracks can compromise silane coatings. This will be an area of future study.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Mixture Designs of All Samples
| Sample | Coarse Aggregate | Fine Aggregate | Cement | Air Content % | Fly Ash (Type C) | Water (kg/m3) | Water Reducer (ASTM C-494 Type A/D) | Air-Entrainer (ASTM C-260) | w/b Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Specific Gravity | kg/m3 | Specific Gravity | kg/m3 | kg/m3 | Specific Gravity | kg/m3 | Type | Specific Gravity | kg/m3 | Specific Gravity | kg/m3 | ||||
| 1 | #67 | 2.69 | 1098 | 2.62 | 731 | 335 | 6 | NONE | 148 | A35 | 1.01 | 0.89 | 1.00 | 0.18 | 0.44 | |
| 2 | #67 | 2.69 | 1068 | 2.62 | 709 | 362 | 6 | NONE | 158 | A36 | 1.01 | 1.90 | 1.00 | 0.06 | 0.44 | |
| 3 | #67 | 2.69 | 1092 | 2.62 | 730 | 268 | 6 | 2.65 | 66.4 | 147 | A37 | 1.01 | 1.13 | 1.00 | 0.06 | 0.44 |
| 4 | #67 | 2.69 | 1074 | 2.62 | 718 | 290 | 6 | 2.65 | 72.4 | 148 | A38 | 1.01 | 1.42 | 1.00 | 0.12 | 0.41 |
| 5 | Not Provided | |||||||||||||||
| 6 | ||||||||||||||||
| 7 | ||||||||||||||||
| 8 | ||||||||||||||||
| 9 | #67 | 2.67 | 1068 | 2.62 | 709 | 362 | 6 | NONE | 158 | A36 | 1.01 | 1.90 | 1.00 | 0.06 | 0.44 | |
| 10 | #67 | 2.67 | 1072 | 2.62 | 714 | 290 | 6 | 2.65 | 72.4 | 148 | A38 | 1.01 | 1.42 | 1.00 | 0.12 | 0.41 |
| 11 | #57 | 2.65 | 1009 | 2.63 | 777 | 268 | 6.5 ± 1.5 | 2.65 | 67.0 | 148 | A35 | Not Provided | Not Provided | 0.44 | ||
| 12 | #57 | 2.65 | 1009 | 2.63 | 777 | 268 | 6.5 ± 1.5 | 2.65 | 67.0 | 148 | A35 | 0.44 | ||||
| 13 | #57 | 2.65 | 1020 | 2.63 | 777 | 335 | 6.5 ± 1.5 | NONE | 148 | A36 | 0.44 | |||||
| 14 | #57 | 2.65 | 1020 | 2.63 | 777 | 335 | 6.5 ± 1.5 | NONE | 148 | A37 | 0.44 | |||||
Appendix B. Transmission X-ray Microscopy (TXM) Technique
Appendix C. Silane and Non-Silane Profiles of All Samples

References
- Koch, G.H.; Brongers, M.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Cost and Preventive Strategies in the United States. March 2002. Available online: https://rosap.ntl.bts.gov/view/dot/40697 (accessed on 25 March 2019).
- Khanzadeh-Moradllo, M.; Meshkini, M.H.; Eslamdoost, E.; Sadati, S.; Shekarchi, M. Effect of Wet Curing Duration on Long-Term Performance of Concrete in Tidal Zone of Marine Environment. Int. J. Concr. Struct. Mater. 2015, 9, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Khanzadeh Moradllo, M.; Hu, Q.; Ley, M.T. Using X-ray imaging to investigate in-situ ion diffusion in cementitious materials. Constr. Build. Mater. 2017, 136, 88–98. [Google Scholar] [CrossRef]
- Ley, T.M.; Sudbrink, B.; Kotha, H.; Materer, N.; Applett, A. Expected Life of Silane Water Repellant Treatments on Bridge Decks (FHWA-OK-12-08). 2012. Available online: https://trid.trb.org/view/1237835 (accessed on 25 March 2019).
- Ley, M.T.; Materer, N.F.; Sudbrink, B.; Khanzadeh Moradllo, M.; Hu, Q.; Ley, M.T.; Davis, J.M.; Materer, N.F.; Apblett, A. Imaging the presence of silane coatings in concrete with micro X-ray fluorescence. Cem. Concr. Res. 2017, 92, 121–127. [Google Scholar] [CrossRef]
- Ray, I.; Gong, Z.; Davalos, J.F.; Kar, A. Shrinkage and cracking studies of high performance concrete for bridge decks. Constr. Build. Mater. 2012, 28, 244–254. [Google Scholar] [CrossRef]
- Khanzadeh Moradllo, M.; Sudbrink, B.; Ley, M.T. Determining the effective service life of silane treatments in concrete bridge decks. Constr. Build. Mater. 2016, 116, 121–127. [Google Scholar] [CrossRef]
- Basham, K.; Meredith, J. Measuring water penetration. Mag. Mason. Constr. 1995, 8, 539–543. [Google Scholar]
- Mehta, P.K. Durability of Concrete—Fifty Years of Progress? Spec. Publ. 1991, 126, 1–32. [Google Scholar] [CrossRef]
- Portland Cement Association. Types and Causes of Concrete Deterioration; Portland Cement Association: Skokie, IL, USA, 2002. [Google Scholar]
- De Domenico, D.; Messina, D.; Recupero, A. A Combined Experimental-Numerical Framework for Assessing the Load-Bearing Capacity of Existing PC Bridge Decks Accounting for Corrosion of Prestressing Strands. Materials 2021, 14, 4914. [Google Scholar] [CrossRef]
- Lu, S.; Landis, E.N.; Keane, D.T. X-ray microtomographic studies of pore structure and permeability in Portland cement concrete. Mater. Struct. 2006, 39, 611–620. [Google Scholar] [CrossRef]
- Dean, S.W.; Sant, G.; Weiss, J. Using X-ray Absorption to Assess Moisture Movement in Cement-Based Materials. J. ASTM Int. 2009, 6, 1–15. [Google Scholar] [CrossRef]
- Hasan, N. Durability and Sustainability of Concrete: Case Studies for Concrete Exposures; Springer Nature: Berlin/Heidelberg, Germany, 2020; ISBN 3030515737. [Google Scholar]
- Bentur, A.; Berke, N.; Diamond, S. Steel Corrosion in Concrete: Fundamentals and Civil Engineering Practice; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Khanzadeh Moradllo, M.; Shekarchi, M.; Hoseini, M. Time-dependent performance of concrete surface coatings in tidal zone of marine environment. Constr. Build. Mater. 2012, 30, 198–205. [Google Scholar] [CrossRef]
- Attanayaka, U.; Ng, S.; Aktan, H. Criteria and Benefits of Penetrating Sealants for Concrete Bridge Decks; Michigan Department of Transportation: Lansing, MI, USA, 2002. [Google Scholar]
- Anderson, R.; Arkles, B.; Larson, G. Silicon Compounds Register and Review, Petrarch Product Catalog; Petrarch Systems, Inc.: Bristol, PA, USA, 1987. [Google Scholar]
- Carter, P.D. Evaluation of Dampproofing Performance and Effective Penetration Depth of Silane Sealers in Concrete. Spec. Publ. 1994, 151, 95–118. [Google Scholar] [CrossRef]
- Bush, T.D., Jr.; Kamel, A.A.; Kalluri, P.A. Influence of Field Variables on Laboratory Performance of Silane Treated Concrete. ACI Mater. J. 1997, 94, 193–202. [Google Scholar] [CrossRef]
- Kerkhoff, B. Effects of Substances on Concrete and Guide to Protective Treatments; Portland Cement Association: Skokie, IL, USA, 2007. [Google Scholar]
- Pfeifer, D.W.; Scali, M.J. Concrete Sealers for Protection of Bridge Structures; Transportation Research Board: Washington, DC, USA, 1981. [Google Scholar]
- Medeiros, M.; Helene, P. Efficacy of surface hydrophobic agents in reducing water and chloride ion penetration in concrete. Mater. Struct. 2007, 41, 59–71. [Google Scholar] [CrossRef]
- Medeiros, M.H.F.; Helene, P. Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption. Constr. Build. Mater. 2009, 23, 1476–1484. [Google Scholar] [CrossRef]
- Dunn, J.R. Performance of Concrete Bridge Deck Sealers; University of Alabama: Tuscaloosa, AL, USA, 2015. [Google Scholar]
- Ren, Z.J. Influence of Water Repellent Treatment on Corrosion of Steel in Concrete with Chloride Ingress. Adv. Mater. Res. 2012, 368–373, 2430–2436. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Giannakou, A.; Jones, M.R. Comparative performance of chloride attenuating and corrosion inhibiting systems for reinforced concrete. Mater. Struct. Constr. 2004, 37, 671–679. [Google Scholar] [CrossRef]
- Cady, P.D. Sealers for Portland Cement Conrete Highway Facilities; Transportation Research Board: Washington, DC, USA, 1994. [Google Scholar]
- Cairns, J.; Melville, C. The effect of concrete surface treatments on electrical measurements of corrosion activity. Constr. Build. Mater. 2003, 17, 301–309. [Google Scholar] [CrossRef]
- Oklahoma Department of Transportation. State Planning and Research Work Program FFY 2020. 2019. Available online: https://oklahoma.gov/content/dam/ok/en/odot/documents/research/2020-spr-workbook.pdf (accessed on 22 August 2020).
- Christodoulou, C.; Goodier, C.I.; Austin, S.A.; Webb, J.; Glass, G.K. Long-term performance of surface impregnation of reinforced concrete structures with silane. Constr. Build. Mater. 2013, 48, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.G.; Akira, Y.; Wittmann, F.H.; Yokota, H.; Zhang, P. Water repellent surface impregnation for extension of service life of reinforced concrete structures in marine environments: The role of cracks. Cem. Concr. Compos. 2010, 32, 101–109. [Google Scholar] [CrossRef]
- Weyers, R.E.; Zemajtis, J.; Drumm, R.O. Service Lives of Concrete Sealers; Transportation Research Board: Washington, DC, USA, 1995; Available online: https://trid.trb.org/view/452552 (accessed on 22 August 2020).
- Behravan, A.; Ley, M.T.; Rywelski, A.; Berke, N. Changes in the rate of ion penetration of alternative cementitious materials with time. Mater. Des. 2021, 197, 109236. [Google Scholar] [CrossRef]
- Scheidegger, A.M.; Vespa, M.; Grolimund, D.; Wieland, E.; Harfouche, M.; Bonhoure, I.; Dähn, R. The use of (micro)-X-ray absorption spectroscopy in cement research. Waste Manag. 2006, 26, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.S.; Huang, C.; Kim, M.; Safron, N.S.; Arnold, M.S.; Wong, B.M.; Gopalan, P.; Himpsel, F.J. Orientation of a Monolayer of Dipolar Molecules on Graphene from X-ray Absorption Spectroscopy. Langmuir 2014, 30, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Bentz, D.P.; Quenard, D.A.; Kunzel, H.M.; Baruchel, J.; Peyrin, F.; Martys, N.S.; Garboczi, E.J. Microstructure and transport properties of porous building materials. II: Three-dimensional X-ray tomographic studies. Mater. Struct. Constr. 2000, 33, 147–153. [Google Scholar] [CrossRef]
- Darma, I.S.; Sugiyama, T.; Promentilla, M.A.B. Application of X-ray CT to study diffusivity in cracked concrete through the observation of tracer transport. J. Adv. Concr. Technol. 2013, 11, 266–281. [Google Scholar] [CrossRef] [Green Version]
- Roels, S.; Carmeliet, J. Analysis of moisture flow in porous materials using microfocus X-ray radiography. Int. J. Heat Mass Transf. 2006, 49, 4762–4772. [Google Scholar] [CrossRef]
- Bentz, D.P.; Hansen, K.K. Preliminary observations of water movement in cement pastes during curing using X-ray absorption. Cem. Concr. Res. 2000, 30, 1157–1168. [Google Scholar] [CrossRef]
- Cavé, L.; Al, T.; Xiang, Y.; Vilks, P. A technique for estimating one-dimensional diffusion coefficients in low-permeability sedimentary rock using X-ray radiography: Comparison with through-diffusion measurements. J. Contam. Hydrol. 2009, 103, 1–12. [Google Scholar] [CrossRef]
- Tidwell, V.C.; Meigs, L.C.; Christian-Frear, T.; Boney, C.M. Effects of spatially heterogeneous porosity on matrix diffusion as investigated by X-ray absorption imaging. J. Contam. Hydrol. 2000, 42, 285–302. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.; Huang, R.; Hsu, H.M.; Zou, S.Y.; Teng, L.W. Evaluation of penetration depth and protective effectiveness of concrete-penetrating sealer materials. J. Mar. Sci. Technol. 2016, 24, 244–249. [Google Scholar] [CrossRef]
- Teng, L.W.; Lin, W.T.; Chen, J.; Cheng, A.; Hsu, H.M. The Component Analysis of Penetration Sealer Materials. Adv. Mater. Res. 2014, 842, 74–77. [Google Scholar] [CrossRef]
- Teng, L.W.; Huang, R.; Hsu, H.M.; Chen, J.; Chao, S.J.; Chen, S.H. The Research of Concrete Sealer Penetrating Depths. Adv. Mater. Res. 2014, 1025–1026, 703–708. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Sudbrink, B.; Hu, Q.; Aboustait, M.; Tabb, B.; Ley, M.T.; Davis, J.M. Using micro X-ray fluorescence to image chloride profiles in concrete. Cem. Concr. Res. 2017, 92, 128–141. [Google Scholar] [CrossRef]
- Khanzadeh Moradllo, M.; Ley, M.T.; Moradllo, M.K.; Ley, M.T. Quantitative measurement of the influence of degree of saturation on ion penetration in cement paste by using X-ray imaging. Constr. Build. Mater. 2017, 141, 113–129. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parameter | TXM Setting |
|---|---|
| Pixel size (mm) | 8.8 |
| Voltage (keV) | 100 |
| Current (mA) | 100 |
| Filter | 0.5 mm Al + Cu |
| Acquisition time | 8 s |
| Chamber condition | Air |
| Parameter | Setting |
|---|---|
| Resolution (µm/pixel) | 15.29 |
| Source energy (keV) | 110 |
| Current (µA) | 90 |
| Optical magnification | 0.4× |
| Number of projections | 1014 |
| Exposure time (s) | 2.5 |
| Total scan time | 4 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behravan, A.; Aqib, S.M.; Delatte, N.J.; Ley, M.T.; Rywelski, A. Performance Evaluation of Silane in Concrete Bridge Decks Using Transmission X-ray Microscopy. Appl. Sci. 2022, 12, 2557. https://doi.org/10.3390/app12052557
Behravan A, Aqib SM, Delatte NJ, Ley MT, Rywelski A. Performance Evaluation of Silane in Concrete Bridge Decks Using Transmission X-ray Microscopy. Applied Sciences. 2022; 12(5):2557. https://doi.org/10.3390/app12052557
Chicago/Turabian StyleBehravan, Amir, Syed Muhammad Aqib, Norbert J. Delatte, M. Tyler Ley, and Anna Rywelski. 2022. "Performance Evaluation of Silane in Concrete Bridge Decks Using Transmission X-ray Microscopy" Applied Sciences 12, no. 5: 2557. https://doi.org/10.3390/app12052557
APA StyleBehravan, A., Aqib, S. M., Delatte, N. J., Ley, M. T., & Rywelski, A. (2022). Performance Evaluation of Silane in Concrete Bridge Decks Using Transmission X-ray Microscopy. Applied Sciences, 12(5), 2557. https://doi.org/10.3390/app12052557







