Research Progress in the Early Warning of Chicken Diseases by Monitoring Clinical Symptoms
Abstract
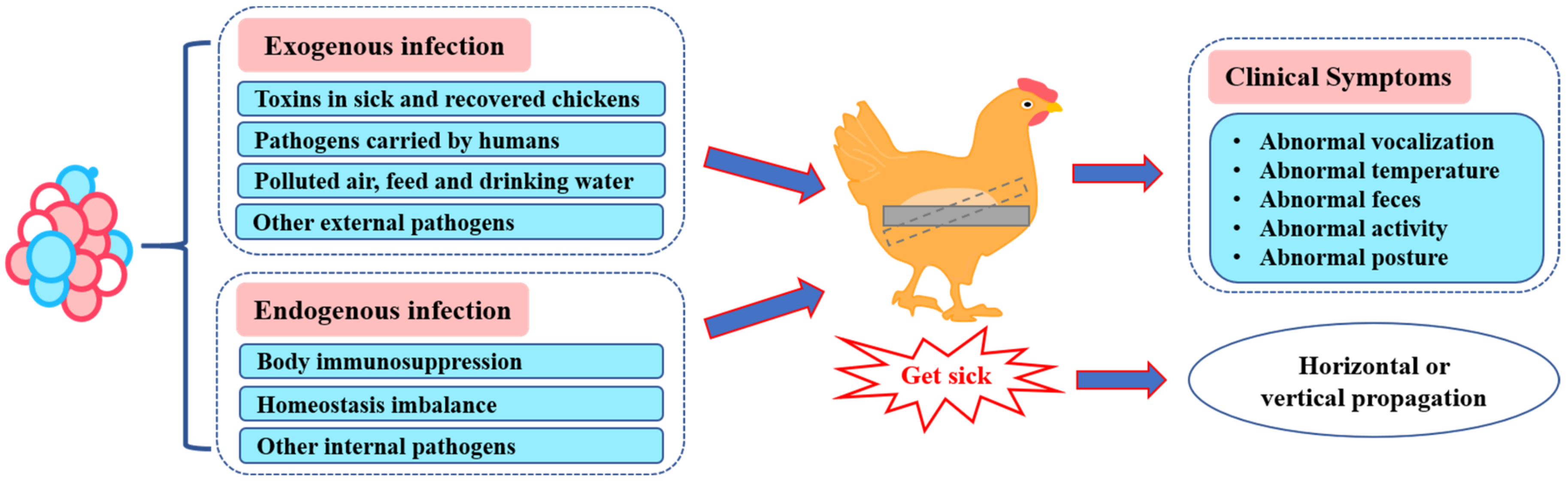
:1. Introduction
2. Early Diseases Detection through Physiological Characteristics
2.1. Abnormal Vocalization
2.2. Abnormal Body Temperature
2.3. Abnormal Feces
3. Early Disease Detection through Behavioral Characteristics
3.1. Abnormal Activity
3.2. Abnormal Posture
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mottet, A.; Tempio, G. Global poultry production: Current state and future outlook and challenges. Worlds Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.H.; Li, D.F. Impact of increased demand for animal protein products in Asian countries: Implications on global food security. Anim. Front. 2013, 3, 48–55. [Google Scholar] [CrossRef]
- Daghir, N.; Diab El Harake, M.; Kharroubi, S. Poultry production and its effects on food security in the Middle Eastern and North African region. J. Appl. Poult. Res. 2021, 30, 100110. [Google Scholar] [CrossRef]
- Astill, J.; Dara, R.A.; Fraser, E.D.G.; Roberts, B.; Sharif, S. Smart poultry management: Smart sensors, big data, and the internet of things. Comput. Electron. Agric. 2020, 170, 105291. [Google Scholar] [CrossRef]
- Jie, H.; Liu, Y.P. Breeding for disease resistance in poultry: Opportunities with challenges. Worlds Poult. Sci. J. 2011, 67, 687–696. [Google Scholar] [CrossRef]
- Gavora, J.S.; Spencer, J.L. Breeding for immune responsiveness and disease resistance. Worlds Poult. Sci. J. 1983, 53, 469–500. [Google Scholar]
- Lamont, S.J. The chicken major histocompatibility complex and disease. Rev. Off. Int. Epizoot. 1998, 17, 128–142. [Google Scholar] [CrossRef]
- Knap, P.W.; Doeschl-Wilson, A. Why breed disease-resilient livestock, and how? Genet. Sel. Evol. 2020, 52, 60. [Google Scholar] [CrossRef]
- Blake, D.P.; Tomley, F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Lillehoj, H.; Liu, Y.H.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalthoff, D.; Globig, A.; Beer, M. (Highly pathogenic) avian influenza as a zoonotic agent. Vet. Microbiol. 2010, 140, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiris, J.S.M.; Cowling, B.J.; Wu, J.T.; Feng, L.Z.; Guan, Y.; Yu, H.J.; Leung, G.M. Interventions to reduce zoonotic and pandemic risks from avian influenza in Asia. Lancet Infect. Dis. 2016, 16, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Jiao, H.U.; Liu, X. Endemicity of H9N2 and H5N1 avian influenza viruses in poultry in China poses a serious threat to poultry industry and public health. Front. Agric. Sci. Eng. 2016, 3, 11–24. [Google Scholar]
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Cervantes, H.M. Antibiotic-free poultry production: Is it sustainable? J. Appl. Poult. Res. 2015, 24, 91–97. [Google Scholar] [CrossRef]
- Weeks, C.A.; Brown, S.N.; Richards, G.J.; Wilkins, L.J.; Knowles, T.G. Levels of mortality in hens by end of lay on farm and in transit to slaughter in Great Britain. Vet. Rec. 2012, 170, 647. [Google Scholar] [CrossRef]
- Burch, D. Laying hen mortality by system—A welfare guide? Vet. Rec. 2012, 171, 649–650. [Google Scholar] [CrossRef]
- Vidic, J.; Manzano, M.; Chang, C.M.; Jaffrezic-Renault, N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017, 48, 11. [Google Scholar] [CrossRef] [Green Version]
- Ben Sassi, N.; Averos, X.; Estevez, I. Technology and Poultry Welfare. Animals 2016, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Ren, Z.; Li, D.; Zeng, L. Review: Automated techniques for monitoring the behaviour and welfare of broilers and laying hens: Towards the goal of precision livestock farming. Animal 2020, 14, 617–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astill, J.; Dara, R.A.; Fraser, E.D.G.; Sharif, S. Detecting and Predicting Emerging Disease in Poultry With the Implementation of New Technologies and Big Data: A Focus on Avian Influenza Virus. Front. Vet. Sci. 2018, 5, 263. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Li, J.W.; Ma, W.H.; Gao, R.H.; Yu, L.G.; Ding, L.Y.; Yu, Q.Y. Research Progress of Intelligent Sensing Technology for Diagnosis of Livestock and Poultry Diseases. Sci. Agric. Sin. 2021, 54, 2445–2463. [Google Scholar]
- Wang, K.Y.; Zhao, X.Y.; He, Y. Review on noninvasive monitoring technology of poultry behavior and physiological information. Trans. Chin. Soc. Agric. Eng. 2017, 33, 197–209. [Google Scholar]
- Wu, J.P.; Yang, Y.F.; Cheng, L.; Wu, J.; Xi, L.L.; Ma, Y.; Zhang, P.Y.; Xu, X.Y.; Zhang, D.K.; Li, S.Y. GCdiscrimination: Identification of gastric cancer based on a milliliter of blood. Brief Bioinform. 2021, 22, 536–544. [Google Scholar] [CrossRef]
- Stergiou, G.; Kollias, A.; Parati, G.; O’Brien, E. Office Blood Pressure Measurement The Weak Cornerstone of Hypertension Diagnosis. Hypertension 2018, 71, 813–815. [Google Scholar] [CrossRef]
- Liu, W.; Tao, Z.W.; Wang, L.; Yuan, M.L.; Liu, K.; Zhou, L.; Wei, S.; Deng, Y.; Liu, J.; Liu, H.G.; et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. 2020, 133, 1032–1038. [Google Scholar] [CrossRef]
- Carpentier, L.; Berckmans, D.; Youssef, A.; Berckmans, D.; van Waterschoot, T.; Johnston, D.; Ferguson, N.; Earley, B.; Fontana, I.; Tullo, E.; et al. Automatic cough detection for bovine respiratory disease in a calf house. Biosyst. Eng. 2018, 173, 45–56. [Google Scholar] [CrossRef]
- Aziz, N.A.; Bin Othman, M.F. Binary Classification using SVM for Sick and Healthy Chicken based on Chicken’s Excrement Image. Pertanika J. Sci. Technol. 2017, 25, 315–324. [Google Scholar]
- Li, X.H.; Nie, C.S.; Liu, Y.C.; Chen, Y.; Lv, X.Z.; Wang, L.; Zhang, J.W.; Yang, W.F.; Li, K.Y.; Zheng, C.W.; et al. The Genetic Architecture of Early Body Temperature and Its Correlation With Salmonella Pullorum Resistance in Three Chicken Breeds. Front. Genet. 2020, 10, 1287. [Google Scholar] [CrossRef]
- Manteuffel, G.; Puppe, B.; Schön, P.C. Vocalization of farm animals as a measure of welfare. Appl. Anim. Behav. Sci. 2004, 88, 163–182. [Google Scholar] [CrossRef]
- Du, X.D.; Carpentier, L.; Teng, G.H.; Liu, M.L.; Wang, C.Y.; Norton, T. Assessment of Laying Hens’ Thermal Comfort Using Sound Technology. Sensors 2020, 20, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, I.; Tullo, E.; Butterworth, A.; Guarino, M. An innovative approach to predict the growth in intensive poultry farming. Comput. Electron. Agric. 2015, 119, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Aydin, A.; Berckmans, D. Using sound technology to automatically detect the short-term feeding behaviours of broiler chickens. Comput. Electron. Agric. 2016, 121, 25–31. [Google Scholar] [CrossRef]
- Gonzalez, J.J.; Nasirahmadi, A.; Knierim, U. Automatically Detected Pecking Activity in Group-Housed Turkeys. Animals 2020, 10, 2034. [Google Scholar] [CrossRef]
- Nasirahmadi, A.; Gonzalez, J.; Sturm, B.; Hensel, O.; Knierim, U. Pecking activity detection in group-housed turkeys using acoustic data and a deep learning technique. Biosyst. Eng. 2020, 194, 40–48. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.X.; Liu, L.S.; Lu, M.S.; He, C.L.; Li, J.W. Research of detection method for broiler chicken feed intake based on audio technology. J. South China Agric. Univ. 2018, 39, 118–124. [Google Scholar]
- Carroll, B.T.; Anderson, D.V.; Daley, W.; Harbert, S.; Britton, D.F.; Jackwood, M.W. Detecting Symptoms of Diseases in Poultry through Audio Signal Processing. In Proceedings of the 2014 IEEE Global Conference on Signal and Information Processing, Atlanta, GA, USA, 3–5 December 2014; pp. 1132–1135. [Google Scholar]
- Lee, C.H.; Chou, C.H.; Han, C.C.; Huang, R.Z. Automatic recognition of animal vocalizations using averaged MFCC and linear discriminant analysis. Pattern Recognit. Lett. 2006, 27, 93–101. [Google Scholar] [CrossRef]
- Kotsiantis, S.B. Decision trees: A recent overview. Artif. Intell. Rev. 2013, 39, 261–283. [Google Scholar] [CrossRef]
- Whitaker, B.M.; Carroll, B.T.; Daley, W.; Anderson, D.V. Sparse Decomposition of Audio Spectrograms for Automated Disease Detection in Chickens. In Proceedings of the 2014 IEEE Global Conference on Signal and Information Processing, Atlanta, GA, USA, 3–5 December 2014; pp. 1122–1126. [Google Scholar]
- Rizwan, M.; Carroll, B.T.; Anderson, D.V.; Daley, W.; Harbert, S.; Britton, D.F.; Jackwood, M.W. Identifying Rale Sounds in Chickens Using Audio Signals for Early Disease Detection in Poultry. In Proceedings of the 2016 IEEE Global Conference on Signal and Information Processing, Washington, DC, USA, 7–9 December 2016; pp. 55–59. [Google Scholar]
- Banakar, A.; Sadeghi, M.; Shushtari, A. An intelligent device for diagnosing avian diseases: Newcastle, infectious bronchitis, avian influenza. Comput. Electron. Agric. 2016, 127, 744–753. [Google Scholar] [CrossRef]
- Carpentier, L.; Vranken, E.; Berckmans, D.; Paeshuyse, J.; Norton, T. Development of sound-based poultry health monitoring tool for automated sneeze detection. Comput. Electron. Agric. 2019, 162, 573–581. [Google Scholar] [CrossRef]
- Mahdavian, A.; Minaei, S.; Marchetto, P.M.; Almasganj, F.; Rahimi, S.; Yang, C. Acoustic features of vocalization signal in poultry health monitoring. Appl. Acoust. 2021, 175, 107756. [Google Scholar] [CrossRef]
- Spanhol, F.A.; Oliveira, L.S.; Petitjean, C.; Heutte, L. Breast Cancer Histopathological Image Classification using Convolutional Neural Networks. In Proceedings of the 2016 International Joint Conference on Neural Networks, Vancouver, BC, Canada, 24–29 July 2016; pp. 2560–2567. [Google Scholar]
- Cuan, K.X.; Zhang, T.M.; Huang, J.D.; Fang, C.; Guan, Y. Detection of avian influenza-infected chickens based on a chicken sound convolutional neural network. Comput. Electron. Agric. 2020, 178, 105688. [Google Scholar] [CrossRef]
- Cuan, K.X.; Zhang, T.M.; Li, Z.Y.; Huang, J.D.; Ding, Y.B.; Fang, C. Automatic Newcastle disease detection using sound technology and deep learning method. Comput. Electron. Agric. 2022, 194, 106740. [Google Scholar] [CrossRef]
- Du, X.D.; Lao, F.D.; Teng, G.H. A Sound Source Localisation Analytical Method for Monitoring the Abnormal Night Vocalisations of Poultry. Sensors 2018, 18, 2906. [Google Scholar] [CrossRef] [Green Version]
- Giloh, M.; Shinder, D.; Yahav, S. Skin surface temperature of broiler chickens is correlated to body core temperature and is indicative of their thermoregulatory status. Poult. Sci. 2012, 91, 175–188. [Google Scholar] [CrossRef]
- Youssef, A.; Exadaktylos, V.; Berckmans, D. Modelling and quantification of the thermoregulatory responses of the developing avian embryo: Electrical analogies of a physiological system. J. Therm. Biol. 2014, 44, 14–19. [Google Scholar] [CrossRef]
- Shepherd, E.M.; Fairchild, B.D. Footpad dermatitis in poultry. Poult. Sci. 2010, 89, 2043–2051. [Google Scholar] [CrossRef]
- Hoffmann, G.; Ammon, C.; Volkamer, L.; Suerie, C.; Radko, D. Sensor-based monitoring of the prevalence and severity of foot pad dermatitis in broiler chickens. Br. Poult. Sci. 2013, 54, 553–561. [Google Scholar] [CrossRef]
- Moe, R.O.; Bohlin, J.; Flo, A.; Vasdal, G.; Erlandsen, H.; Guneriussen, E.; Sjokvist, E.C.; Stubsjoen, S.M. Effects of subclinical footpad dermatitis and emotional arousal on surface foot temperature recorded with infrared thermography in turkey toms (Meleagris gallopavo). Poult. Sci. 2018, 97, 2249–2257. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Patterson, J.; Cheng, H.W. Use of thermography to screen for subclinical bumblefoot in poultry. Poult. Sci. 2009, 88, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Wang, F.J.; Liu, Y.H.; Wu, Y.Y.; Lu, H.S.; Yan, H.W. Comparative study on surface temperature between diseased and healthy layers. China Poult. 2017, 39, 53–56. [Google Scholar]
- Shen, P.N.; Lei, P.K.; Liu, Y.C.; Haung, Y.J.; Lin, J.J. Development of a temperature measurement system for a broiler flock with thermal imaging. Eng. Agric. Environ. Food. 2016, 9, 291–295. [Google Scholar] [CrossRef]
- Wu, K.; Klein, T.; Krishna, V.D.; Su, D.; Perez, A.M.; Wang, J.P. Portable GMR Handheld Platform for the Detection of Influenza A Virus. ACS Sens. 2017, 2, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.S.; Yao, D.; Chen, J.F.; Ye, W.; Ou, X.H.; Chen, T.M.; Sun, B.C. Development and evaluation of a real-time RT-PCR assay for detection of a novel avian influenza A (H5N6) virus. J. Virol. Methods 2018, 257, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Liu, D.; Hu, J.; Sun, W.Q.; Liu, K.T.; Li, J.; Xu, H.X.; Liu, J.; He, L.H.; Jiang, D.X.; et al. Multiplex one-step real-time PCR assay for rapid simultaneous detection of velogenic and mesogenic Newcastle disease virus and H5-subtype avian influenza virus. Arch. Virol. 2019, 164, 1111–1119. [Google Scholar] [CrossRef]
- Okada, H.; Itoh, T.; Suzuki, K.; Tsukamoto, K. Wireless sensor system for detection of avian influenza outbreak farms at an early stage. In Proceedings of the 2009 IEEE Sensors, Christchurch, New Zealand, 25–28 October 2009; pp. 1374–1377. [Google Scholar]
- Noh, J.Y.; Kim, K.J.; Lee, S.H.; Kim, J.B.; Kim, D.H.; Youk, S.; Song, C.S.; Nahm, S.S. Thermal Image Scanning for the Early Detection of Fever Induced by Highly Pathogenic Avian Influenza Virus Infection in Chickens and Ducks and Its Application in Farms. Front. Vet. Sci. 2021, 8, 616755. [Google Scholar] [CrossRef]
- Blas, A.; Diezma, B.; Moya, A.; Gomez, C. Early detection of mortality in poultry production using high resolution thermography. In Proceedings of the Vii Congreso Iberico de Agroingenieria y Ciencias Horticolas: Innovar y Producir para el Futuro. Innovating and Producing for the Future, Madrid, Spain, 26–29 August 2013; pp. 1499–1504. [Google Scholar]
- Ducatelle, R.; Goossens, E.; De Meyer, F.; Eeckhaut, V.; Antonissen, G.; Haesebrouck, F.; Van Immerseel, F. Biomarkers for monitoring intestinal health in poultry: Present status and future perspectives. Vet. Res. 2018, 49, 107756. [Google Scholar] [CrossRef] [Green Version]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wang, Y.K.; Niu, M.M.; Wang, C.J.; Wang, Z.F. Machine learning for characterizing risk of type 2 diabetes mellitus in a rural Chinese population: The Henan Rural Cohort Study. Sci. Rep. 2020, 10, 4406. [Google Scholar] [CrossRef]
- Khaldi, B.; Aiadi, O.; Kherfi, M.L. Combining colour and grey-level co-occurrence matrix features: A comparative study. IET Image Process 2019, 13, 1401–1410. [Google Scholar] [CrossRef]
- Wang, J.T.; Shen, M.X.; Liu, L.S.; Xu, Y.; Okinda, C. Recognition and Classification of Broiler Droppings Based on Deep Convolutional Neural Network. J. Sens. 2019, 2019, 3823515. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lin, Y.; Liu, Y.; Li, Z.; Li, Z.; Hu, S.; Liu, Z.; Lin, D.; Wu, Z. Cascaded-Automatic Segmentation for Schistosoma japonicum eggs in images of fecal samples. Comput. Biol. Med. 2014, 52, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, R.; Wu, Y.; Chu, K.; Xu, Q.; Sun, M.; Smith, Z.J. A low-cost, automated parasite diagnostic system via a portable, robotic microscope and deep learning. J. Biophotonics 2019, 12, e201800410. [Google Scholar] [CrossRef] [PubMed]
- Thevenoux, R.; Le, V.L.; Villessèche, H.; Buisson, A.; Beurton-Aimar, M.; Grenier, E.; Folcher, L.; Parisey, N. Image based species identification of Globodera quarantine nematodes using computer vision and deep learning. Comput. Electron. Agric. 2021, 186, 106058. [Google Scholar] [CrossRef]
- Liu, Y.L.; Windham, W.R.; Lawrence, K.C.; Park, B. Simple algorithms for the classification of visible/near-infrared and hyperspectral imaging spectra of chicken skins, feces, and fecal contaminated skins. Appl. Spectrosc. 2003, 57, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.C.; Park, B.; Lawrence, K.C.; Windham, W.R.; Heitschmidt, G.W. Line-scan hyperspectral imaging system for real-time inspection of poultry carcasses with fecal material and ingesta. Comput. Electron. Agric. 2011, 79, 159–168. [Google Scholar] [CrossRef]
- Chowdhury, E.U.; Morey, A. Application of optical technologies in the US poultry slaughter facilities for the detection of poultry carcase condemnation. Br. Poult. Sci. 2020, 61, 646–652. [Google Scholar] [CrossRef]
- De Jong, I.C.; Hindle, V.A.; Butterworth, A.; Engel, B.; Ferrari, P.; Gunnink, H.; Moya, T.P.; Tuyttens, F.A.M.; van Reenen, C.G. Simplifying the Welfare Quality((R)) assessment protocol for broiler chicken welfare. Animal 2016, 10, 117–127. [Google Scholar] [CrossRef]
- Knowles, T.G.; Kestin, S.C.; Haslam, S.M.; Brown, S.N.; Green, L.E.; Butterworth, A.; Pope, S.J.; Pfeiffer, D.; Nicol, C.J. Leg Disorders in Broiler Chickens: Prevalence, Risk Factors and Prevention. PLoS ONE 2008, 3, e1545. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, H.H.; Cornou, C. Automatic detection of deviations in activity levels in groups of broiler chickens—A pilot study. Biosyst. Eng. 2011, 109, 369–376. [Google Scholar] [CrossRef]
- Aydin, A. Using 3D vision camera system to automatically assess the level of inactivity in broiler chickens. Comput. Electron. Agric. 2017, 135, 4–10. [Google Scholar] [CrossRef]
- Aydin, A. Development of an early detection system for lameness of broilers using computer vision. Comput. Electron. Agric. 2017, 136, 140–146. [Google Scholar] [CrossRef]
- Silvera, A.M.; Knowles, T.G.; Butterworth, A.; Berckmans, D.; Vranken, E.; Blokhuis, H.J. Lameness assessment with automatic monitoring of activity in commercial broiler flocks. Poult. Sci. 2017, 96, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, M.S.; Roberts, S.J.; Cain, R.J.; Nickson, T.; Donnelly, C.A. Early warning of footpad dermatitis and hockburn in broiler chicken flocks using optical flow, bodyweight and water consumption. Vet. Rec. 2017, 180, 499. [Google Scholar] [CrossRef] [Green Version]
- Colles, F.M.; Cain, R.J.; Nickson, T.; Smith, A.L.; Roberts, S.J.; Maiden, M.C.J.; Lunn, D.; Dawkins, M.S. Monitoring chicken flock behaviour provides early warning of infection by human pathogen Campylobacter. Proc. Royal Soc. B 2016, 283, 20152323. [Google Scholar] [CrossRef] [Green Version]
- Malladi, S.; Weaver, J.T.; Clouse, T.L.; Bjork, K.E.; Trampel, D.W. Moving-Average Trigger for Early Detection of Rapidly Increasing Mortality in Caged Table-Egg Layers. Avian Dis. 2011, 55, 603–610. [Google Scholar] [CrossRef]
- Kozak, M.; Tobalske, B.; Springthorpe, D.; Szkotnicki, B.; Harlander-Matauschek, A. Development of physical activity levels in laying hens in three-dimensional aviaries. Appl. Anim. Behav. Sci. 2016, 185, 66–72. [Google Scholar] [CrossRef]
- Kashiha, M.; Pluk, A.; Bahr, C.; Vranken, E.; Berckmans, D. Development of an early warning system for a broiler house using computer vision. Biosyst. Eng. 2013, 116, 36–45. [Google Scholar] [CrossRef]
- Peña Fernández, A.; Norton, T.; Tullo, E.; van Hertem, T.; Youssef, A.; Exadaktylos, V.; Vranken, E.; Guarino, M.; Berckmans, D. Real-time monitoring of broiler flock’s welfare status using camera-based technology. Biosyst. Eng. 2018, 173, 103–114. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Chai, L.L.; Aggrey, S.E.; Oladeinde, A.; Johnson, J.; Zock, G. A Machine Vision-Based Method for Monitoring Broiler Chicken Floor Distribution. Sensors 2020, 20, 3179. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.L.; Bi, M.N.; Guo, J.L.; Wu, S.Y.; Zhang, T.M. Development of an early warning algorithm to detect sick broilers. Comput. Electron. Agric. 2018, 144, 102–113. [Google Scholar] [CrossRef]
- Okinda, C.; Lu, M.Z.; Liu, L.S.; Nyalala, I.; Muneri, C.; Wang, J.T.; Zhang, H.L.; Shen, M.X. A machine vision system for early detection and prediction of sick birds: A broiler chicken model. Biosyst. Eng. 2019, 188, 229–242. [Google Scholar] [CrossRef]
- Zhuang, X.L.; Zhang, T.M. Detection of sick broilers by digital image processing and deep learning. Biosyst. Eng. 2019, 179, 106–116. [Google Scholar] [CrossRef]
- Thenmozhi, M.; Saravanan, M.; Kumar, K.P.M.; Suseela, S.; Deepan, S. Improving the prediction rate of unusual behaviors of animal in a poultry using deep learning technique. Soft Comput. 2020, 24, 14491–14502. [Google Scholar] [CrossRef]
- Lao, F.D.; Du, X.D.; Teng, G.H. Automatic Recognition Method of Laying Hen Behaviors Based on Depth Image Processing. Trans. Chin. Soc. Agric. Mach. 2017, 48, 155–162. [Google Scholar]
- Fang, C.; Zhang, T.M.; Zheng, H.K.; Huang, J.D.; Cuan, K.X. Pose estimation and behavior classification of broiler chickens based on deep neural networks. Comput. Electron. Agric. 2021, 180, 105863. [Google Scholar] [CrossRef]
- Vegad, J.L. A Colour Atlas of Poultry Diseases; VetBooks: Boxmeer, The Netherlands, 2008. [Google Scholar]
- Intervet International BV. Important Poultry Diseases; VetBooks: Boxmeer, The Netherlands, 2009. [Google Scholar]


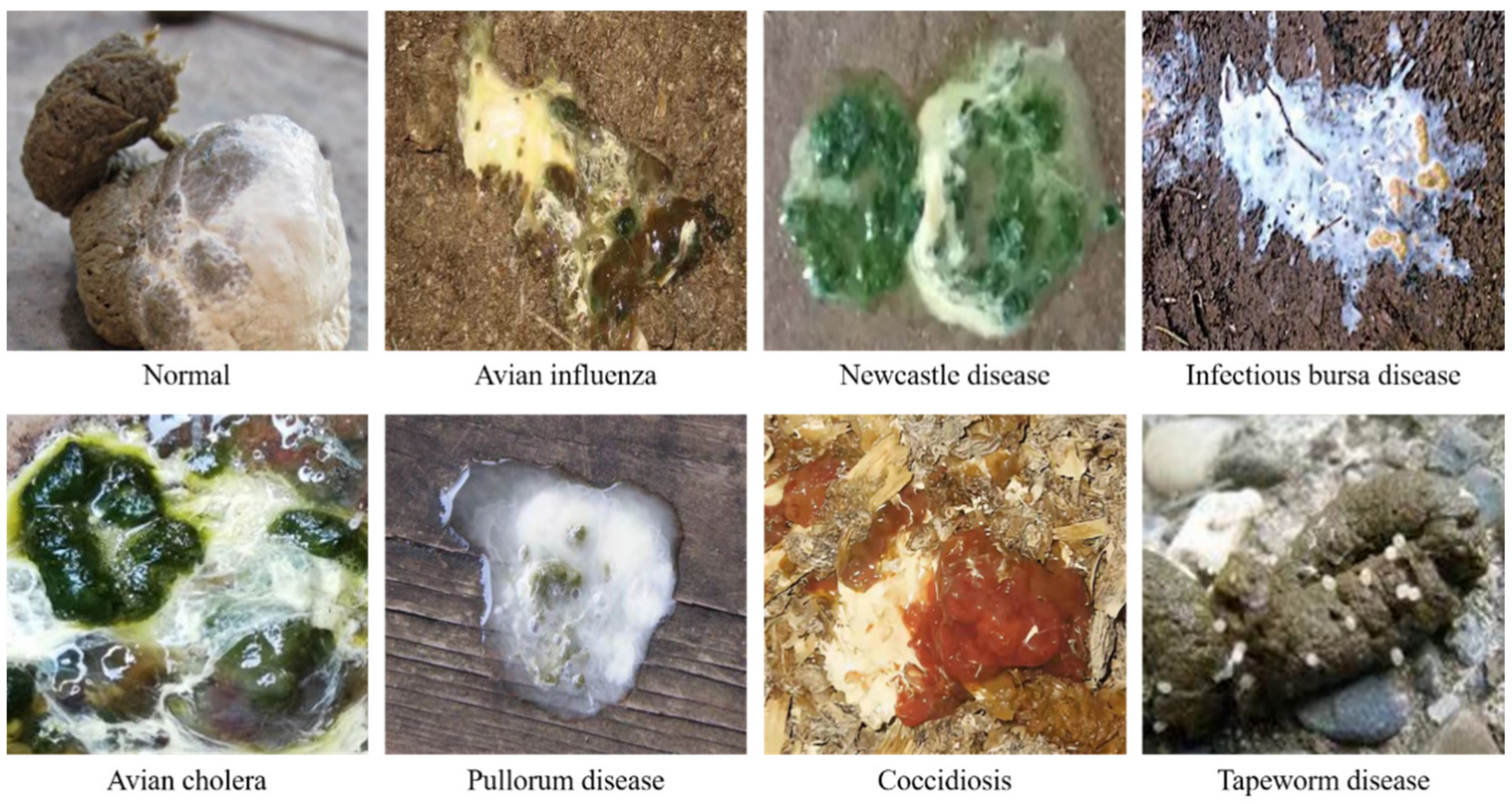
| Name of Disease | Vulnerable Time | Degree of Hazard * | Characteristics of Feces |
|---|---|---|---|
| Avian influenza | All ages | ★★★★ | Yellow-brown and watery |
| Newcastle disease | All ages | ★★★★ | Yellow-green and watery |
| Infectious bursa disease | 3–6 weeks | ★★★ | Lime watery |
| Infectious bronchitis | 3–7 weeks | ★★★ | White and watery |
| Avian cholera | Adult | ★★★ | Grass-green and watery |
| Pullorum disease | 0–2 weeks | ★★ | White mushy |
| Coccidiosis | 4–6 weeks | ★★ | Brown-red thin or blood |
| Tapeworm disease | All ages | ★ | Tapeworm eggs |
| Clinical Symptoms | Collection Devices | Monitoring Level | Advantages | Disadvantages |
|---|---|---|---|---|
| Vocalization | Microphone and camera | Group |
|
|
| Temperature | Temperature sensor | Individual |
|
|
| Infrared thermal imager | Group |
|
| |
| Feces | Camera | Group |
|
|
| Spectroscope | Group |
|
| |
| Activity | Acceleration sensor | Individual |
|
|
| Camera | Group |
|
| |
| Posture | Camera | Individual |
|
|
| Diseases | Causative Agent | Severity | Signs/Symptoms |
|---|---|---|---|
| Newcastle disease | Family: Paramvxoviridae Genus: Paramyxovirus | Mortality can be up to 100% | Respiratory signs Gasping and coughing, copious mucoid discharge, edema of tissue around eyes, throat and face, cyanosis of comb and wattles. Nervous signs Convulsions, torticollis, opisthotonus, drooping of wings, paralysis of legs and wings, enteric signs and greenish diarrhea are frequently seen. |
| Avian influenza | Orthomyxoviridae (Virus family) | Mortality can be up to 60% | Pronounced depression and ↓ feed consumption, huddling and ruffled feathers, mild to severe respiratory signs: coughing, sneezing, rales and excessive lacrimation, subcutaneous edema of head and face which may extend to neck and breast, cyanosis of wattles, comb and unfathered skin, areas of diffuse hemorrhage on shanks, nervous disorders, i.e., convulsions, ataxia, mucoid diarrhea (white). |
| Infectious bursal disease | Family: Birnaviridae Genus: Birnavirus | Mortality rate 1–50% | Sudden onset and short duration, the tendency for some birds to pick at their own vents, whitish, watery mucoid diarrhea followed by dehydration, soiled vent feathers, anorexia, severe depression and ruffled feathers, trembling, incoordination, prostration and finally death. |
| Marek’s disease | Marek’s disease virus | Mortality can be up to 100% | Asymmetric progressive paresis. Later complete paralysis of one or more of the extremities. Signs vary from bird to bird as anyone or several nerves may be affected by incoordination and lameness. Bird stretches one leg forward and other backward as a result of paralysis of the leg. Drooping of the limb in case of wing involvement. Dilation of crop and gasping if vagal paralysis. If nerves controlling neck muscles are affected, the head may be held low. |
| Fowl pox | Fowl pox virus Family (Poxviridae) Genus (Avipox) | Mortality rate is 1–5% | Cutaneous form (Dry Pox) Mild forms may remain unnoticed, generalized lesions on wattle, comb and unfeathered parts of the skin. Diphtheritic form (Wet Pox) White or opaque eruptions in the mouth, nares, pharynx, esophagus, larynx and trachea, caseous white patches coalesce and expand rapidly and become ulcerated. Mucous membranes undergo an extensive fibrin-necrotic process and develop diphtheritic membrane. Dyspnea, gasping and suffocation due to caseous material in the larynx. Death occurs due to suffocation. |
| Coccidiosis | Eimeria acervulina, Eimeria necatrix, Eimeria tenella, Eimeria Mitis, Eimeria Tenella, Eimeria maxima and Eimeria brunetti. | Mortality can be up to 50% | Coccidiosis can be divided into 2 groups Cecal coccidiosis: Mainly caused by E. tenella in chickens up to 12 weeks old. Mortality may run as high as 50%. Infected birds are listless, have bloody droppings and a pale comb and show a lack of appetite. Laboratory examination will show hemorrhages in the cecal wall. After severe bleeding, a core will be formed in the lumen. Small intestinal coccidiosis: Caused by E. acervulina, E. brunetti, E. maxima, E. necatrix, E. tenella and E. mitis. May affect birds of any age. E. acervulina is not normally very pathogenic, but in some cases, considerable mortality may be seen. Birds infected show loss of weight, combs may be shriveled and a drop or even cessation of egg production in layers may be seen. |
| Pullorum disease | Salmonella pullorum Family: Enterobacteriaceae | Mortality can be up to 100% | The incubation period is 4–5 days. Large numbers of dead in-shell chicks or chicks die shortly after hatching. Loss of appetite and huddling together. Sagging of wings and distorted body appearance, pot-bellied, chalky white excreta (white diarrhea), vent pasting, labored breathing or gasping. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, P.; Chen, Z.; Yu, H.; Hayat, K.; He, Y.; Pan, J.; Lin, H. Research Progress in the Early Warning of Chicken Diseases by Monitoring Clinical Symptoms. Appl. Sci. 2022, 12, 5601. https://doi.org/10.3390/app12115601
He P, Chen Z, Yu H, Hayat K, He Y, Pan J, Lin H. Research Progress in the Early Warning of Chicken Diseases by Monitoring Clinical Symptoms. Applied Sciences. 2022; 12(11):5601. https://doi.org/10.3390/app12115601
Chicago/Turabian StyleHe, Pengguang, Zhonghao Chen, Hongwei Yu, Khawar Hayat, Yefan He, Jinming Pan, and Hongjian Lin. 2022. "Research Progress in the Early Warning of Chicken Diseases by Monitoring Clinical Symptoms" Applied Sciences 12, no. 11: 5601. https://doi.org/10.3390/app12115601
APA StyleHe, P., Chen, Z., Yu, H., Hayat, K., He, Y., Pan, J., & Lin, H. (2022). Research Progress in the Early Warning of Chicken Diseases by Monitoring Clinical Symptoms. Applied Sciences, 12(11), 5601. https://doi.org/10.3390/app12115601






