Abstract
The quality of liquid egg white (LEW) during storage is critical for the development of the egg industry. In order to effectively control its storage quality, the effects of packaging materials and storage conditions on the quality of LEW were investigated. High-throughput sequencing (HTS) was applied to explore changes in bacterial population proportions and microflora in the spoilage of LEW. The shelf life of LEW packaged with glass (LEW-PG), plastic (LEW-PP), and tinplate (LEW-PT) was preliminarily determined to be 8, 5, and 7 days, respectively. LEW-PG possessed superior sensory scores (65) and L values (87.5), and a lower growth rate of total volatile basic nitrogen (TVB-N) content among the three samples on the last day of shelf life, and was chosen for further study. During 24 days of storage, the sensory scores of the LEW-PG in 10 °C and 4 °C groups decreased by 32.7% and 25.7%, respectively. There was no significant difference in foaming properties of LEW-PG between the 10 °C and 4 °C groups (p > 0.05). HTS analysis showed that the abundance of species composition in the 10 °C samples was higher than that in the 4 °C samples, though the latter possessed a higher community diversity. At the genus level, the dominant bacteria in the 10 °C group were Pseudomonas (21.79%), others (19.21%), and Escherichia (11.21%), while others (37.5%), Escherichia (30.40%), and Bifidobacterium (17.72%) were highly abundant in the 4 °C samples. It is hoped that this study could provide theoretical support for quality control of LEW during storage.
1. Introduction
Liquid egg product is a substitute for shell egg consumption, which is obtained by shell-breaking, shell-separation, egg-yolk separation, filtration, homogenization and pasteurization. It has a more controllable quality, more convenient use, and greater safety and convenience during storage and transportation in comparison with shelled egg [1]. Owing to these advantages, the consumption of liquid egg products has been increasing worldwide [2]. Globally, liquid egg products mainly contain whole liquid egg, liquid egg yolk, liquid egg white (LEW), special liquid egg with salt or sugar, high-whip egg whites, and different proportions of egg white and yolk mixtures [3]. LEW, which possesses superior functional properties, including gelling, foaming, and emulsifying properties, has been widely used in meat products, surimi products, and flour products [4].
LEW is generally preserved as a refrigerated product. Hence, the critical issue is to guarantee safety and maintain the functional properties of the LEW, especially in the packaging and storage processes. During the storage process, the properties of LEW are affected by multiple factors, such as storage temperature, packaging, and microbiological status [4]. Changes in LEW properties are complex, and mainly include sensory quality, total volatile basic nitrogen (TVB-N), pH, and albumen content, which leads to a decrease in functional properties [5]. In order to control these changes, several researchers have studied the effects of storage conditions on functional qualities, such as rheological behavior [6,7], and other physicochemical characteristics [8,9]. At present, many studies have highlighted several emerging sterilization technologies to improve microbial safety, as well as functionality and nutritionality of LEW products, such as ohmic heating [10], high hydrostatic pressure [11], and ultrahigh temperature [12]. Apart from these approaches, other important issues for the preservation of the quality of LEW are packaging materials and storage conditions. The packaging materials for liquid food in industrial production are mainly plastic, paper, paper–plastic, and tinplate [13]. Plastic and paper packaging materials are widely used in food packaging due to their good performance and low price [14]. The molecular substances in plastic or paper packaging materials may, however, migrate to food or food contents [15]. A certain amount of migration might lead to changes in the flavor of liquid foods, and may even affect human health [16,17]. It has been reported that the properties of food substrates and storage conditions are related to the amount of migration [18] Therefore, it is urgent to find optimal packaging materials and storage conditions that are suitable for LEW that are combined with its characteristics.
Moreover, effective control of pathogenic bacteria is also a potential alternative for maintaining the quality of LEW. In early years, a great deal of researchers established microbiological growth models, aiming to predict shelf life and improve the safety and quality of LEW [19,20]. However, the deterioration mechanism of LEW during storage remains unclear. As a result, the development of prevention and control technology for product storage lacks the necessary theoretical basis. With the rapid development of molecular techniques, high-throughput sequencing (HTS) has been regarded as a powerful tool in numerous fields of research, and contributes to presenting dynamic changes in communities and analyzing the correlations between spoilage bacteria and their metabolic pathways [21]. In recent studies, there has been a lack of applications of HTS in exploring the species of spoilage bacteria in LEW during storage.
Taken together, the first purpose of this study is to choose optimal packaging materials for LEW based on the characteristics. Secondly, the effects of storage conditions on the functional properties of LEW were investigated. Finally, high-throughput proteomics was used to investigate changes in the bacterial population proportion and microflora in the context of spoilage for LEW, aiming to provide theoretical support for quality control of LEW during storage.
2. Materials and Methods
2.1. Materials and Reagents
Eggs were purchased from Charoen Pokphand Egg Industry Co. Ltd. (Wuhan, China). An AxyPrep DNA Gel Extraction Kit was purchased from Axygen Biotechnology Co. Ltd. (Hangzhou, China). A DNA extraction kit, NEXTFLEX Rapid DNA-Seq Kit, was purchased from BIOO Scientific Co. Ltd. (Pomona, CA, American). Agar, ethanol, boric acid, methylene blue, magnesium oxide, hydrochloric, sulfuric acid, sodium hydroxide, and sodium dodecyl sulfate (SDS) were purchased from the China National Pharmaceutical Group Co. Ltd. (Analytical Reagent, Beijing, China). Tinplate (thickness 0.4 mm), glass (thickness 2 mm), and plastic (PET, thickness 0.5 mm) packaging materials were supplied by Hubei Shendan Healthy Food Co., Ltd. (Wuhan, China).
2.2. Preparation of LEW
LEW was prepared according to previous methods [22] with a slight modification. Eggs were rinsed with running water, scrubbed with 75% alcohol, and dried on a bench top. An egg splitter was used to separate the white from the yolk. Then, the egg white was dispersed uniformly using a high-speed disperser (12,500 rpm, 2 min) at room temperature, filtered with six layers of gauze, and subsequently divided into the three different packing materials (glass, plastic, and tinplate). The three packaged LEWs were pasteurized, cooled to room temperature, and stored at different temperatures (room temperature, 4 °C or 10 °C) for further study.
2.3. Determination of total Bacterial Count
The total bacterial count of LEWs was measured using a previous method with a slight modification [23]. A 25 mL sample was placed into a sterile conical flask containing 225 mL of phosphate buffer solution. This solution was flapped using a flapping homogenizer for 2 min, diluted properly, and added to a sterile Petri dish containing 15–20 mL of agar medium. After agar medium solidification, the Petri dish was flipped and cultured at 37 °C for 48 h. Then, the total bacterial count for each group was determined using a bacterial colony counter. The total count of bacteria in the LEW did not exceed the limits for pathogenic bacteria [24] during the storage days, which was called the shelf life of the LEW.
2.4. Measurement of Sensory Quality
The sensory quality of the LEW was scored from four aspects: taste, status, odor, and color. The assessment methods referred to a previous article with a slight modification [25], and a description of each level is shown in Table 1. The sensory assessment group consisted of 10 teachers and students who were trained in egg sensory analysis. During sensory assessment, all participants adhered to evaluation principles, such as isolation, non-communication, and rinsing with water before evaluation. Organoleptic assessments were carried out in a sensory-assessment laboratory. Additionally, the total sensory scores were the sum of all four aspects.

Table 1.
Scoring rules for the LEW.
2.5. Determination of TVB-N Content
TVB-N content was measured using semi-micro nitrogen determination [26]. First, LEW was dispersed uniformly with a disperser, 100 mL of distilled water was added, and then it was soaked for 30 min and then filtered. Second, 10 mL of boric acid solution (20 g/L) and 5 drops of mixed indicator solution (methyl red ethanol solution:methylene blue ethanol solution = 2:1) were added to the receiving flask. A condensing tube was inserted under the liquid level. A total of 10 mL of sample and 5 mL of magnesium oxide suspension were separately injected into the reaction chamber, and the rod glass plug was tightened. After distillation for 5 min, the receiving bottle of distillate was moved, and distilled for 1 min. Then, the lower end of the condensing tube was rinsed with water, and the receiving bottle was removed. Finally, the end point was titrated with hydrochloric or sulfuric acid standard titration solution (0.0100 mol/L) and the final color was magenta. The used calculation formula was from a previous study [27] with slight modifications, as follows:
X is the TVB-N content; V1 is the volume of hydrochloric or sulfuric acid standard titration solution consumed by the sample solution; V2 is the volume of hydrochloric or sulfuric acid standard titration solution consumed by the blank solution; C is the concentration of hydrochloric or sulfuric acid standard titration solution; 14 is the mass of nitrogen equivalent in the standard titration solution of 1.0 mL hydrochloric acid or sulfuric acid, m is the quality of the sample; V is the volume of the filtrate; V0 is the total volume of the sample solution; and 100 is conversion coefficient.
2.6. Measurement of Chromatic Aberration
The determination of chromatic aberration was conducted according to a previous method [28]. Briefly, a colorimeter was preheated for 30 min, and then calibrated with a slight amount of hydrazine and a white board. The light source was a D65/10. The parameters, including lightness (L), redness (a), and yellowness (b), were obtained. L denoted the brightness, a and b denoted the parallel color, which is to say that a was a red-green bias and b was a yellowish-blue bias. When L was 0, it denoted black; when L was 100, it denoted white. As for a, positive values represented red and negative values represented green. Positive b denoted yellow, and a negative b denoted blue.
2.7. Determination of Foaming Capacity and Stability
The procedure for foaming capacity and stability was conducted according to previous published articles [29]. LEW was diluted 10 times with boric acid–sodium hydroxide buffer solution (pH 9.0). A total of 200 mL of diluent was agitated with a waring blender at a speed of 12,000 rpm/min at room temperature for 1 min. Foam volume V1 was measured immediately, and foam volume V2 was measured after being left at room temperature for 25 min. The foaming capacity (Fc) and stability (Fs) of LEW were calculated using the following equations, respectively [30].
V0 is the diluent volume of the sample; V1 is the volume of foam after stirring for 1 min; V2 is the foam volume after being left standing for 25 min.
2.8. Measurement of Emulsifying Activity and Stability
The emulsifying activity and stability of LEW was determined using a previous method with a slight modification (Bai et al., 2014). A total of 5 mL of LEW and 100 mL of deionized water were uniformly mixed and was regarded as the sample solution. A total of 24 mL of sample solution and 8 mL of vegetable oil were placed into a beaker. Then, the mixture was stirred for 2 min at a speed of 10,000 rpm/min, and 100 μL of emulsion was quickly taken from the bottom of the liquid and put into a test tube containing 10 mL SDS solution (0.1%) at 0 min and then at 10 min. The absorbance values were measured at 500 nm, and the SDS solution was regarded as a blank control. The calculation formulas for the emulsifying activity (EA) and stability (ES) were as follows [31]:
A0 is the absorbance value measured at 0 min; DF is a dilution ratio; θ is the proportion of oil used to form the emulsion; L is the thickness of the color plate; C is the concentration of LEW; and A10 is the absorbance value measured at 10 min.
2.9. DNA Extraction, Amplicon Amplification
According to previous articles, LEW reached spoilage over 40 d under storage at 4 °C [32]. In order to investigate the changes in the bacterial population structure and the microflora of spoilage of LEW, LEW was stored in different storage environments (4 °C and 10 °C) for 50 days. The total DNA of each sample was extracted using the AxyPrep DNA Gel Extraction Kit, according to the manufacturer’s protocol. The quality of extracted genomic DNA was detected using 1% agarose gel electrophoresis. The universal and specific primers for the 16S rRNA gene in the V3–V4 region were synthesized using a real-time PCR instrument (Thermo Fisher Scientific, USA). PCR amplification cycles included an initial denaturation for 15 s at 95 °C, followed by annealing for 15 s at an appropriate Tm, and then extension for 1 min at 72 °C for 40 cycles. Each sample was replicated three times. PCR products from the same sample were mixed and detected with 2% agarose gel electrophoresis.
2.10. High-Throughput Sequencing
High-quality amplified fragments from each sample were then pooled at the same concentration and sent to Illumina’s MiSeq/HiSeq platform for sequencing according to Novogene’s manufacturer’s instructions. Then, paired-end (PE) reads obtained from the MiSeq sequencing were stitched according to the overlap relationship, and the quality of the sequences was also controlled and filtered. After the samples were distinguished, operational taxonomic unit (OUT) cluster analysis and species taxonomic analysis were performed. The diversity index of the OUT could be analyzed based on the results of OTU cluster analysis. The community structure could also be statistically analyzed at each taxonomic level, based on taxonomic information.
2.11. Statistical Analysis
Data were presented as mean ± standard deviation (SD). Statistical analysis was performed using one-way analysis of ANOVA with Duncan’s multiple range test. p-values < 0.05 were considered as statistically significant. Different lowercase letters in the figures and tables indicate significant differences.
3. Results and Discussion
3.1. Effect of Different Packaging Materials on LEW
3.1.1. Total Bacterial Count and TVB-N Analysis
As shown in Figure 1a, there was no significant difference in total bacterial count for the LEW among the three different packaging materials when stored at room temperature for 0–3 days. These results might be attributed to the inhibition of anti-bacterial substances, such as lysozyme, ovotransferrin, etc. With an increase in storage time, the total bacterial count of LEW-PG (6th day), LEW-PP (3rd day), and LEW-PT (4th day) began to increase sharply, which may be related to the decrease of antibacterial substance and thick white as well as the increase in thin white and dominant spoilage organisms (Salmonella enterica and Carnobacteriaceae family [33]. Additionally, the total bacterial count of LEW-PG (9th day), LEW-PP (6th day), and LEW-PT (8th day) were greater than 1*106 CFU/mL, which exceeded the limit of pathogenic bacteria in food [24]. Hence, the shelf life of LEW-PG, LEW-PP, and LEW-PT was preliminarily determined to be 8, 5, and 7 days, respectively. Follow-up studies of egg white liquid were carried out within the shelf life.
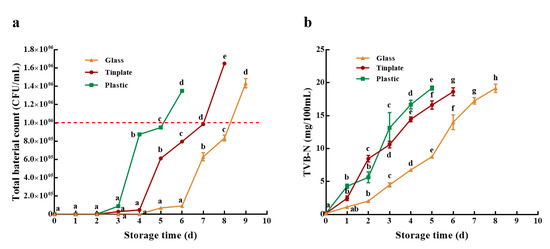
Figure 1.
The effect of different packaging materials on (a) total bacterial count, (b) TVB-N value of LEW during storage at room temperature. Different lowercase letters in the figure indicate significant differences within groups (p < 0.05).
TVB-N is commonly used to measure the freshness index of protein-rich foods [9]. It was reported that a TVB-N content of 30 mg/100 mL was regarded as the upper acceptable limit for human intake [34]. As shown in Figure 1b, the TVB-N content of LEW-PP, LEW-PT, and LEW-PG showed an increasing trend with the increase in storage time, and the TVB-N values on the 5th, 7th and 8th days were 19.15, 19.18, 18.64 mg/100 mL, respectively. A similar phenomenon was also found in previous work [9]. It could be inferred that these results may be due to ammonia production in LEW during storage. In addition, the growth rate of TVB-N content in LEW-PG was lower than that of LEW-PP and LEW-PT, presumably because of the slow microbial growth, to some extent.
3.1.2. Sensory Quality
Sensory evaluation is one of the common approaches for judging the quality and acceptance of egg product [35]. The results of the total sensory scores, including color, odor, status, and taste, are shown in Table 2, Table 3 and Table 4. Sensory qualities on day zero of storage were satisfied for all groups; while the total sensory scores of LEW-PG, LEW-PP, and LEW-PT decreased significantly with the increase in storage time. Decomposition of proteins and fats, as well as microbial activities (Pseudomonas spp., Shewanella putrefasciens provisions) might be the main cause for the decrease in total sensory scores [36]. As presented in Table 4, LEW-PT had the lowest color, odor, and total sensory scores on the last day of shelf life. These results might be due to the reaction between acid metabolites produced during the storage and the tinplate packaging, leading to a separation of metal ions and undesirable changes in sensory qualities [37].

Table 2.
Changes in sensory quality of LEW-PG with room temperature storage.

Table 3.
Sensory quality of LEW-PP with room temperature storage.

Table 4.
Sensory quality of LEW-PT with room temperature storage.
3.1.3. Color Difference
Color values (L, a, b) of LEW-PG, LEW-PP, and LEW-PT are shown in Figure 2a–c. L represents lightness, and the value range is 0–100, where 0 = black and 100 = white. As presented in Figure 2a, the L values of LEW-PG, LEW-PP, and LEW-PT decreased significantly with the increase in storage time (p < 0.05), and the values were reduced by 3.73%, 1.42%, and 39.24%, respectively, on the last day of shelf life. A positive value represents red hues, and a negative value represents green hues. Less statistical fluctuation of a value in the LEW-PG and LEW-PP groups was observed throughout the storage time, showing a visible green tint (Figure 2b). The value of LEW-PT was elevated significantly and reached a positive value (as well as presenting a visible red hue), which was consistent with color changes in the context of sensory quality. This phenomenon might be associated with the formation of Fe3+-acid metabolite complexes in the tinplate package [38]. A positive b value represented yellow tints, and a negative b value represented blue tints. As shown in Figure 2c, the b value of these three groups showed an irregular trend, and was mainly affected by multiple factors, such as gas tightness and light reflection [39].
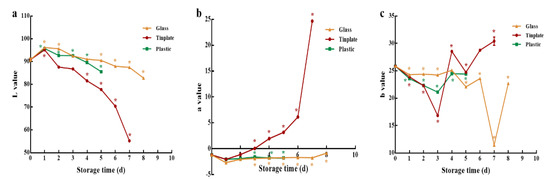
Figure 2.
The effect of different packaging materials on chromatic aberration of LEW during storage at room temperature. (a) L value (lightness), (b) a value (redness), (c) b value (yellowness). * p < 0.05 versus fresh LEW.
Overall, the glass packaging material was suitable for the preservation of LEW at room temperature, and was chosen for further study.
3.2. Effect of Different Storage Condition on LEW-PG
3.2.1. Sensory Analyses
The effect of storage time on the sensory quality of LEW-PG was investigated under storage temperatures of 10 °C and 4 °C. As shown in Figure 3a,b, the sensory quality of these two groups showed a decreasing trend throughout the 24 days of storage. The sensory scores of the 4 °C group decreased slowly compared with the 10 °C group, demonstrating a preservative effect of low temperature against undesirable changes during the storage time. These results may be attributed to the inhibition of enzyme activity and microbial growth due to the lower temperature to some extent [40].
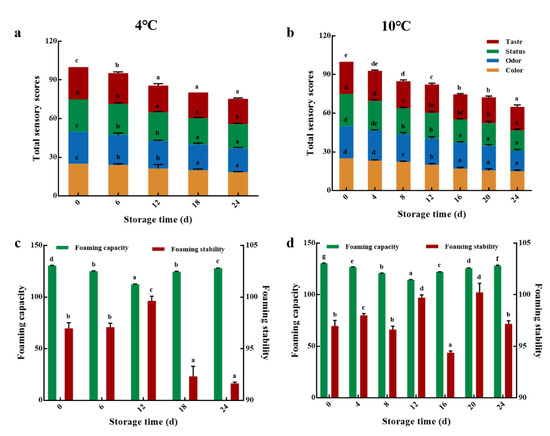
Figure 3.
Sensory quality of LEW under (a) 4 °C and (b) 10 °C storage conditions. Foaming properties of LEW under (c) 4 °C and (d) 10 °C storage conditions. Different lowercase letters in the figure indicate significant differences within groups (p < 0.05).
3.2.2. Foaming Properties
It is accepted that egg white possesses excellent foaming properties, and has been widely used in food processing [4]. As shown in Figure 3c,d, the foaming capacity of the 10 °C and 4 °C groups first decreased and then increased with the increase in storage time. As such, the foaming capacity of these two groups both reached a minimum value of 128.23% and 127.9% on the 12th day. There are probably two reasons for this phenomenon. One of reasons might be due to the degeneration and decreased solubility of egg white protein, caused by heat treatment, resulting in a decrease in foaming [41]. On the other hand, numerous insoluble flocculus in water appeared throughout a storage time of 0–12 d, which makes it difficult for egg white proteins to expand and absorb at the gas–liquid interface, leading to a gradual decline in egg white foamability [42,43]. While, the foaming capacity of LEW began to increase after 12 d, this might be attributed to the dissociation of egg white protein from the complex with the prolongation of storage time (12–24 d) [44,45], leading to the higher protein solubility and foaming capacity of LEW.
As presented in Figure 3c,d, the foaming stability of LEW in 10 °C and 4 °C group both showed a decreased trend and then an increased trend throughout storage time (0–24 d). As such, the foaming stability of the two groups gradually increased during a storage time of 0–8 d. This may be due to the denaturation precipitation of proteins with poor thermal stability caused by pasteurization, protein surface exposure of hydrophobic groups, and quick protein absorption on the water–gas interface, resulting in a stronger mechanical interface strength and elasticity and increased foam stability [46]. Meanwhile, the interaction between protein molecules was enhanced, leading to the formation of a more stable network structure among protein molecules through non-covalent bonds, followed by an increase in foaming stability of LEW [42,47]. After 8 d, the foaming stability of LEW in the 10 °C and 4 °C groups both reduced significantly (p < 0.05), and the foaming stability of the former was higher than that of the latter. These results might be due to the further exposure of hydrophobic groups inside the protein, he formation of more aggregates between protein molecules, and the breakdown of the stability of the optimal water–air interface, which resulted in a decline in foaming stability [45].
In general, the total sensory quality of the 4 °C group was superior to that of the 10 °C group after 24 days of storage; there was no significant difference in the sensory and foaming properties of LEW-PG between the 10 °C and 4 °C groups. Regarding the results of sensory quality and the economic costs of storage, 10 °C and 4 °C were both suitable for the preservation of LEW packaged with glass, to some extent. Hence, LEW-PG samples stored at 10 °C and 4 °C were used for further study.
3.3. High-Throughput Sequencing Analysis
3.3.1. OTU Analysis
The dilution curve can reflect the sampling depth of samples in the HTS process, so it is a common indicator to evaluate whether sequencing data fully cover a microbial community. In order to investigate the validity of sequencing quantity of egg white, data were randomly selected from the original data obtained by sequencing and the species diversity index was counted to obtain the dilution curve. As shown in Figure 4a, the OTU curve gradually flattened out with an increase in data, showing that the OTU coverage of the samples had basically been saturated, and more data marginally contributed to the discovery of new OTU This result indicated that the sequencing data volume was large enough to reflect the vast majority of microbial diversity information in the samples used in this study. As presented in the rank–abundance curve (Figure 4b), there was an obvious difference between the abundance and uniformity of the microorganisms. As such, the curve of the 10 °C group was relatively smooth with a large horizontal span, indicating the abundance of species composition and the high uniformity of LEW stored at 10 °C. The curve of the 4 °C group was steeper with a small horizontal span, demonstrating the low abundance of microorganisms and a numerical advantage of one or several kinds of microbials [48] in LEW stored at 4 °C.
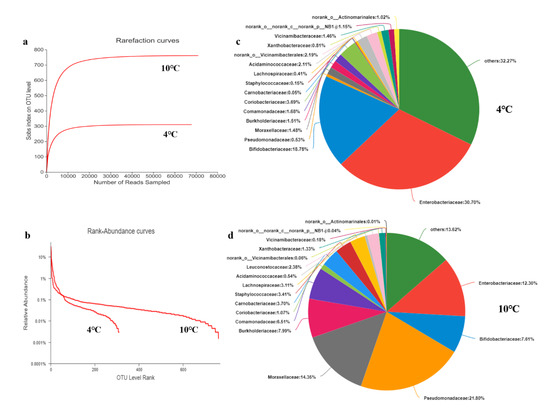
Figure 4.
(a) Dilution curve; (b) rank–abundance curve. Relative abundances of bacterial communities of LEW under (c) 4 °C and (d) 10 °C storage conditions at the family level.
3.3.2. Alpha Diversity Analysis
The diversity and abundance of microbial species in egg white samples were evaluated using the alpha diversity index, including Ace, Chao, Coverage, Shannon, and Simpson (Table 5). Ace and Chao are used to evaluate the abundance of microbials, and the value of these two indexes had a positive correlation with abundance. Shannon and Simpson are used to calculate the diversity of a bacterial community, and their values showed positive and negative correlations with diversity, respectively. As shown in Table 5, the coverage of LEW in the 10 °C and 4 °C groups both reached over 99%, which demonstrated that sequencing could reflect the real situation of the samples. The Ace, Chao, and Shannon of LEW in the 10 °C group were higher than those in the 4 °C group, while the former had a lower Simpson value. These results demonstrated that LEW in the 10 °C group possessed a higher abundance and uniformity of microbials.

Table 5.
Diversity index.
3.3.3. Bacterial Abundance and Distribution
As shown in Figure 4c,d, the 4 °C and 10 °C samples covered 17 and 18 families at the family level, respectively, showing similarity in the invasion species in both groups during the process of egg white spoilage. The dominant bacteria in the 4 °C group were from others (32.27%), Enterobacteriaceae (30.70%), and Bifidobacteriaceae (18.78%). The bacteria in the 10 °C group with the highest abundance belonged to Pseudomonadaceae (21.8%), followed by Moraxellaceae (14.35%), others (13.62%), and Enterobacteriaceae (12.3%). These results suggested that the dominant spoilage bacteria were markedly different in the two groups, which might be attributed to the different optimum growth temperatures of spoilage bacteria [49]. It has been reported that Pseudomonadaceae often exists in the spoilage of cryogenic liquid eggs [50], which is consistent with the results of these experiments. Pseudomonadaceae infection during breeding of poultry might occur through wounds, umbilical cords, environment, or infiltration through eggshells, and was also common in fresh eggs [51].
As shown in Figure 5, Pseudomonas (21.79%), others (19.21%), and Escherichia (11.21%) were highly abundant in the 10 °C samples. The dominant bacteria in the 4 °C group were others (37.5%), Escherichia (30.40%), and Bifidobacterium (17.72%). Among the dominant bacteria in the 10 °C group, Pseudomonas, which is known to play an important role in egg spoilage [50], accounted for the largest proportion. As a saprophytic bacterium, Pseudomonas are able to utilize new nutrients (lactate, succinate) after glucose depletion, giving them an ecological advantage over non-proteolytic bacteria or less-proteolytic bacteria [52]. The relative abundance of Bifidobacterium in the 4 °C sample (17.72%) was higher than that in the 10 °C sample (7.10%). It has been reported that Bifidobacterium has an antagonistic effect on the growth and reproduction of other bacteria [53], which may contribute relatively to extending the shelf life of LEW under a storage temperature of 4 °C. It is accepted that Escherichia is an important contributor to the spoilage of eggs and egg-derived products [54]. In the present study, Escherichia appeared at a relatively high abundance in the two groups, though especially in the 4 °C sample.
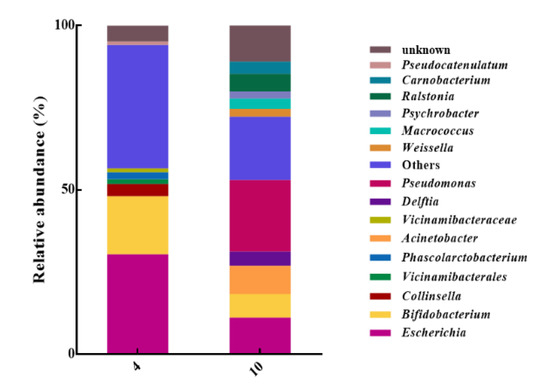
Figure 5.
Relative abundances of bacterial communities of LEW at the genus level.
The relative abundance and distribution of the microbial species showed significant differences in the two groups, which may be attributed to the following reasons. In the early stage of storage, different storage temperatures may be the main cause for the difference in microbial distribution between the two groups. Subsequently, depletion of glucose resources results in a shift from saccharolytic to amino-acid degrading metabolism [52], leading to changes in bacterial species. Additionally, dominant bacteria controlled the population behavior by secreting soluble small molecules and gained survival advantages, which are called microbial quorum sensing [55]. Several studies have shown that Enterobacteriaceae produce a rich array of molecules that might affect bacterial traits, which were involved in the process of product spoilage during storage [56,57].
4. Conclusions
In the present study, the shelf life of LEW-PG, LEW-PP, and LEW-PT was preliminarily determined to be 8, 5, and 7 days, respectively. Our observations revealed that a glass packaging material and cold storage (10 °C or 4 °C) were better for LEW storage. Our findings also clarified and confirmed the marked differences in the diversity and abundance of microbial species between the 10 °C and 4 °C samples. At the genus level, the dominant bacteria in the 10 °C sample were Pseudomonas, others, and Escherichia, while others, Escherichia and Bifidobacterium were highly abundant in the 4 °C group. These results might provide guidance for the optimization and control of the preservation of LEW. Additionally, future studies will focus on the decomposition status of proteins and fats in LEW under the action of extracellular enzymes belonging to specific putrefying bacteria, followed by explanation of the deterioration mechanisms of LEW quality during storage.
Author Contributions
D.G.: methodology, data curation, writing—original draft. Q.P.: conceptualization, methodology, data curation. Q.H.: conceptualization, data curation. Y.Y.: funding acquisition, supervision. H.W.: funding acquisition, supervision. W.X.: writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially Supported by Key Research and Development Project of Hubei Province (Program No. 2020BBA046).
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- Telli, N.; Telli, A.E.; Kahraman, H.A.; Ahmet, G.; Doruer, Y. Comparison of pasteurized liquid egg with shell eggs in terms of some quality characteristics. Eurasian J. Vet. Sci. 2015, 31, 177. [Google Scholar]
- Magdelaine, P. Egg and egg product production and consumption in Europe and the rest of the world. Improv. Saf. Qual. Eggs Egg Prod. 2011, 207, 3–16. [Google Scholar]
- Singh, J.; Sharma, H.K.; Premi, M.; Kumari, K. Effect of storage conditions of egg on rheological properties of liquid whole egg. J. Food Sci. Technol.-Mysore 2014, 51, 543–550. [Google Scholar] [CrossRef]
- Song, H.P.; Kim, B.; Choe, J.H.; Jung, S.; Kim, K.S.; Kim, D.H.; Jo, C. Improvement of foaming ability of egg white product by irradiation and its application. Radiat. Phys. Chem. 2009, 78, 217–221. [Google Scholar] [CrossRef]
- Muhammed, Y.; Asik, H. Texture, rheology, storage stability, and sensory evaluation of meringue’s prepared from lipase enzyme-modified liquid egg white. J. Food Process. Preserv. 2020, 44, e14667. [Google Scholar]
- Strnkova, J.; Nedomova, S.; Kumbar, V.; Buchar, J. Effect of egg storage duration on the rheology of liquid egg products. J. Food Eng. 2015, 156, 45–54. [Google Scholar]
- Yuceer, M.; Caner, C. Effects of protease-hydrolyzed egg white on the meringue batter properties and meringue textural and sensory properties during storage. Int. J. Gastron. Food Sci. 2021, 25, 100409. [Google Scholar] [CrossRef]
- Kang, G.H.; Cho, S.H.; Seong, P.N.; Park, B.Y.; Chae, H.S. Microbial and Physicochemical Properties of Liquid Egg during Cold Storage. Hangug Chugsan Sigpum Haghoeji = Korean J. Food Sci. Anim. Resour. 2011, 31, 557–562. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Gu, L.; Su, Y.; Chang, C.; Xu, L.; Yang, Y.; Li, J. Changes in microbial, physiochemical, and functional properties of pasteurized liquid whole egg during refrigerated storage. J. Sci. Food Agric. 2020, 100, 2873–2879. [Google Scholar] [CrossRef]
- Yildiz, H.; Guven, E. Industrial applications and potential use of ohmic heating for fluid foods. Bulg. Chem. Commun. 2014, 46, 98–102. [Google Scholar]
- Naderi, N.; House, J.D.; Pouliot, Y.; Doyen, A. Effects of High Hydrostatic Pressure Processing on Hen Egg Compounds and Egg Products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 707–720. [Google Scholar] [CrossRef]
- Tu, Y.; Yang, Z.; Ma, C. Touching tastes: The haptic perception transfer of liquid food packaging materials. Food Qual. Prefer. 2015, 39, 124–130. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Qiao, Y.; Sun, X.; Ma, S. Adhesion of liquid food to packaging surfaces: Mechanisms, test methods, influencing factors and anti-adhesion methods. J. Food Eng. 2018, 228, 102–117. [Google Scholar] [CrossRef]
- Babaee, M.; Garavand, F.; Rehman, A.; Jafarazadeh, S.; Amini, E.; Cacciotti, I. Biodegradability, physical, mechanical and antimicrobial attributes of starch nanocomposites containing chitosan nanoparticles. Int. J. Biol. Macromol. 2022, 195, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Yang, C.Y.; Liu, Z.; Zhao, Z.Z. GC-MS Studies on the Contaminants in Paper-Plastic Food Packaging Materials. Adv. Mater. Res. 2012, 380, 282–285. [Google Scholar] [CrossRef]
- Begley, T.H.; Hsu, W.; Noonan, G.; Diachenko, G. Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit. Contam. 2008, 25, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, J.L.; Elizalde, M. Migration of Photoinitiators in Food Packaging: A Review. Packag. Technol. Sci. 2015, 28, 181–203. [Google Scholar] [CrossRef]
- Xu, Y.; Noonan, G.O.; Begley, T.H. Migration of perfluoroalkyl acids from food packaging to food simulants. Food Addit. Contam. Part A-Chem. Anal. Control. Expo. Risk Assess. 2013, 30, 899–908. [Google Scholar] [CrossRef]
- Kim, Y.J.; Moon, H.J.; Lee, S.K.; Song, B.R.; Lim, J.S.; Heo, E.J.; Park, H.J.; Wee, S.H.; Moon, J.S. Development and Validation of Predictive Model for Salmonella Growth in Unpasteurized Liquid Eggs. Korean J. Food Sci. Anim. Resour. 2018, 38, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Sakha, M.Z.; Fujikawa, H. Prediction of Salmonella Enteritidis growth in pasteurized and unpasteurized liquid egg products with a growth model. Biocontrol Sci. 2013, 18, 89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, N.; Zhang, Y.X.; Wu, Q.P.; Gu, Q.H.; Chen, M.T.; Zhang, Y.Z.; Sun, X.L.; Zhang, J.M. High-throughput sequencing analysis of bacterial community composition and quality characteristics in refrigerated pork during storage. Food Microbiol. 2019, 83, 86–94. [Google Scholar] [CrossRef]
- Souza, P.M.D.; Fernández, A. Consumer acceptance of UV-C treated liquid egg products and preparations with UV-C treated eggs. Innov. Food Sci. Emerg. Technol. 2012, 14, 107–114. [Google Scholar] [CrossRef]
- Zihang, S.; Xingmin, L. Effect of modified atmosphere packaging, cleaning coating and ultraviolet sterilization on egg quality. Food Sci. Technol. 2019, 44, 73–79. [Google Scholar]
- Liu, R.; Ali, S.; Haruna, S.A.; Ouyang, Q.; Chen, Q. Development of a fluorescence sensing platform for specific and sensitive detection of pathogenic bacteria in food samples. Food Control. 2021, 131, 108419. [Google Scholar] [CrossRef]
- Necidová, L.; Bursová, Š.; Ježek, F.; Haruštiaková, D.; Vorlová, L.; Golian, J. Effect of preservatives on the shelf-life and sensory characteristics of pasteurized liquid whole egg stored at 4 °C. Poult. Sci. 2019, 98, 5940–5948. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.C.; Chen, Y. Evaluation on Uncertainty of Determination of Total Volatile Basic Nitrogen in Fresh Meat. J. Beijing Technol. Bus. Univ. 2011, 29, 37–40. [Google Scholar]
- Özoǧul, F.; Özoǧul, Y. Comparision of Methods Used for Determination of Total Volatile Basic Nitrogen (TVB-N) in Rainbow Trout (Oncorhynchus mykiss). Turk. J. Zool. 2014, 24, 113–120. [Google Scholar]
- Zhu, S.; Ramaswamy, H.S.; Simpson, B.K. Effect of high-pressure versus conventional thawing on color, drip loss and texture of Atlantic salmon frozen by different methods. LWT-Food Sci. Technol. 2004, 37, 291–299. [Google Scholar] [CrossRef]
- Su, Y.J.; Yang, X.Y.; Di, Z.; Yang, Y.J. Effect of Heat-treatment on the Properties of Egg Yolk. Food Ferment. Ind. 2012, 38, 70–75. [Google Scholar]
- Raymundo, A.; Empis, J.; Sousa, I. Method to evaluate foaming performance. J. Food Eng. 1998, 36, 445–452. [Google Scholar] [CrossRef]
- Bai, J.; Peng, Y.J.; Yu-Mei, L.I.; Tao, G.Q.; Liu, L.S.; Tian, X.; Guo, H. Effects of ultra High pressure processing on microorganisms and quality attributes of liquid egg during storage. Food Sci. Technol. 2014, 38, 564–568. [Google Scholar]
- Zu, L.; Ma, M. Optimization of whole egg liquid sterilization technology using high pressure CO2. Trans. Chin. Soc. Agric. Eng. 2017, 33, 299–307. [Google Scholar] [CrossRef]
- Wilson, A.; Chandry, P.S.; Turner, M.S.; Courtice, J.M.; Fegan, N. Comparison between cage and free-range egg production on microbial composition, diversity and the presence of Salmonella enterica. Food Microbiol. 2021, 97, 103754. [Google Scholar] [CrossRef] [PubMed]
- Yerlıkaya, N. Determination of the shelf life of marinated sardine (Sardina pilchardus) stored at 4 °C. Food Control 2004, 15, 1–4. [Google Scholar]
- Jasour, M.S.; Ehsani, A.; Mehryar, L.; Naghibi, S.S. Chitosan coating incorporated with the lactoperoxidase system: An active edible coating for fish preservation. J. Sci. Food Agric. 2015, 95, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, A.; Naghibi, S.S.; Aminzare, M.; Keykhosravi, K.; Hashemi, M. Extraction of specific egg yolk antibodies and application in chitosan coating: Effect on microbial and sensory properties of rainbow trout fillet during chilled storage. J. Sci. Food Agric. 2019, 99, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Grassino, A.N.; Grabaric, Z.; Pezzani, A.; Squitieri, G.; Berkovic, K. Corrosion inhibition with different protective layers in tinplate cans for food preservation. J. Sci. Food Agric. 2010, 90, 2419–2426. [Google Scholar] [CrossRef]
- Xia, D.; Song, S.; Wang, J.; Bi, H.; Han, Z. Corrosion Behavior of Tinplates in a Functional Beverage. Acta Phys.-Chim. Sin. 2012, 28, 121–126. [Google Scholar] [CrossRef]
- Eleroğlu, H.; Yıldırım, A.; Duman, M.; Okur, N. Effect of Eggshell Color on the Egg Characteristics and Hatchability of Guinea Fowl (Numida meleagris) Eggs. Rev. Bras. Cienc. Avic. 2016, 18, 61–68. [Google Scholar] [CrossRef][Green Version]
- Arnosti, C.; Jorgensen, B.B. High activity and low temperature optima of extracellular enzymes in Arctic sediments: Implications for carbon cycling by heterotrophic microbial communities. Mar. Ecol. Prog. 2003, 249, 15–24. [Google Scholar] [CrossRef]
- Talansier, E.; Loisel, C.; Dellavalle, D.; Desrumaux, A.; Legrand, J. Optimization of dry heat treatment of egg white in relation to foam and interfacial properties. LWT-Food Sci. Technol. 2009, 42, 496–503. [Google Scholar] [CrossRef]
- Wei, X.U.; Dai, Y.; Wang, H.; Meng, Q.; Jiang, P.; Lei, M. Effect of Frozen Treatment on Foaming and Gelatinability of Egg White Liquid. Food Ind. 2019, 40, 96–98. [Google Scholar]
- Sadahira, M.S.; Rodrigues, M.I.; Akhtar, M.; Murray, B.S.; Netto, F.M. Effect of egg white protein-pectin electrostatic interactions in a high sugar content system on foaming and foam rheological properties. Food Hydrocoll. 2016, 58, 1–10. [Google Scholar] [CrossRef]
- Wang, M.P.; Chen, X.W.; Guo, J.; Yang, J.; Wang, J.M.; Yang, X.Q. Stabilization of foam and emulsion by subcritical water-treated soy protein: Effect of aggregation state. Food Hydrocoll. 2019, 87, 619–628. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, L.; Gouda, M.; Ma, M. Impact of ultrasound treatment on the foaming and physicochemical properties of egg white during cold storage. LWT 2019, 113, 108303. [Google Scholar] [CrossRef]
- Nicorescu, I.; Vial, C.; Talansier, E.; Lechevalier, V.; Loisel, C.; Valle, D.D.; Riaublanc, A.; Djelveh, G.; Legrand, J. Comparative effect of thermal treatment on the physicochemical properties of whey and egg white protein foams. Food Hydrocoll. 2011, 25, 797–808. [Google Scholar] [CrossRef]
- Radványi, D.; Juhász, R.; Nemeth, C.; Suhajda, A.; Barta, J. Evaluation of the Stability of Whipped Egg White. Czech J. Food Sci. 2012, 30, 412–420. [Google Scholar] [CrossRef]
- Liu, T.; Awasthi, M.K.; Jiao, M.; Awasthi, S.K.; Zhang, Z. Changes of fungal diversity in fine coal gasification slag amendment pig manure composting. Bioresour. Technol. 2021, 325, 124703. [Google Scholar] [CrossRef]
- Shetty, T.S.; Setty, T.; Ravishankar, C.N. Temperature Growth Response of Spoilage Bacteria Isolated from Indian Oil Sardine (Sardinella longiceps) Stored in Chilled Sea Water. Fish. Technol. 1992, 29, 158–161. [Google Scholar]
- Gongora-Nieto, M.M.; Pedrow, P.D.; Swanson, B.G.; Barbosa-Canovas, G.V. Energy analysis of liquid whole egg pasteurized by pulsed electric fields. J. Food Eng. 2003, 57, 209–216. [Google Scholar] [CrossRef]
- Reu, K.D.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Herman, L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef]
- Nychas, G.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Zambori, C.; Morvay, A.A.; Sala, C.; Licker, M.; Tirziu, E. Antimicrobial effect of probiotics on bacterial species from dental plaque. J. Infect. Dev. Ctries. 2016, 10, 214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pasquali, F.; Manfreda, G.; Olivi, P.; Rocculi, P.; Sirri, F.; Meluzzi, A. Modified-atmosphere packaging of hen table eggs: Effects on pathogen and spoilage bacteria. Poult. Sci. 2012, 91, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Abisado, R.G.; Benomar, A.S.; Klaus, A.J.R.; Dandekar, A.A.A.; Chandlera, B.J.R. Bacterial Quorum Sensing and Microbial Community Interactions. Mbio 2018, 9, e02331-17. [Google Scholar] [CrossRef]
- Martins, M.L.; Pinto, U.M.; Riedel, K.; Vanetti, M. Quorum Sensing and Spoilage Potential of Psychrotrophic Enterobacteriaceae Isolated from Milk. BioMed Res. Int. 2018, 2018, 2723157. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, A.; Feng, L.; Gao, H. Quorum sensing signals affect spoilage of refrigerated large yellow croaker (Pseudosciaena crocea) by Shewanella baltica. Int. J. Food Microbiol. 2016, 217, 146–155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).