Clinical Effects of Locally Delivered Lactobacillus reuteri as Adjunctive Therapy in Patients with Periodontitis: A Split-Mouth Study
Abstract
1. Introduction
2. Materials and Methods
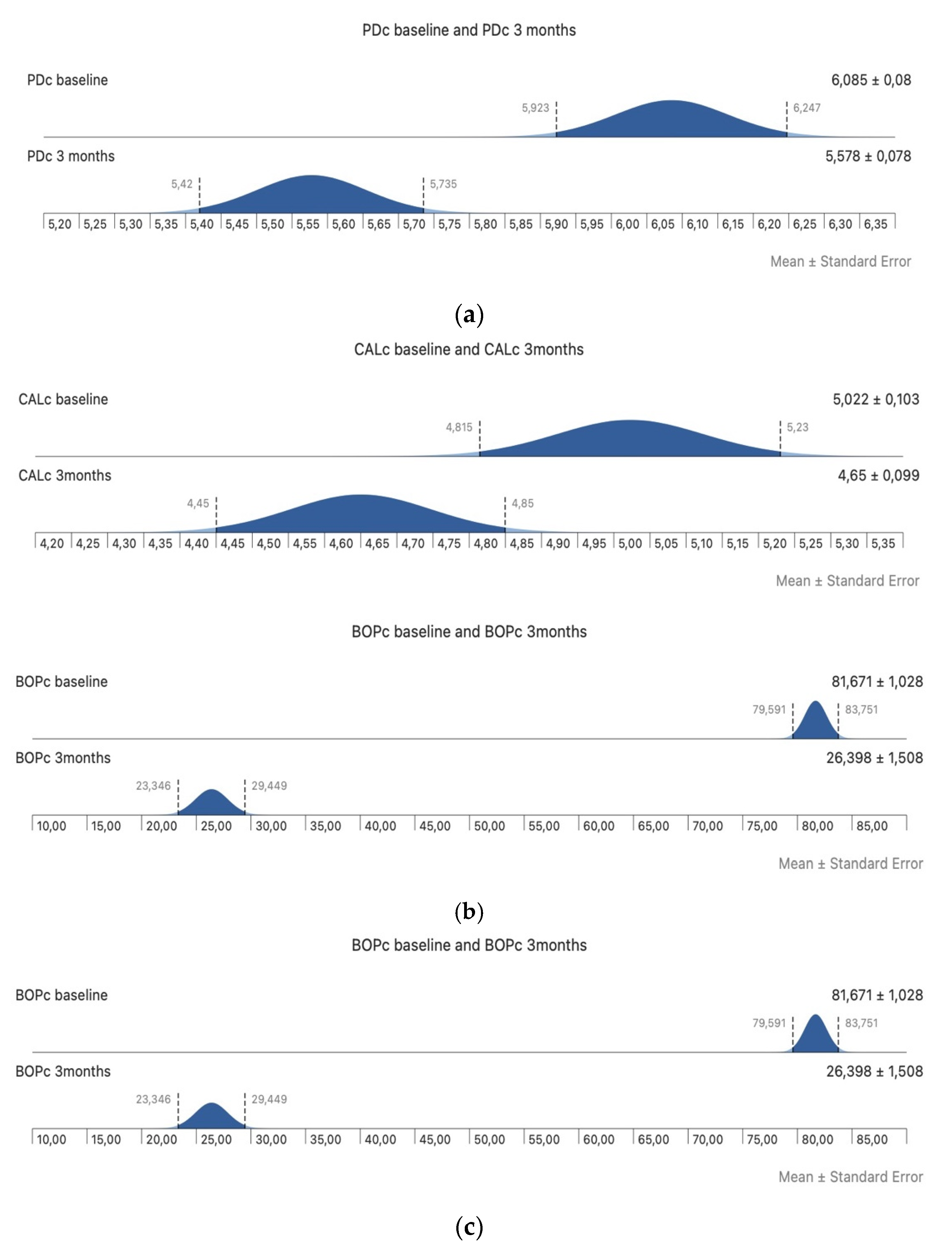
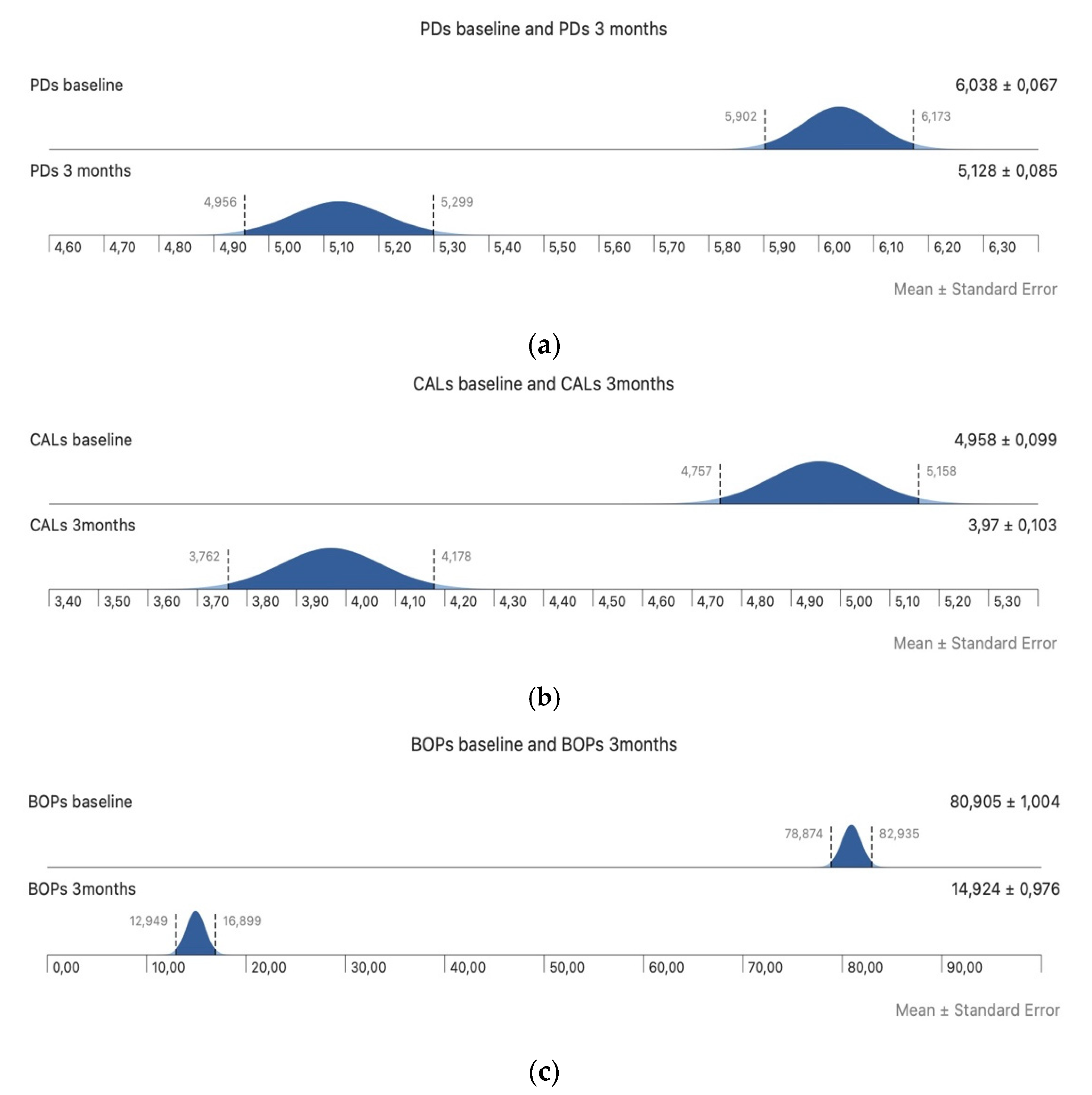
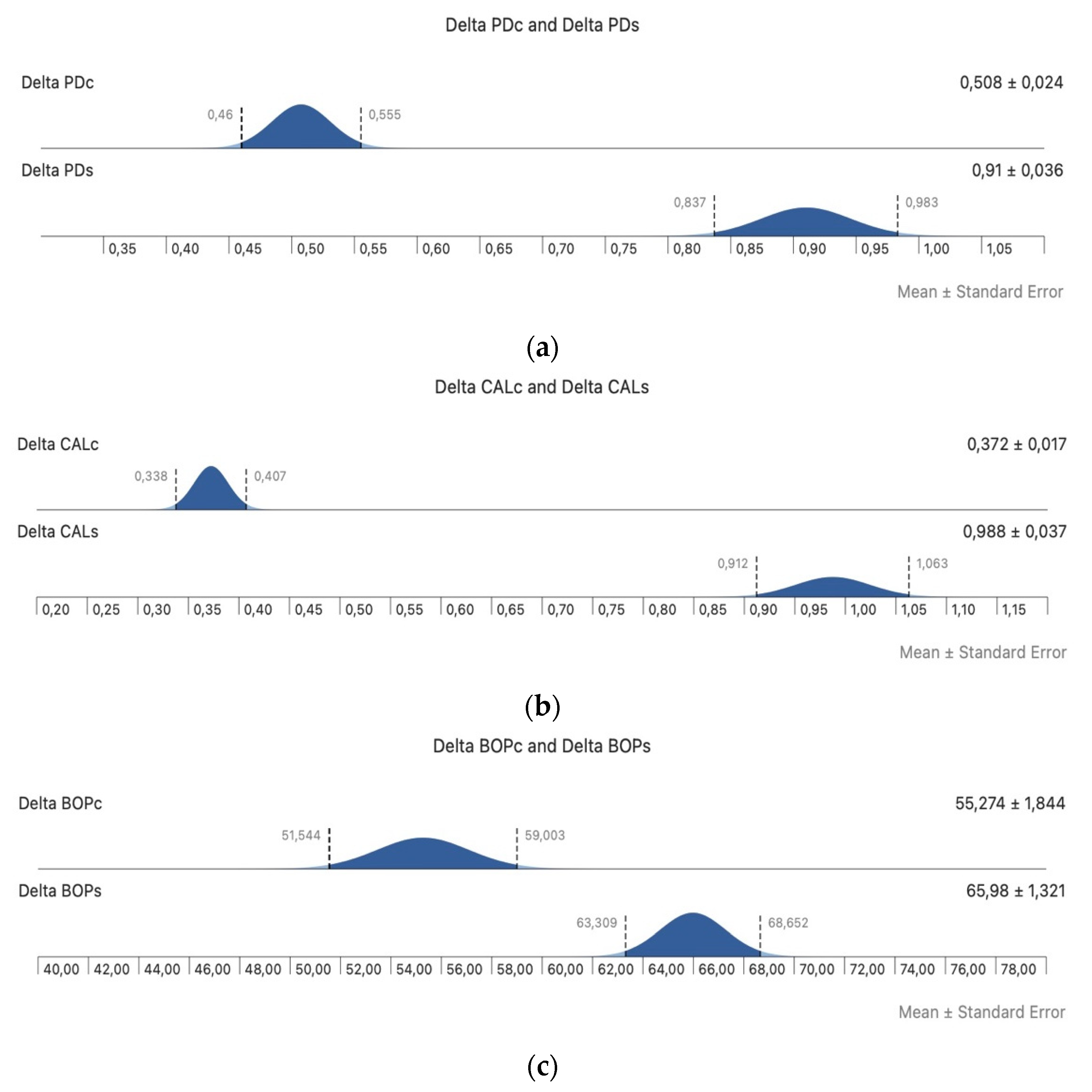
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Fragkioudakis, I.; Riggio, M.P.; Apatzidou, D.A. Understanding the microbial components of periodontal disease and periodontal treatment-induced microbiological shifts. J. Med. Microbiol. 2021, 70, 001247. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Leite, F.R.M.; Scheutz, F.; López, R. Periodontitis: From infection to inflammation. Curr. Oral Health Rep. 2017, 4, 301–308. [Google Scholar] [CrossRef]
- Teodorescu, A.C.; Martu, I.; Teslaru, S.; Kappenberg-Nitescu, D.C.; Goriuc, A.; Luchian, I.; Martu, M.A.; Solomon, S.M.; Martu, S. Assessment of salivary levels of RANKL and OPG in aggressive versus chronic periodontitis. J. Immunol. Res. 2019, 2019, 6195258. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Slots, J. Primer on etiology and treatment of progressive/severe periodontitis: A systemic health perspective. Periodontol. 2000 2020, 83, 272–276. [Google Scholar] [CrossRef]
- Alassy, H.; Pizarek, J.A.; Kormas, I.; Pedercinic, A.; Wolff, L.F. Antimicrobial adjuncts in the management of periodontal and peri-implant diseases and conditions: A narrative review. Front. Oral Maxillofac. Med. 2021, 3, 16. [Google Scholar] [CrossRef]
- Mocanu, R.C.; Martu, M.A.; Luchian, I.; Sufaru, I.G.; Maftei, G.A.; Ioanid, N.; Martu, S.; Tatarciuc, M. Microbiologic profiles of patients with dental prosthetic treatment and periodontitis before and after photoactivation therapy—Randomized clinical trial. Microorganisms 2021, 9, 713. [Google Scholar] [CrossRef]
- Allaker, R.P.; Stephen, A.S. Use of probiotics and oral health. Curr. Oral Health Rep. 2017, 4, 309–318. [Google Scholar] [CrossRef]
- Martin-Cabezas, R.; Davideau, J.L.; Tenenbaum, H.; Huck, O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 520–530. [Google Scholar] [CrossRef]
- Meurman, J.H. Probiotics: Do they have a role in oral medicine and dentistry? Eur. J. Oral Sci. 2005, 113, 188–196. [Google Scholar] [CrossRef]
- Devine, D.A.; Marsh, P.D.; Meade, J. Modulation of host responses by oral commensal bacteria. J. Oral Microbiol. 2015, 7, 26941. [Google Scholar] [CrossRef] [PubMed]
- Alok, A.; Singh, I.D.; Singh, S.; Kishore, M.; Jha, P.C.; Iqubal, M.A. Probiotics: A new era of biotherapy. Adv. Biomed. Res. 2017, 6, 31. [Google Scholar] [CrossRef]
- Valeur, N.; Engel, P.; Carbajal, N.; Connolly, E.; Ladefoged, K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl. Environ. Microbiol. 2004, 70, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Rosander, A.; Connolly, E.; Roos, S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl. Environ. Microbiol. 2008, 74, 6032–6040. [Google Scholar] [CrossRef]
- Hallström, H.; Lindgren, S.; Yucel-Lindberg, T.; Dahlén, G.; Renvert, S.; Twetman, S. Effect of probiotic lozenges on inflammatory reactions and oral biofilm during experimental gingivitis. Acta Odontol. Scand. 2013, 71, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Durukan, A.; Ozcelik, O.; Pauwels, M.; Quirynen, M.; Haytac, M.C. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J. Clin. Periodontol. 2013, 40, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Vicario, M.; Santos, A.; Violant, D.; Nart, J.; Giner, L. Clinical changes in periodontal subjects with the probiotic Lactobacillus reuteri Prodentis: A preliminary randomized clinical trial. Acta Odontol. Scand. 2013, 71, 813–819. [Google Scholar] [CrossRef]
- Penala, S.; Kalakonda, B.; Pathakota, K.R.; Jayakumar, A.; Koppolu, P.; Lakshmi, B.V.; Pandey, R.; Mishra, A. Efficacy of local use of probiotics as an adjunct to scaling and root planing in chronic periodontitis and halitosis: A randomized controlled trial. J. Res. Pharm. Pract. 2016, 5, 86–93. [Google Scholar] [CrossRef]
- Dumitrescu, D.; Fanuta, B.; Stepan, A.E.; Fronie, A.I.; Dumitrescu, C.I.; Martu, M.C.; Surlin, P.; Surlin, V.; Popescu, M. Silent sinus syndrome—Report of a case. Rom. J. Morphol. Embryol. 2015, 56, 229–237. [Google Scholar]
- Kim, J.H.; Kim, S.J.; Choi, J.I.; Lee, J.Y. Periodontal attachment loss of extracted teeth for periodontal reasons. J. Korean Acad. Periodontol. 2006, 36, 1049594. [Google Scholar] [CrossRef][Green Version]
- Roshna, T. Generalized aggressive periodontitis and its treatment options: Case reports and review of literature. Case Rep. Med. 2012, 2012, 535321. [Google Scholar] [CrossRef]
- Maftei, G.A.; Martu, C.M.; Popa, C.; Geletu, G.; Danila, V.; Jelihovschi, I.; Foia, L. The biomechanical properties of suture materials and their relationship to bacterial adherence. Mat. Plast. 2019, 56, 980–985. [Google Scholar] [CrossRef]
- Tekce, M.; Ince, G.; Gursoy, H.; Dirikan Ipci, S.; Cakar, G.; Kadir, T.; Ylmaz, S. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1-year follow-up study. J. Clin. Periodontol. 2015, 42, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Maeda, K.; Hidaka, K.; Nemoto, K.; Hirose, Y.; Deguchi, S. Daily intake of heat-killed Lactobacillus plantarum L-137 decreases the probing Depth in patients undergoing supportive periodontal therapy. Oral Health Prev. Dent. 2016, 14, 207–214. [Google Scholar]
- Laleman, I.; Yilmaz, E.; Ozcelik, O.; Haytac, C.; Pauwels, M.; Herrero, E.R.; Slomka, V.; Quirynen, M.; Alkaya, B.; Teughels, W. The effect of a Streptococci containing probiotic in periodontal therapy: A randomized controlled trial. J. Clin. Periodontol. 2015, 42, 1032–1041. [Google Scholar] [CrossRef]
- İnce, G.; Gürsoy, H.; İpçi, Ş.D.; Cakar, G.; Emekli-Alturfan, E.; Yılmaz, S. Clinical and biochemical evaluation of lozenges containing Lactobacillus reuteri as an adjunct to non-surgical periodontal therapy in chronic periodontitis. J. Periodontol. 2015, 86, 746–754. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, S.J.; Ko, S.H.; Ouwehand, A.C.; Ma, D.S. Modulation of the host response by probiotic Lactobacillus brevis CD2 in experimental gingivitis. Oral Dis. 2015, 21, 705–712. [Google Scholar] [CrossRef]
- Szkaradkiewicz, A.K.; Stopa, J.; Karpinski, T.M. Effect of oral administration involving a probiotic strain of Lactobacillus reuteri on proinflammatory cytokine response in patients with chronic periodontitis. Arch. Immunol. Ther. Exp. 2014, 62, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Ursarescu, I.G.; Martu-Stefanache, M.A.; Solomon, S.M.; Pasarin, L.; Boatca, R.M.; Caruntu, I.D.; Martu, S. The assessment of IL-6 and RANKL in the association between chronic periodontitis and osteoporosis. Rev. Chem. 2016, 67, 386–389. [Google Scholar]
- Anton, D.M.; Martu, M.A.; Maris, M.; Maftei, G.A.; Sufaru, I.G.; Tatarciuc, D.; Luchian, I.; Ioanid, N.; Martu, S. Study on the effects of melatonin on glycemic control and periodontal parameters in patients with type II diabetes mellitus and periodontal disease. Medicina 2021, 57, 140. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; Invernizzi, M.; Ferrillo, M.; Gimigliano, F.; Baricich, A.; Cisari, C.; De Marchi, F.; Foglio Bonda, P.L.; Mazzini, L.; Migliario, M. Functional status and oral health in patients with amyotrophic lateral sclerosis: A cross-sectional study. Neuro Rehabil. 2021, 48, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ferrillo, M.; Migliario, M.; Roccuzzo, A.; Molinero-Mourelle, P.; Falcicchio, G.; Umano, G.R.; Pezzotti, F.; Foglio Bonda, P.L.; Calafiore, D.; de Sire, A. Periodontal disease and vitamin D deficiency in pregnant women: Which correlation with preterm and low-weight birth? J. Clin. Med. 2021, 10, 4578. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sufaru, I.-G.; Lazar, L.; Sincar, D.-C.; Martu, M.-A.; Pasarin, L.; Luca, E.-O.; Stefanescu, A.; Froicu, E.-M.; Solomon, S.-M. Clinical Effects of Locally Delivered Lactobacillus reuteri as Adjunctive Therapy in Patients with Periodontitis: A Split-Mouth Study. Appl. Sci. 2022, 12, 2470. https://doi.org/10.3390/app12052470
Sufaru I-G, Lazar L, Sincar D-C, Martu M-A, Pasarin L, Luca E-O, Stefanescu A, Froicu E-M, Solomon S-M. Clinical Effects of Locally Delivered Lactobacillus reuteri as Adjunctive Therapy in Patients with Periodontitis: A Split-Mouth Study. Applied Sciences. 2022; 12(5):2470. https://doi.org/10.3390/app12052470
Chicago/Turabian StyleSufaru, Irina-Georgeta, Luminita Lazar, Dorina-Cerasella Sincar, Maria-Alexandra Martu, Liliana Pasarin, Elena-Odette Luca, Ada Stefanescu, Eliza-Maria Froicu, and Sorina-Mihaela Solomon. 2022. "Clinical Effects of Locally Delivered Lactobacillus reuteri as Adjunctive Therapy in Patients with Periodontitis: A Split-Mouth Study" Applied Sciences 12, no. 5: 2470. https://doi.org/10.3390/app12052470
APA StyleSufaru, I.-G., Lazar, L., Sincar, D.-C., Martu, M.-A., Pasarin, L., Luca, E.-O., Stefanescu, A., Froicu, E.-M., & Solomon, S.-M. (2022). Clinical Effects of Locally Delivered Lactobacillus reuteri as Adjunctive Therapy in Patients with Periodontitis: A Split-Mouth Study. Applied Sciences, 12(5), 2470. https://doi.org/10.3390/app12052470







