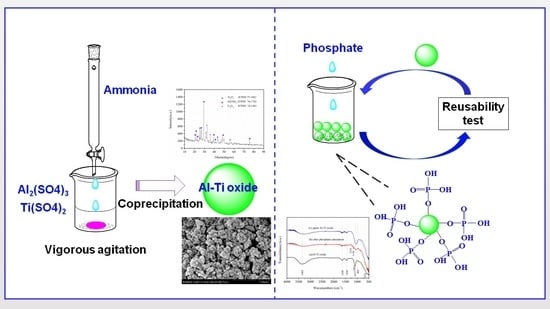
Phosphate Adsorption onto an Al-Ti Bimetal Oxide Composite in Neutral Aqueous Solution: Performance and Thermodynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Al-Ti Oxide
2.2. Characterization
2.3. Batch Experiments
3. Results and Discussion
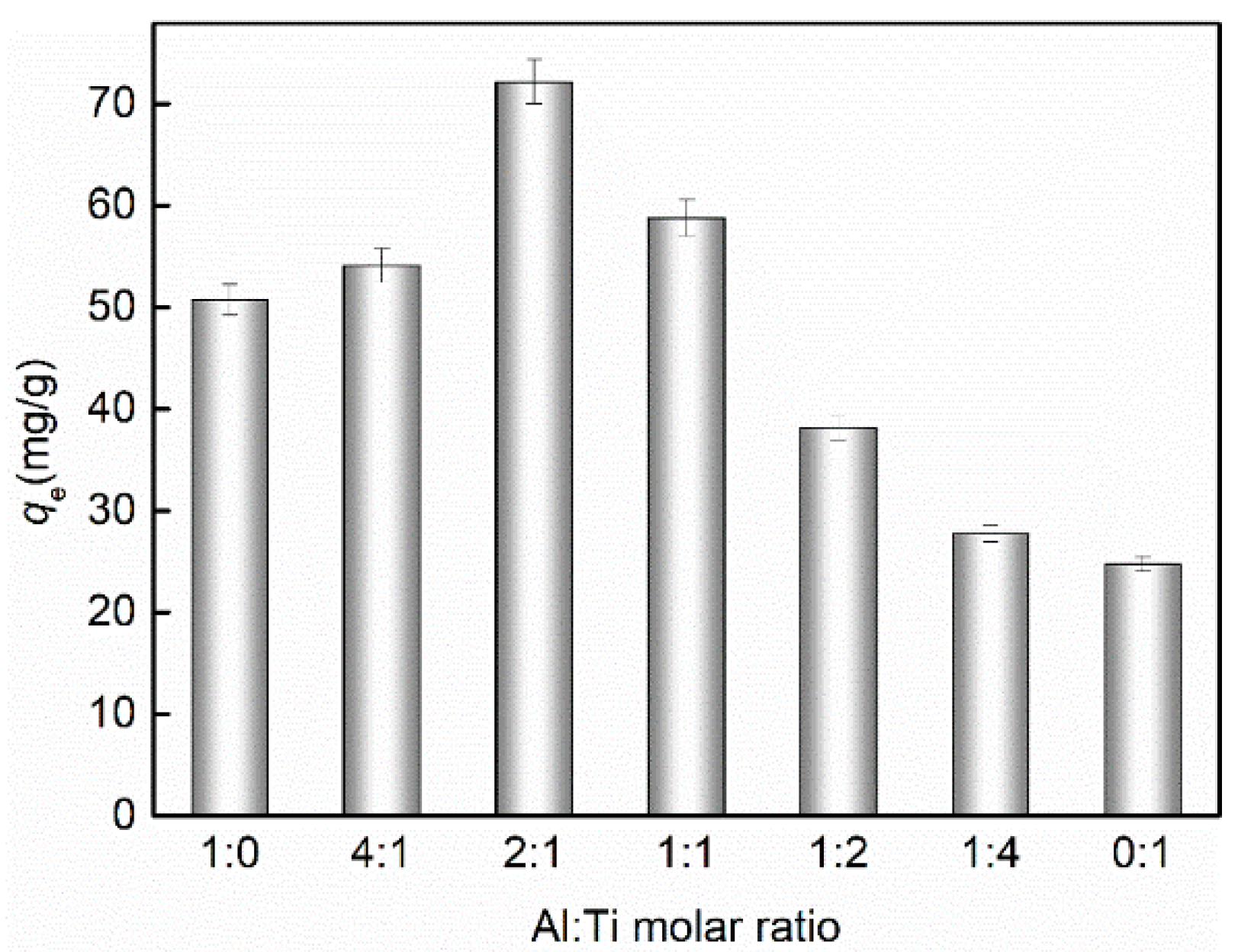
3.1. Optimization of Al-Ti Oxide for Adsorption of Phosphate
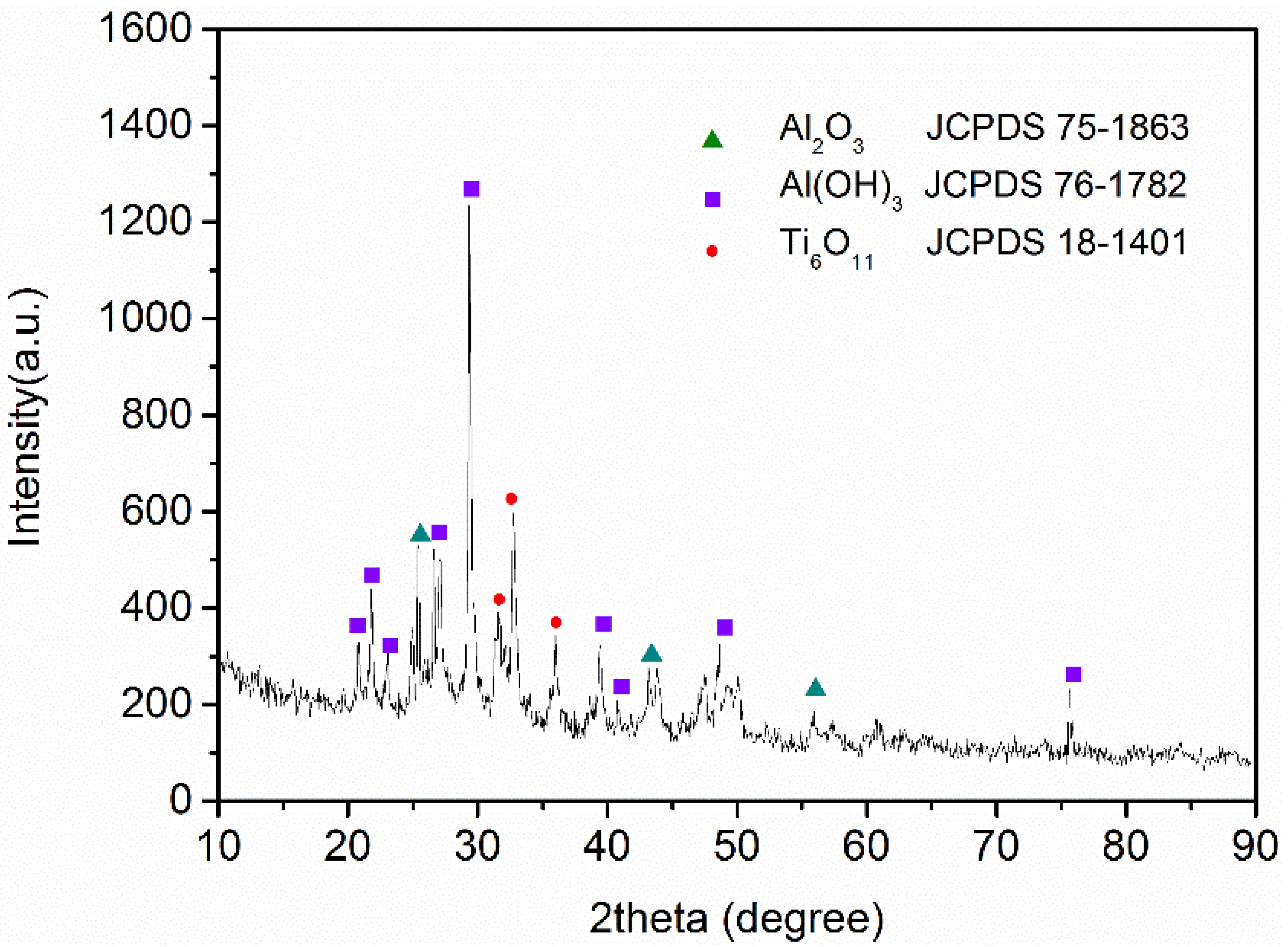
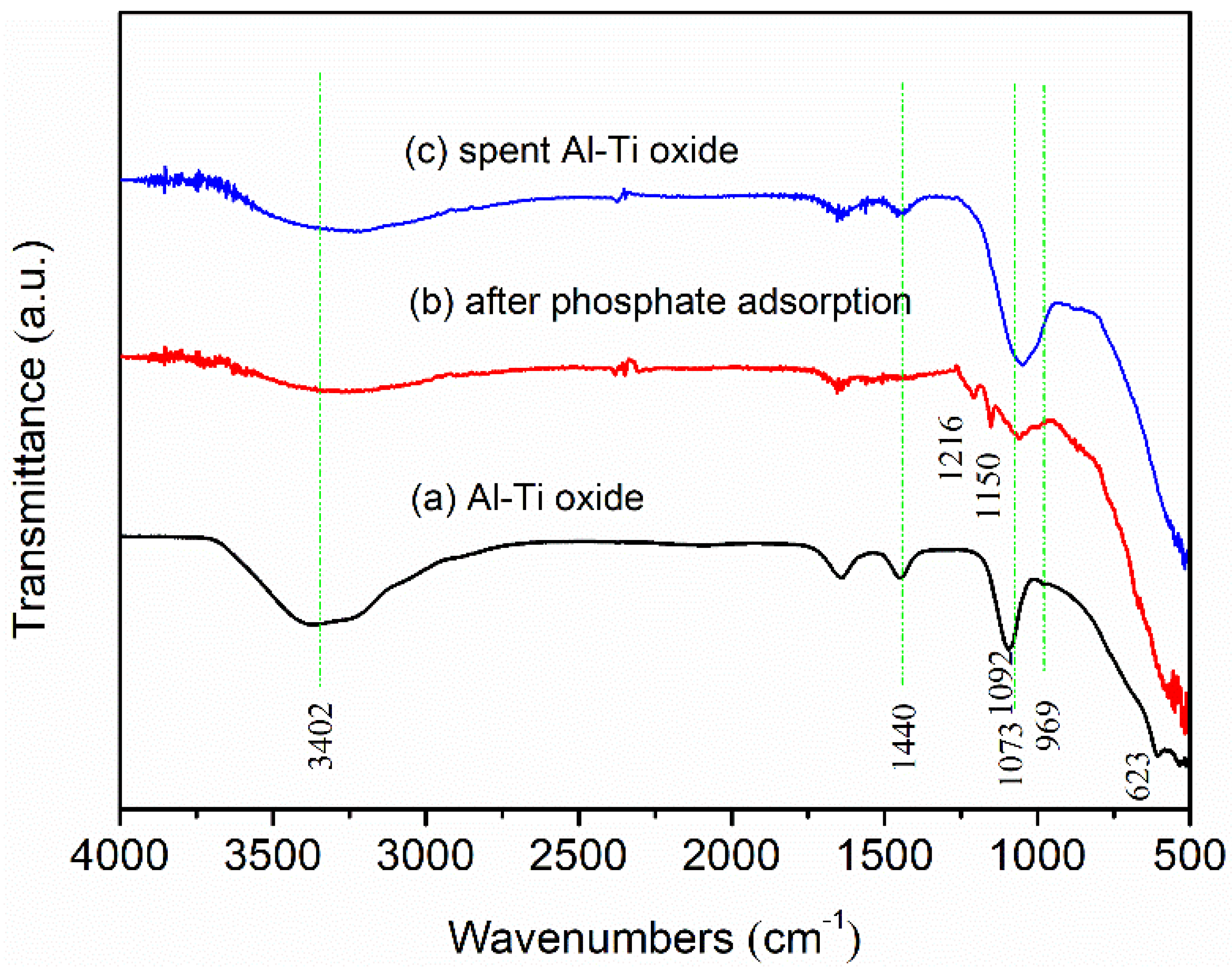
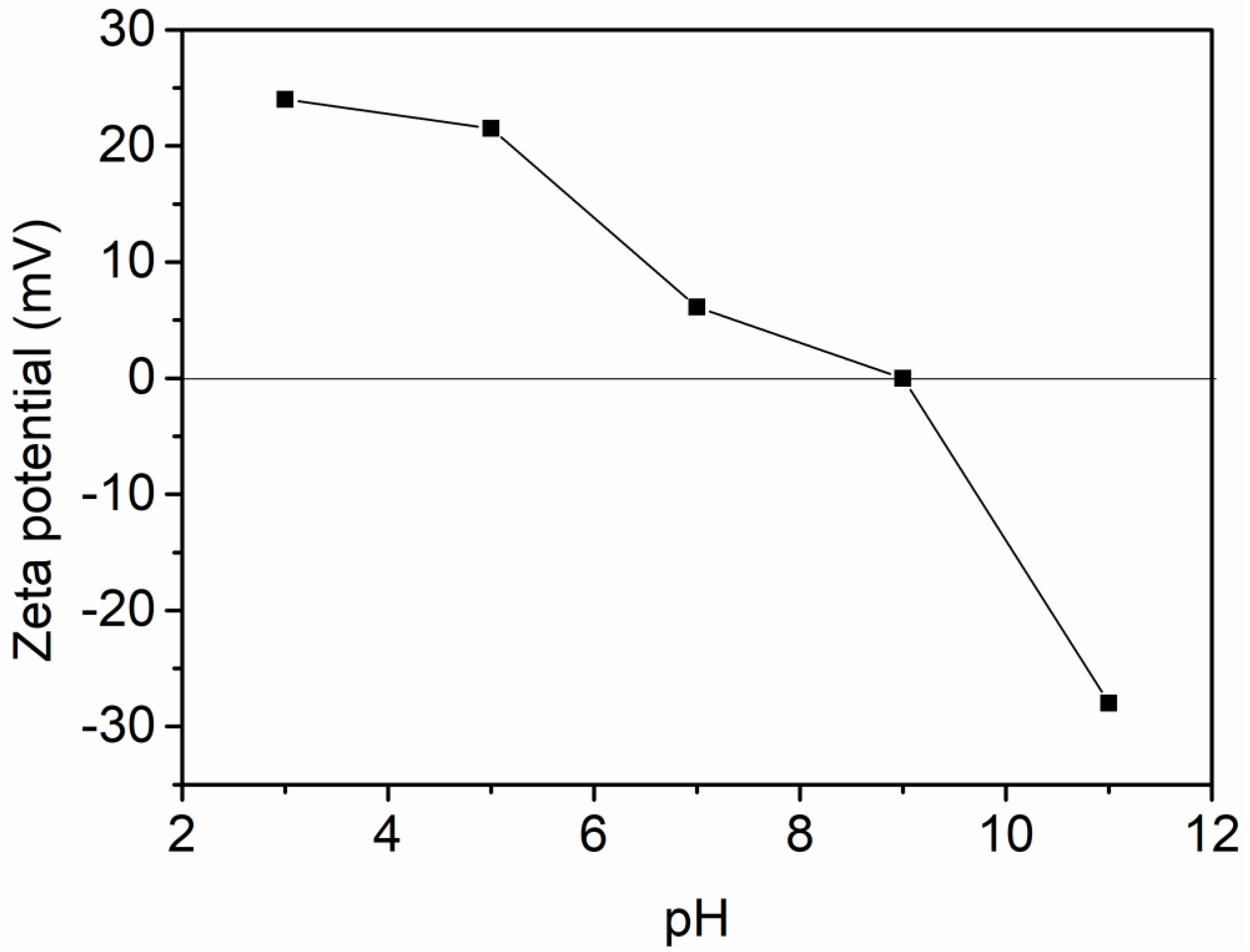
3.2. Characterization of Al-Ti Oxide
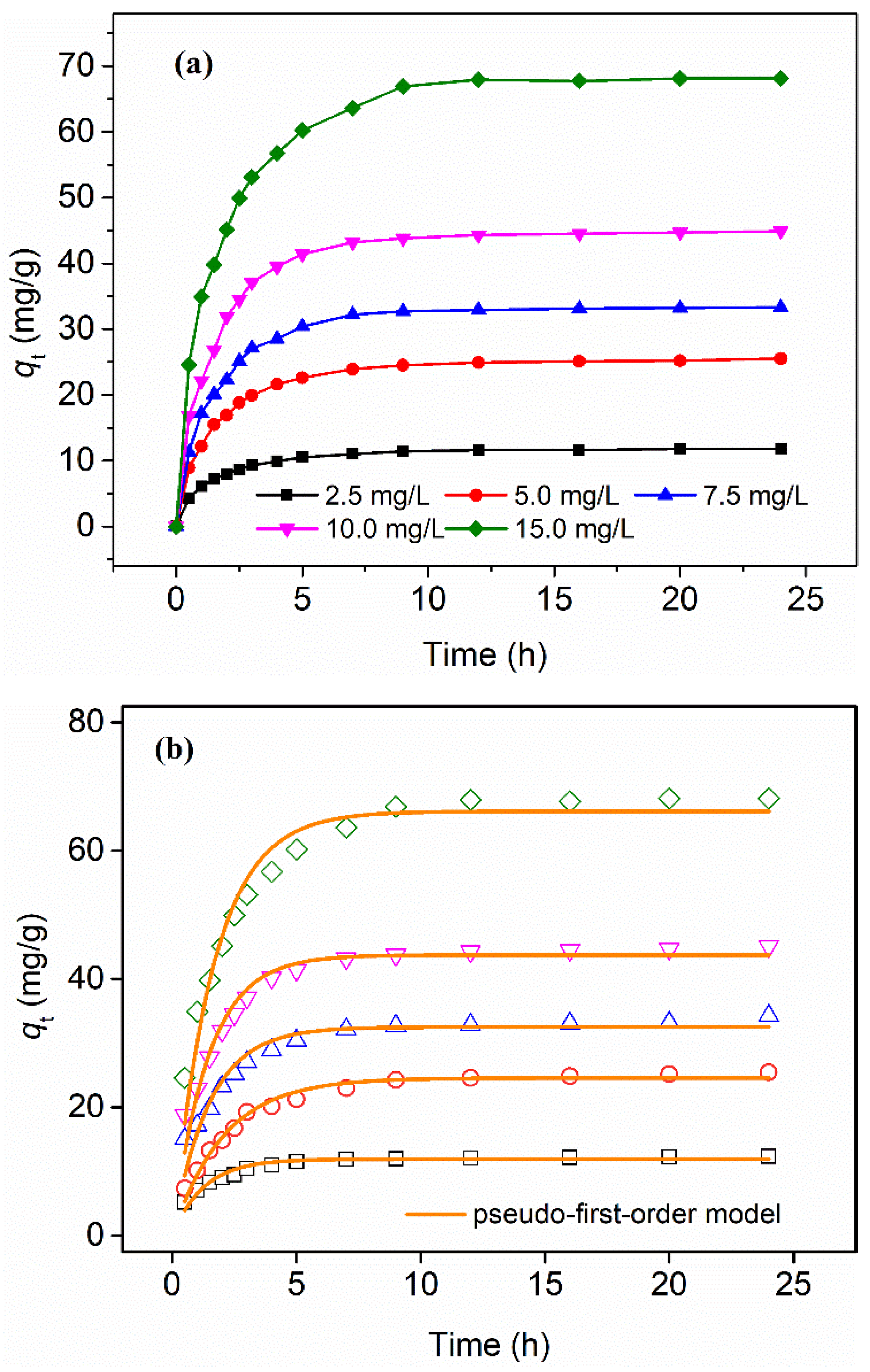
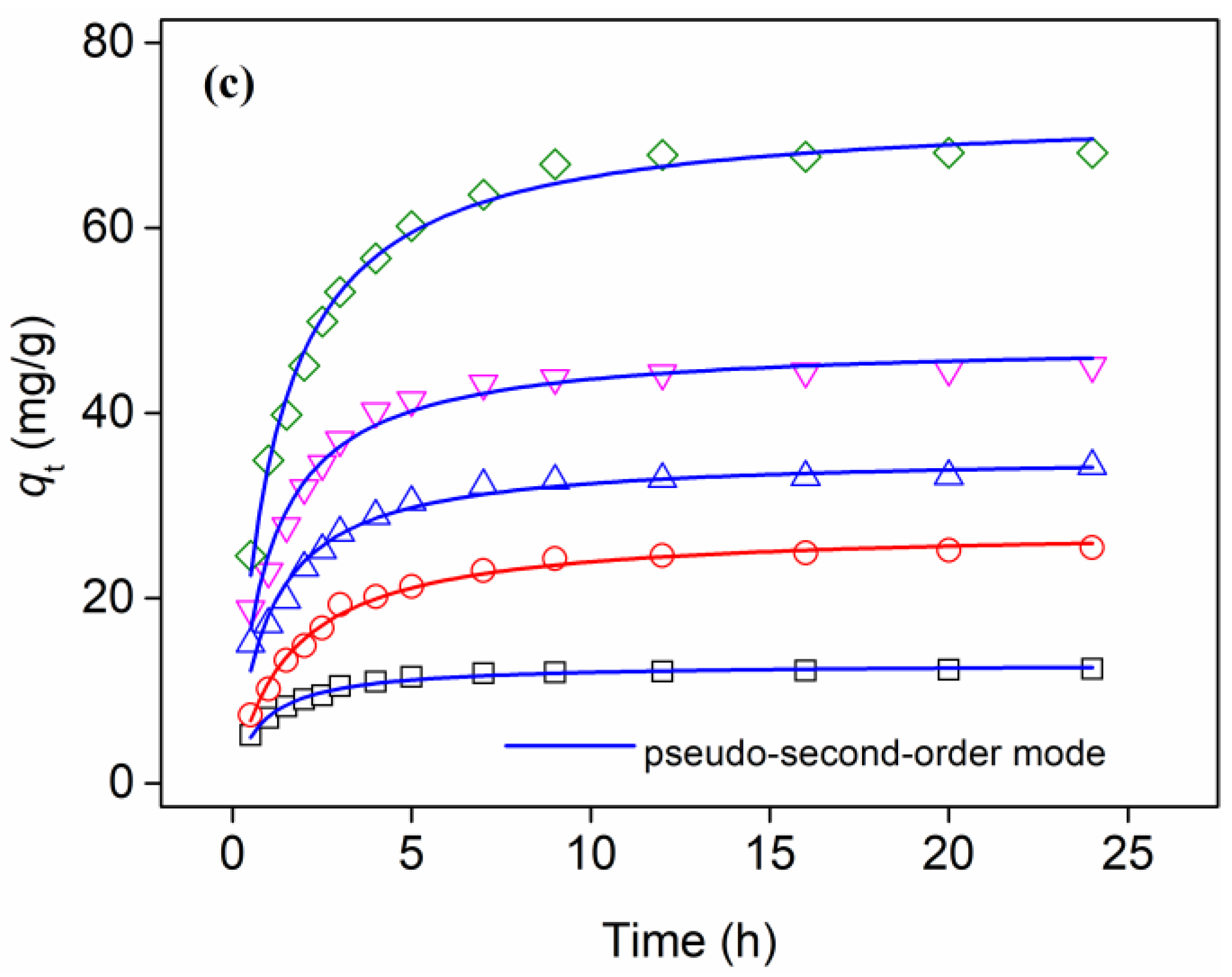
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.4.1. Langmuir Isotherm
3.4.2. Freundlich Isotherm
3.4.3. Dubinin–Radushkevick (D-R) Isotherm
3.4.4. Temkin Isotherm
3.4.5. Frumkin Isotherm
3.4.6. Harkin–Jura Isotherm
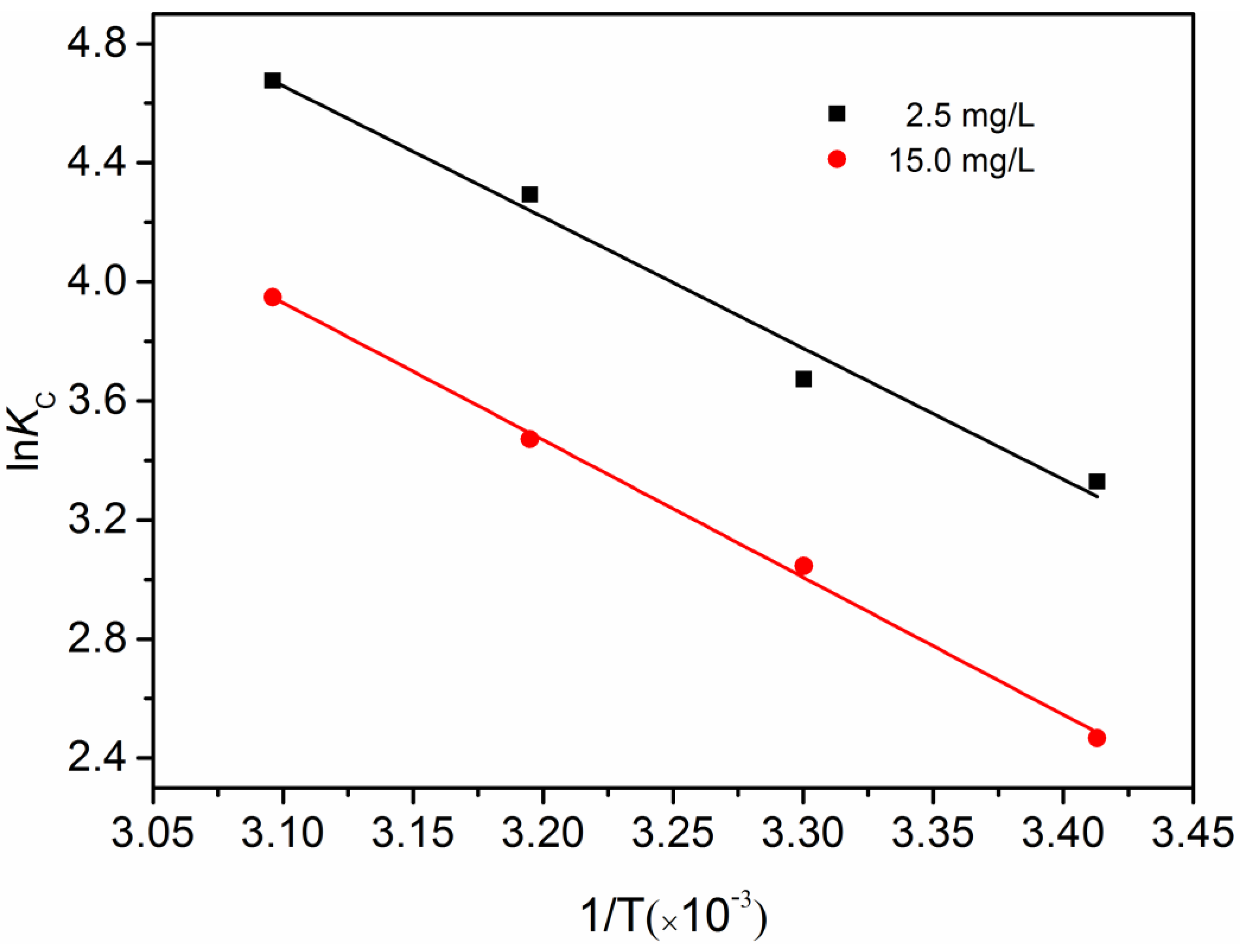
3.5. Thermodynamic Studies
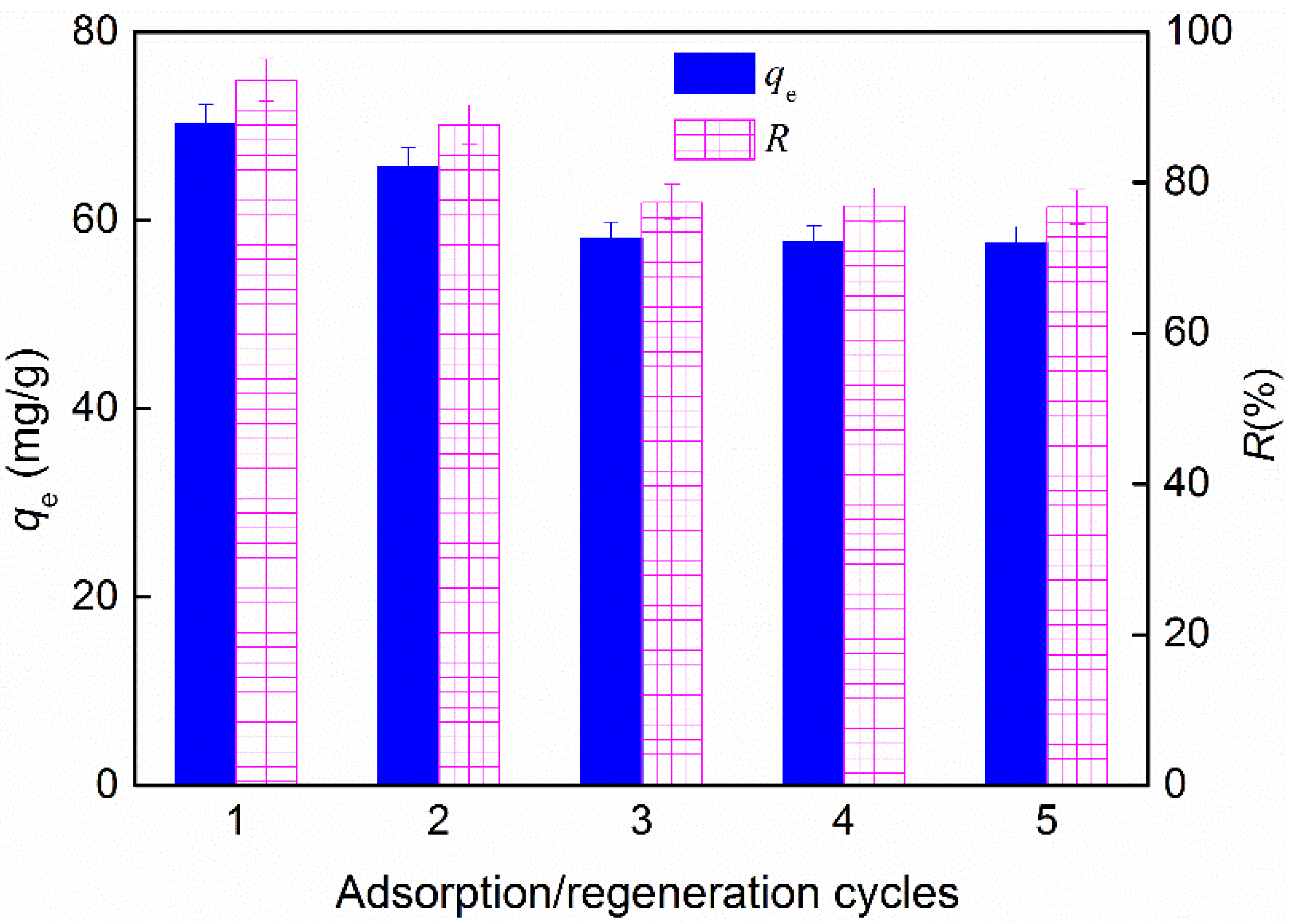
3.6. Regeneration of Spent Sorbent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Lee, D.J.; Nguyen, P.D.; Bui, X.T. Modification of agricultural waste/by-products for enhanced phosphate removal and recovery: Potential and obstacles. Bioresour. Technol. 2014, 169, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, S.; Wang, Y.; Ji, J. Phosphate adsorption from aqueous solution by lanthanum-iron hydroxide loaded with expanded graphite. Environ. Technol. 2018, 39, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, N.; Yu, Y.; Hu, W.; Feng, C. Investigation on the adsorption of phosphorus by Fe-loaded ceramic adsorbent. J. Colloid Interface Sci. 2016, 464, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L.; Bryhn, A.C.; Hytteborn, J.K. On the issue of limiting nutrient and predictions of cyanobacteria in aquatic systems. Sci. Total Environ. 2007, 379, 89–108. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Begum, S.A.; Hyder, A.; Hicklen, Q.; Crocker, T.; Oni, B. Adsorption characteristics of ammonium onto biochar from an aqueous solution. J. Water Supply Res. Technol. AQUA 2020, 70, 113–122. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Chase, J.M.; Dosch, K.L.; Hartson, R.B.; Gross, J.A.; Larson, D.J.; Sutherland, D.R.; Carpenter, S.R. Aquatic eutrophication promotes pathogenic infection in amphibians. Proc. Natl. Acad. Sci. USA 2007, 104, 15781–15786. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, X.; Sun, D.; Ma, W.; Wang, X. Adsorptive removal of phosphate from secondary effluents in WWTPs by ZnAl layered double hydroxides granules. Desalin. Water Treat. 2015, 54, 1216–1225. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Zhang, P.; Zhang, D.; Gao, F. Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chem. Eng. J. 2017, 307, 696–706. [Google Scholar] [CrossRef]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A comprehensive review of phosphorus recovery from wastewater by crystallization processes. Chemosphere 2018, 197, 768–781. [Google Scholar] [CrossRef]
- Lee, W.H.; Bishop, P.L. In situ microscale analyses of activated sludge flocs in the enhanced biological phosphate removal process by the use of microelectrodes and fluorescent in situ hybridization. J. Environ. Eng. 2010, 136, 561–567. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review. Environ. Sci. Pollut. Res. 2017, 24, 26297–26309. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, Y.; Dai, C. Removal of phosphate from aqueous solution using nanoscale zerovalent iron (nZVI). Colloids Surf. A 2014, 457, 433–440. [Google Scholar] [CrossRef]
- Karaca, S.; Gürses, A.; Ejder, M.; Açıkyıldız, M. Kinetic modeling of liquid-phase adsorption of phosphate on dolomite. J. Colloid Interface Sci. 2004, 277, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Moharami, S.; Jalali, M. Use of modified clays for removal of phosphorus from aqueous solutions. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, K.; Ghimire, K.N.; Ohta, S.; Harada, H.; Ohto, K.; Kawakita, H. The adsorption of phosphate from an aquatic environment using metal-loaded orange waste. J. Colloid Interface Sci. 2007, 312, 214–223. [Google Scholar] [CrossRef]
- Tran, D.N.H.; Kabiri, S.; Wang, L.S.; Losic, D. Engineered graphene-nanoparticle aerogel composites for efficient removal of phosphate from water. J. Mater. Chem. A 2015, 3, 6844–6852. [Google Scholar] [CrossRef]
- Li, G.; Gao, S.; Zhang, G.; Zhang, X. Enhanced adsorption of phosphate from aqueous solution by nanostructured iron(III)–copper(II) binary oxides. Chem. Eng. J. 2014, 235, 124–131. [Google Scholar] [CrossRef]
- Meng, W.N.; Xie, J.; Wu, D.Y.; Zhang, Z.J.; Kong, H.N. Study on phosphate removal and recovery by activated alumina. Environ. Sci. 2013, 34, 231–236. (In Chinese) [Google Scholar]
- Vodyanitskii, Y.N. Iron hydroxides in soils: A review of publications. Eurasian Soil Sci. 2010, 43, 1244–1254. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, J.-K.; Keller, A.A. Removal of arsenic and phosphate from aqueous solution by metal (hydr-)oxide coated sand. ACS Sustainable Chem. Eng. 2014, 2, 1128–1138. [Google Scholar] [CrossRef] [Green Version]
- Kropacheva, T.N.; Antonova, A.S.; Kornev, V.I. The influence of aminopolycarboxylates on the sorption of copper (II) cations by (Hydro)oxides of iron, Aluminum, and manganese. Eurasian Soil Sci. 2016, 49, 765–772. [Google Scholar] [CrossRef]
- Antelo, J.; Avena, M.; Fiol, S.; Lopez, R.; Arce, F. Effects of pH and ionic strength on the adsorption of phosphate and arsenate at the goethite-water interface. J. Colloid Interface Sci. 2005, 285, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Tofik, A.S.; Taddesse, A.M.; Tesfahun, K.T.; Girma, G.G. Fe–Al binary oxide nanosorbent: Synthesis, characterization and phosphate sorption property. J. Environ. Chem. Eng. 2016, 4, 2458–2468. [Google Scholar] [CrossRef]
- Lu, J.; Liu, H.; Zhao, X.; Jefferson, W.; Cheng, F.; Qu, J. Phosphate removal from water using freshly formed Fe–Mn binary oxide: Adsorption behaviors and mechanisms. Colloids Surf. A 2014, 455, 11–18. [Google Scholar] [CrossRef]
- Wan, S.; Wang, S.; Li, Y.; Gao, B. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. [Google Scholar] [CrossRef]
- Kang, S.A.; Li, W.; Lee, H.E.; Phillips, B.L.; Lee, Y.J. Phosphate uptake by TiO2: Batch studies and NMR spectroscopic evidence for multisite adsorption. J. Colloid Interface Sci. 2011, 364, 455–461. [Google Scholar] [CrossRef]
- Park, H.S.; Kwak, S.H.; Mahardika, D.; Mameda, N.; Choo, K.H. Mixed metal oxide coated polymer beads for enhanced phosphorus removal from membrane bioreactor effluents. Chem. Eng. J. 2017, 319, 240–247. [Google Scholar] [CrossRef]
- Lu, J.; Liu, D.; Hao, J.; Zhang, G.; Lu, B. Phosphate removal from aqueous solutions by a nano-structured Fe–Ti bimetal oxide sorbent. Chem. Eng. Res. Des. 2015, 93, 652–661. [Google Scholar] [CrossRef]
- He, Z.; Honeycutt, C.W. A modified molybdenum blue method for orthophosphate determination suitable for investigating enzymatic hydrolysis of organic phosphates. Commun. Soil Sci. Plant Anal. 2005, 36, 1373–1383. [Google Scholar] [CrossRef]
- Wang, X.-H.; Liu, F.-F.; Lu, L.; Yang, S.; Zhao, Y.; Su, L.-B.; Wang, S.-G. Individual and competitive adsorption of Cr(VI) and phosphate onto synthetic Fe–Al hydroxides. Colloids Surf. A 2013, 423, 42–49. [Google Scholar] [CrossRef]
- Novillo, C.; Guaya, D.; Allen-Perkins Avendaño, A.; Armijos, C.; Cortina, J.L.; Cota, I. Evaluation of phosphate removal capacity of Mg/Al layered double hydroxides from aqueous solutions. Fuel 2014, 38, 72–79. [Google Scholar] [CrossRef]
- Mostafa, M.S.; Bakr, A.A.; Eshaq, G.; Kamel, M.M. Novel Co/Mo layered double hydroxide: Synthesis and uptake of Fe(II) from aqueous solutions (Part 1). Desalin. Water Treat. 2015, 56, 239–247. [Google Scholar] [CrossRef]
- Deng, L.; Zeng, H.; Shi, Z.; Zhang, W.; Luo, J. Sodium dodecyl sulfate intercalated and acrylamide anchored layered double hydroxides: A multifunctional adsorbent for highly efficient removal of Congo red. J. Colloid Interface Sci. 2018, 521, 172–182. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, H.; Wang, L.; Zhang, X.; Yuan, Y.; Li, R.; Wan, H.; Guan, G. Facile synthesis of pure phase γ-AlOOH and γ-Al2O3 nanofibers in a recoverable ionic liquid via a low temperature route. RSC Adv. 2015, 5, 104884–104890. [Google Scholar] [CrossRef]
- Bessaha, H.; Bouraada, M.; Demenorval, L.C. Removal of indigo carmine and green bezanyl-F2B from water using calcined and uncalcined Zn/Al + Fe layered double hydroxide. J. Water Reuse. Desal. 2017, 7, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-W.; Lee, S.-Y.; Park, K.-Y.; Lee, K.-B.; Kim, D.-J.; Lee, S.-H. Investigation of phosphorous removal from wastewater through ion exchange of mesostructure based on inorganic material. Desalination 2011, 266, 281–285. [Google Scholar] [CrossRef]
- Brigante, M.; Schulz, P.C. Adsorption of paraquat on mesoporous silica modified with titania: Effects of pH, ionic strength and temperature. J. Colloid Interface Sci. 2011, 363, 355–361. [Google Scholar] [CrossRef]
- Gad, H.M.H.; El-Sayed, A.A. Activated carbon from agricultural by-products for the removal of Rhodamine-B from aqueous solution. J. Hazard. Mater. 2009, 168, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. About the theory of so called adsorption of soluble substances. Handlingar 1898, 24, 1–39. [Google Scholar]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J. Colloid Interface Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The constitution and fundamental properties of solids and liquids Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Hyder, A.H.M.G.; Begum, S.A.; Egiebor, N.O. Adsorption isotherm and kinetic studies of hexavalent chromium removal from aqueous solution onto bone char. J. Environ. Chem. Eng. 2015, 3, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.A.; da Silva, M.L.C.P. An investigation of phosphate adsorption from aqueous solution onto hydrous niobium oxide prepared by co-precipitation method. Colloids Surf. A 2009, 334, 191–196. [Google Scholar] [CrossRef]
- Tran, N.; Drogui, P.; Blais, J.-F.; Mercier, G. Phosphorus removal from spiked municipal wastewater using either electrochemical coagulation or chemical coagulation as tertiary treatment. Sep. Purif. Technol. 2012, 95, 16–25. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Zeitschrift für Physikalische Chemie 1906, 57, 385–471. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of activated charcoal. Proc. Acad. Sci. Phys. Chem. Sect. 1947, 55, 327–329. [Google Scholar]
- Kajjumba, G.W.; Yıldırım, E.; Aydın, S.; Emik, S.; Ağun, T.; Osra, F.; Wasswa, J. A facile polymerisation of magnetic coal to enhanced phosphate removal from solution. J. Environ. Manag. 2019, 247, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Tempkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Basar, C.A. Applicability of the various adsorption models of three dyes adsorption onto activated carbon prepared waste apricot. J. Hazard. Mater. 2006, 135, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Staroń, P.; Chwastowski, J. Raphia-microorganism composite biosorbent for lead ion removal from aqueous solutions. Materials 2021, 14, 7482. [Google Scholar] [CrossRef]
- Frumkin, A.N. A Multicomponent Isotherm for Gas Adsorption. Z. Phys. Chem. 1925, 116, 466–485. [Google Scholar] [CrossRef]
- Harkins, W.D.; Jura, G. The decrease of free surface energy as a basis for the development of equations for adsorption isotherms; and the existence of two condensed phases in films on solids. J. Chem. Phys. 1944, 12, 112–113. [Google Scholar] [CrossRef]
- Su, Y.; Cui, H.; Li, Q.; Gao, S.; Shang, J.K. Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res. 2013, 47, 5018–5026. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yang, Q.; Wang, D.; Xu, Q.; Yao, F.; Chen, F.; Tao, Z.; Huang, X. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents. J. Environ. Manag. 2019, 231, 370–379. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Mashhadi, S.; Ghasemi, M. Kinetics and thermodynamics of malachite green dye removal from aqueous phase using iron nanoparticles loaded on ash. J. Mol. Liq. 2016, 223, 1340–1347. [Google Scholar] [CrossRef]
- Fernandes, A.N.; Almeida, C.A.P.; Debacher, N.A.; Sierra, M.M.D.S. Isotherm and thermodynamic data of adsorption of methylene blue from aqueous solution onto peat. J. Mol. Struct. 2010, 982, 62–65. [Google Scholar] [CrossRef]
- Seki, Y.; Yurdakoç, K. Adsorption of promethazine hydrochloride with KSF montmorillonite. Adsorption 2006, 12, 89–100. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F. Tragacanth gum based hydrogel nanocomposites for the adsorption of methylene blue: Comparison of linear and non-linear forms of different adsorption isotherm and kinetics models. Int. J. Biol. Macromol. 2019, 133, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.C.; Paulino, F.A.; Cardoso, V.A.; Pereira, A.G.; Fajardo, A.R.; Rodrigues, F.H. Cellulose nanowhiskers improve the methylene blue adsorption capacity of chitosan-g-poly(acrylic acid) hydrogel. Carbohydr. Polym. 2018, 181, 358–367. [Google Scholar] [CrossRef] [PubMed]
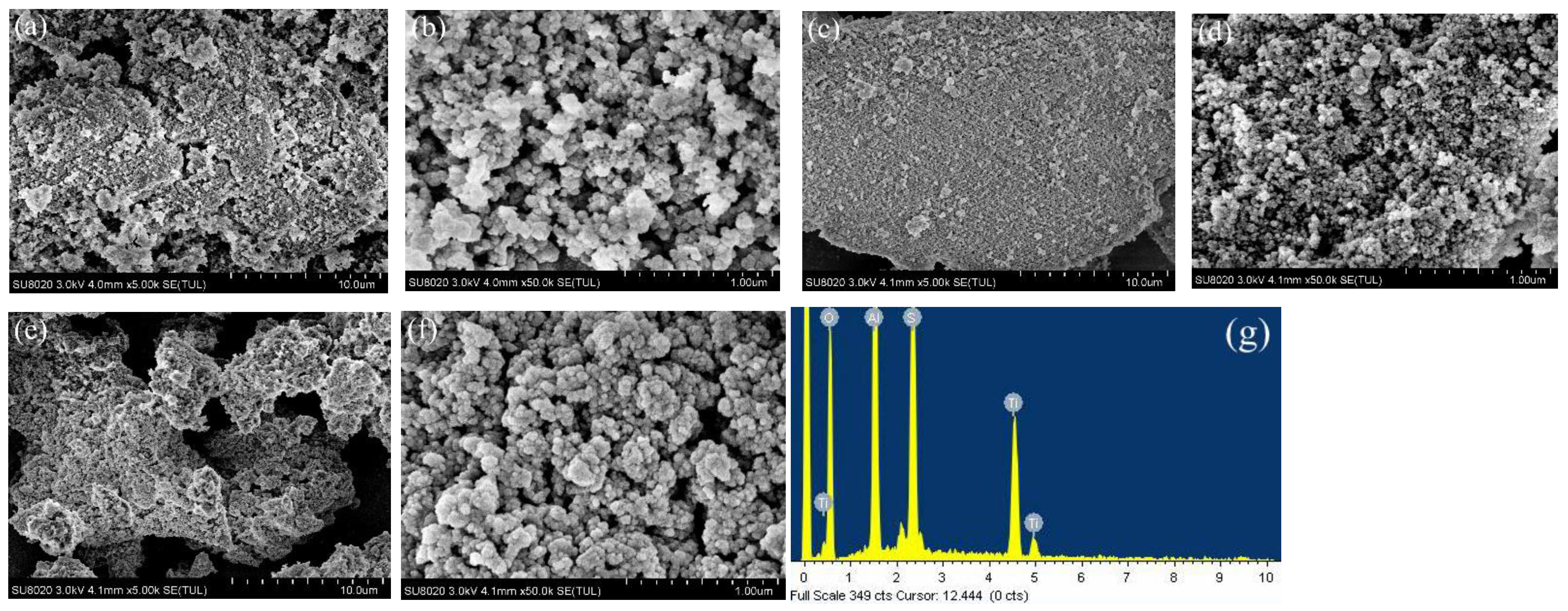
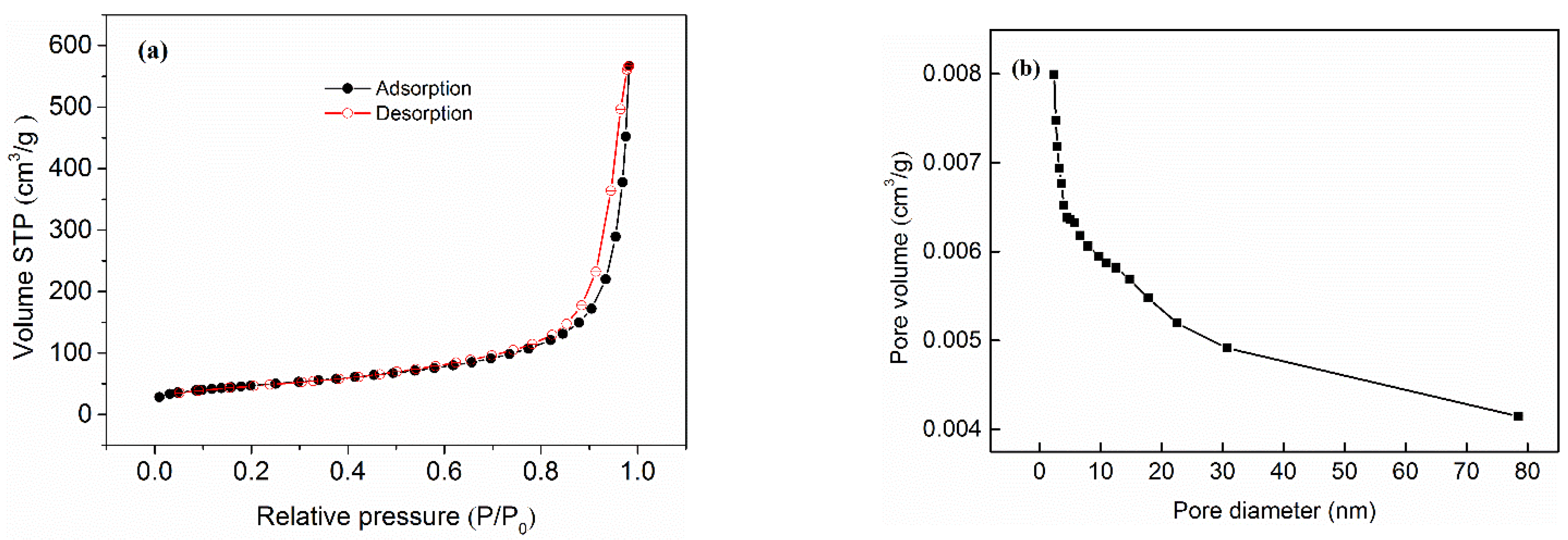

| BET Surface Area (m2/g) | Micropore Area (m2/g) | Average Pore Diameter (nm) | |
|---|---|---|---|
| Al-Ti oxide | 3.94 | 1.79 | 12.1 |
| Al oxide | 5.52 | 3.43 | 20.2 |
| Ti oxide | 2.36 | 1.26 | 10.6 |
| Initial P Concentration, mg/L | Pseudo-First-Order Model | Pesudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| k1 (1/h) | qe (mg/g) | R2 | k2 g/(mg·h) | qe (mg/g) | R2 | |
| 2.5 | 0.801 | 11.9 | 0.941 | 0.0971 | 13.0 | 0.990 |
| 5.0 | 0.488 | 24.6 | 0.977 | 0.0236 | 27.6 | 0.991 |
| 7.5 | 0.679 | 32.5 | 0.907 | 0.0294 | 35.5 | 0.968 |
| 10.0 | 0.701 | 43.7 | 0.949 | 0.0225 | 47.7 | 0.980 |
| 15.0 | 0.612 | 66.1 | 0.948 | 0.0123 | 72.8 | 0.991 |
| Parameter | 293 K | 303 K | 313 K | 323 K |
|---|---|---|---|---|
| Langmuir | ||||
| Qm (mg/g) | 68.2 | 63.0 | 60.5 | 70.7 |
| b (L/mg) | 0.691 | 0.759 | 0.701 | 0.958 |
| RL | 0.028 | 0.026 | 0.028 | 0.020 |
| r2 | 0.998 | 0.995 | 0.996 | 0.998 |
| Freundlich | ||||
| KF (L/g) | 33.4 | 1.23 | 1.24 | 1.36 |
| n | 4.31 | 4.80 | 4.67 | 3.25 |
| r2 | 0.768 | 0.810 | 0.942 | 0.795 |
| D-R | ||||
| QDR (mg/g) | 51.3 | 56.2 | 68.2 | 61.9 |
| (mol2/kJ2) | 12.7 | 19.2 | 25.7 | 26.3 |
| E (kJ/mol) | 6.28 | 5.10 | 4.41 | 4.36 |
| r2 | 0.955 | 0.977 | 0.975 | 0.952 |
| Temkin | ||||
| KT (L/mg) | 4.75 | 5.22 | 5.21 | 1.88 |
| B (kJ/mol) | 7.93 | 6.80 | 6.63 | 10.83 |
| r2 | 0.919 | 0.931 | 0.993 | 0.944 |
| Frumkin | ||||
| a | −4.72 | −4.49 | −5.10 | −4.93 |
| ln k | 2.00 | 2.52 | 2.78 | 0.169 |
| ΔG (kJ/mol) | 4.87 | 6.34 | 7.24 | 0.455 |
| r2 | 0.834 | 0.869 | 0.988 | 0.836 |
| Harkin–Jura | ||||
| 1.97 | 2.38 | 2.41 | 1.59 | |
| B | 1.50 | 1.87 | 1.98 | 1.47 |
| r2 | 0.603 | 0.457 | 0.263 | 0.384 |
| Adsorbent | pH | Temp (K) | Dose (g/L) | qm (mg/g) | References |
|---|---|---|---|---|---|
| Fe-Ti bimetal oxide | 4.5 | 293 | 0.2 | 32.95 | [31] |
| Fe-Al hydroxide | 6.0 | 298 | 1.0 | 51.80 | [33] |
| Mg/Al LDHS | 6.0 | 298 | 0.6 | 54.90 | [34] |
| Amorphous ZrO2 | 6.2 | 298 | 0.1 | 99.00 | [57] |
| Modified La2O3 | 5.6 | 298 | 0.5 | 58.70 | [58] |
| Ai-Ti bimetal oxide | 6.8 | 293 | 0.2 | 68.20 | This study |
| Concentration (mg/L) | −ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/mol K) | ||
|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | |||
| 2.5 | 8.11 | 9.25 | 11.17 | 36.62 | 152.25 |
| 15.0 | 6.01 | 7.67 | 9.03 | 38.36 | 151.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Miao, J.; Lv, Z.; Wan, X.; Zhang, N.; Zhang, R.; Peng, S. Phosphate Adsorption onto an Al-Ti Bimetal Oxide Composite in Neutral Aqueous Solution: Performance and Thermodynamics. Appl. Sci. 2022, 12, 2309. https://doi.org/10.3390/app12052309
Wei X, Miao J, Lv Z, Wan X, Zhang N, Zhang R, Peng S. Phosphate Adsorption onto an Al-Ti Bimetal Oxide Composite in Neutral Aqueous Solution: Performance and Thermodynamics. Applied Sciences. 2022; 12(5):2309. https://doi.org/10.3390/app12052309
Chicago/Turabian StyleWei, Xuefeng, Juan Miao, Zhen Lv, Xiaoyang Wan, Ning Zhang, Ruichang Zhang, and Shuge Peng. 2022. "Phosphate Adsorption onto an Al-Ti Bimetal Oxide Composite in Neutral Aqueous Solution: Performance and Thermodynamics" Applied Sciences 12, no. 5: 2309. https://doi.org/10.3390/app12052309
APA StyleWei, X., Miao, J., Lv, Z., Wan, X., Zhang, N., Zhang, R., & Peng, S. (2022). Phosphate Adsorption onto an Al-Ti Bimetal Oxide Composite in Neutral Aqueous Solution: Performance and Thermodynamics. Applied Sciences, 12(5), 2309. https://doi.org/10.3390/app12052309