Chronic Effects of Fluoxetine on Danio rerio: A Biochemical and Behavioral Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Chemicals and Test Solutions Preparation
2.2. Test Organisms
2.3. Animal Exposure
2.4. Behavior Tests
2.4.1. Locomotor and Thigmotactic Behavior
2.4.2. Novel Tank/Exploratory Swimming Test
2.4.3. Social Test
2.5. Biochemical Analysis
2.6. Statistical Analysis
3. Results
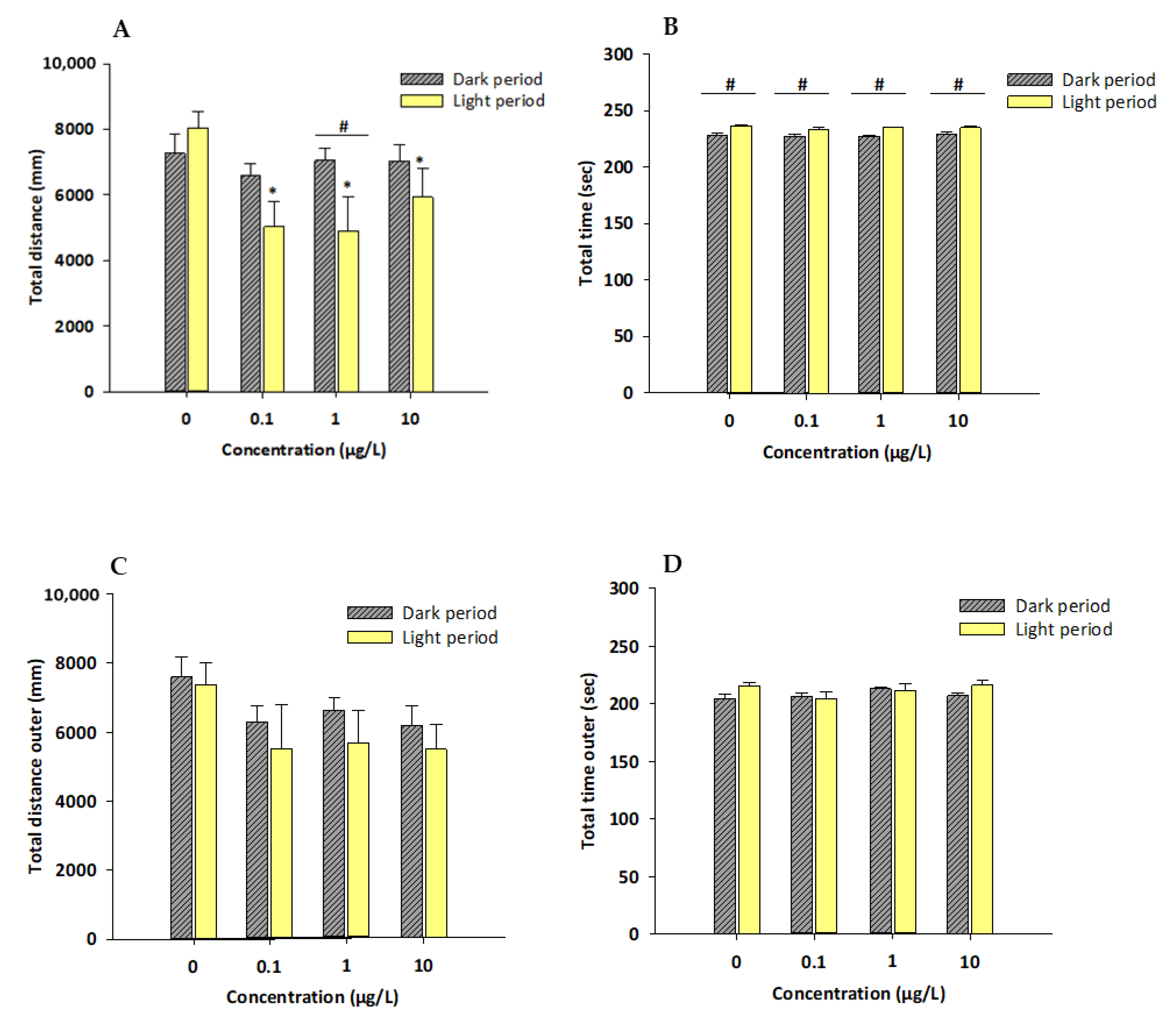
3.1. Locomotor Activity and Thigmotactic Behavior
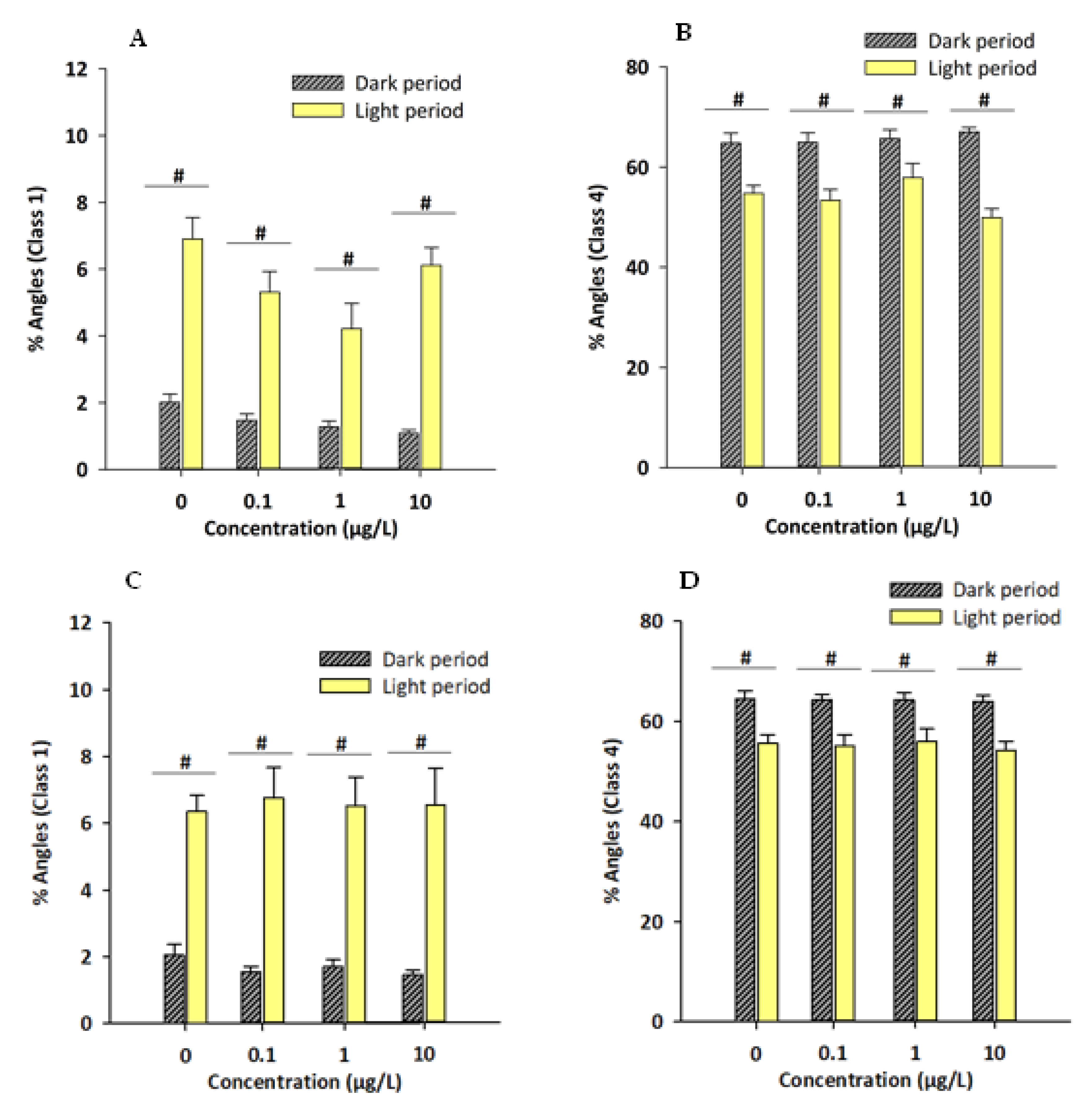
3.2. Novel Tank/Exploratory Swimming Test
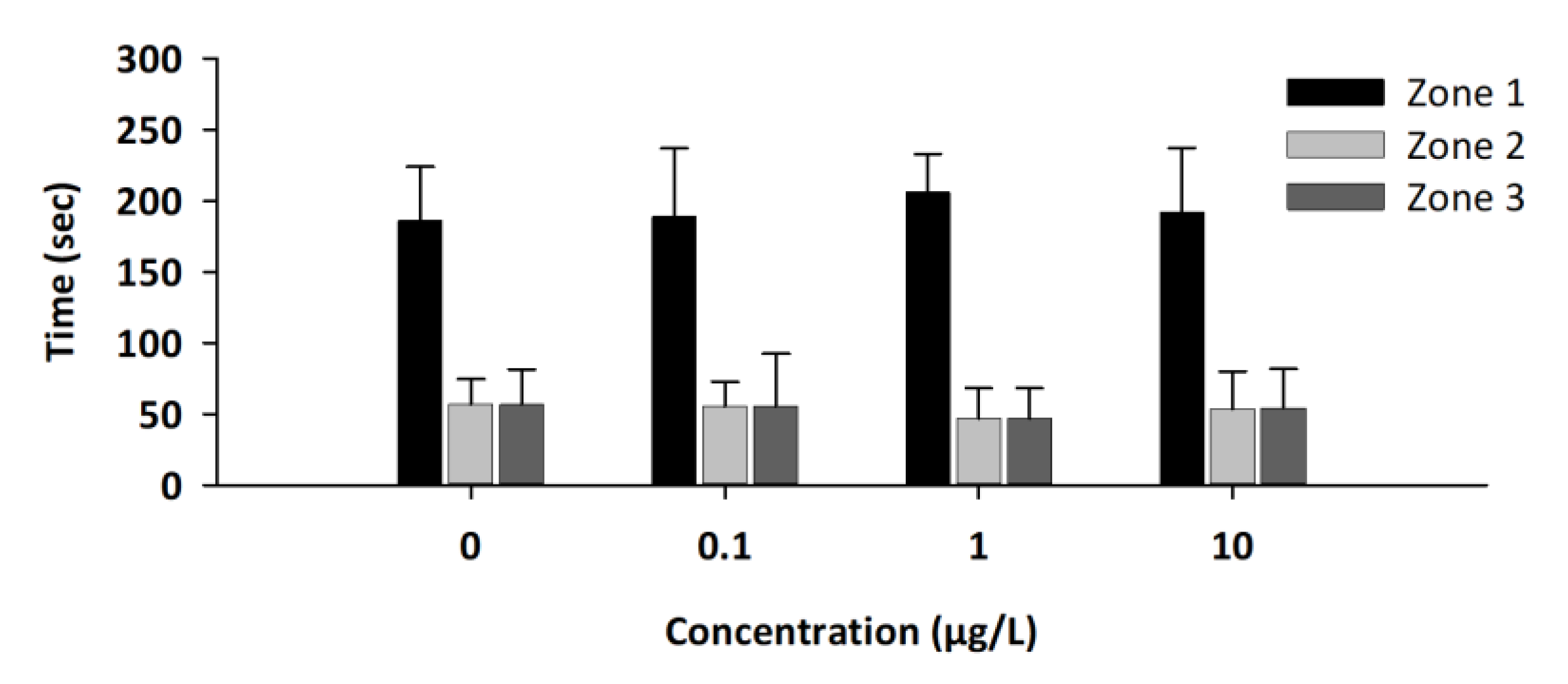
3.3. Social Test
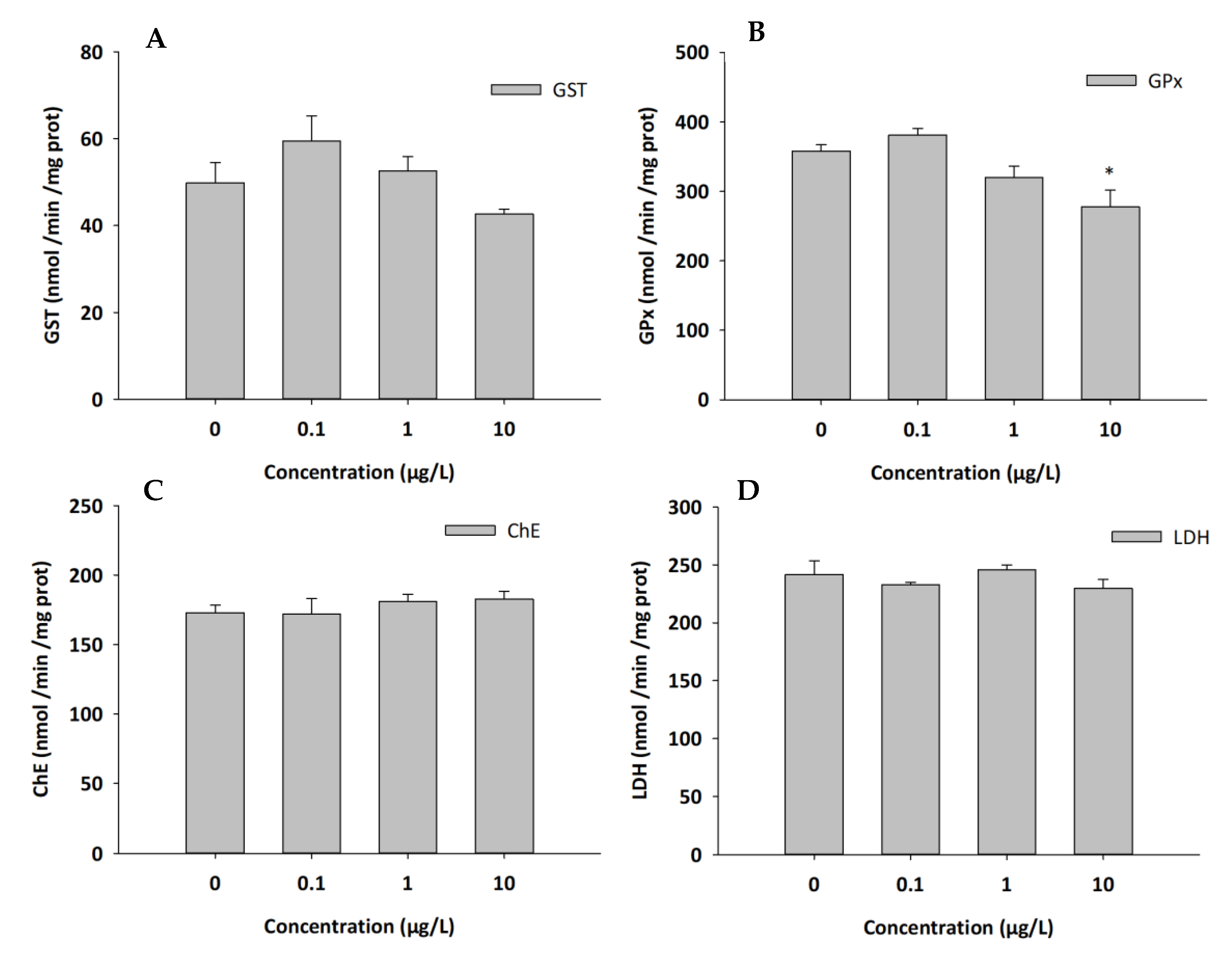
3.4. Biochemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammadkhanizadeh, A.; Nikbakht, F. Investigating the potential mechanisms of depression induced-by COVID-19 infection in patients. J. Clin. Neurosci. 2021, 91, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Al Shuraiqi, A.; Al-Habsi, A.; Barry, M.J. Time-, dose- and transgenerational effects of fluoxetine on the behavioural responses of zebrafish to a conspecific alarm substance. Environ. Pollut. 2021, 270, 116164. [Google Scholar] [CrossRef] [PubMed]
- De Castro-Català, N.; Muñoz, I.; Riera, J.L.; Ford, A.T. Evidence of low dose effects of the antidepressant fluoxetine and the fungicide prochloraz on the behavior of the keystone freshwater invertebrate Gammarus pulex. Environ. Pollut. 2017, 231, 406–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Farias, N.O.; Oliveira, R.; Sousa-Moura, D.; de Oliveira, R.C.S.; Rodrigues, M.A.C.; Andrade, T.S.; Domingues, I.; Camargo, N.S.; Muehlmann, L.A.; Grisolia, C.K.; et al. Exposure to low concentration of fluoxetine affects development, behaviour and acetylcholinesterase activity of zebrafish embryos. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 215, 1–8. [Google Scholar] [CrossRef]
- deFarias, N.O.; Oliveira, R.; Moretti, P.N.S.; e Pinto, J.M.; Oliveira, A.C.; Santos, V.L.; Rocha, P.S.; Andrade, T.S.; Grisolia, C.K.; de Farias, N.O.; et al. Fluoxetine chronic exposure affects growth, behavior and tissue structure of zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108836. [Google Scholar] [CrossRef]
- Parolini, M.; Ghilardi, A.; De Felice, B.; Del Giacco, L. Environmental concentration of fluoxetine disturbs larvae behavior and increases the defense response at molecular level in zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2019, 26, 34943–34952. [Google Scholar] [CrossRef]
- Theodoridi, A.; Tsalafouta, A.; Pavlidis, M. Acute Exposure to Fluoxetine Alters Aggressive Behavior of Zebrafish and Expression of Genes Involved in Serotonergic System Regulation. Front. Neurosci. 2017, 11, 223. [Google Scholar] [CrossRef]
- Abreu, M.S.; Giacomini, A.C.V.; Gusso, D.; Rosa, J.G.S.; Koakoski, G.; Kalichak, F.; Idalêncio, R.; Oliveira, T.A.; Barcellos, H.H.A.; Bonan, C.D.; et al. Acute exposure to waterborne psychoactive drugs attract zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 179, 37–43. [Google Scholar] [CrossRef]
- Ansai, S.; Hosokawa, H.; Maegawa, S.; Kinoshita, M. Chronic fluoxetine treatment induces anxiolytic responses and altered social behaviors in medaka, Oryzias latipes. Behav. Brain Res. 2016, 303, 126–136. [Google Scholar] [CrossRef]
- Cunha, V.; Rodrigues, P.; Santos, M.M.; Moradas-Ferreira, P.; Ferreira, M. Fluoxetine modulates the transcription of genes involved in serotonin, dopamine and adrenergic signalling in zebrafish embryos. Chemosphere 2018, 191, 954–961. [Google Scholar] [CrossRef]
- Dorelle, L.S.; Da Cuña, R.H.; Sganga, D.E.; Rey Vázquez, G.; López Greco, L.; Lo Nostro, F.L. Fluoxetine exposure disrupts food intake and energy storage in the cichlid fish Cichlasoma dimerus (Teleostei, Cichliformes). Chemosphere 2020, 238, 1242609. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.T.; Fong, P.P. The effects of antidepressants appear to be rapid and at environmentally relevant concentrations. Environ. Toxicol. Chem. 2016, 35, 794–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, M.D. An AOP analysis of selective serotonin reuptake inhibitors (SSRIs) for fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 197, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.Y.; Oxendine, S.E.; Godwin, J. Behavioral and neurogenomic transcriptome changes in wild-derived zebrafish with fluoxetine treatment. BMC Genomics 2013, 14, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vera-Chang, M.N.; St-Jacques, A.D.; Lu, C.; Moon, T.W.; Trudeau, V.L. Fluoxetine Exposure During Sexual Development Disrupts the Stress Axis and Results in Sex- and Time- Dependent Effects on the Exploratory Behavior in Adult Zebrafish Danio rerio. Front. Neurosci. 2019, 13, 1015. [Google Scholar] [CrossRef] [PubMed]
- Vera-Chang, M.N.; St-Jacques, A.D.; Gagné, R.; Martyniuk, C.J.; Yauk, C.L.; Moon, T.W.; Trudeau, V.L. Transgenerational hypocortisolism and behavioral disruption are induced by the antidepressant fluoxetine in male zebrafish Danio rerio. Proc. Natl. Acad. Sci. USA 2018, 115, E12435–E12442. [Google Scholar] [CrossRef] [Green Version]
- Barry, M.J. Effects of fluoxetine on the swimming and behavioural responses of the Arabian killifish. Ecotoxicology 2013, 22, 425–432. [Google Scholar] [CrossRef]
- Tierney, A.J.; Hanzlik, K.N.; Hathaway, R.M.; Powers, C.; Roy, M. Effects of fluoxetine on growth and behavior in the crayfish Orconectes rusticus. Mar. Freshw. Behav. Physiol. 2015, 49, 133–145. [Google Scholar] [CrossRef]
- Mennigen, J.A.; Stroud, P.; Zamora, J.M.; Moon, T.W.; Trudeau, V.L. Pharmaceuticals as Neuroendocrine Disruptors: Lessons Learned from Fish on Prozac. J. Toxicol. Environ. Health Part B Crit. Rev. 2011, 14, 387–412. [Google Scholar] [CrossRef]
- Batt, A.L.; Kostich, M.S.; Lazorchak, J.M. Analysis of ecologically relevant pharmaceuticals in wastewater and surface water using selective solid-phase extraction and UPLC-MS/MS. Anal. Chem. 2008, 80, 5021–5030. [Google Scholar] [CrossRef]
- Vasskog, T.; Anderssen, T.; Pedersen-Bjergaard, S.; Kallenborn, R.; Jensen, E. Occurrence of selective serotonin reuptake inhibitors in sewage and receiving waters at Spitsbergen and in Norway. J. Chromatogr. A 2008, 1185, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Vasskog, T.; Berger, U.; Samuelsen, P.J.; Kallenborn, R.; Jensen, E. Selective serotonin reuptake inhibitors in sewage influents and effluents from Tromsø, Norway. J. Chromatogr. A 2006, 1115, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.A.; Gonçalves, C.; Cunha, E.; Hajšlová, J.; Alpendurada, M.F. Cleanup strategies and advantages in the determination of several therapeutic classes of pharmaceuticals in wastewater samples by SPE-LC-MS/MS. Anal. Bioanal. Chem. 2011, 399, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Chu, S.; Judt, C.; Li, H.; Oakes, K.D.; Servos, M.R.; Andrews, D.M. Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ. Toxicol. Chem. 2010, 29, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Miao, X.S.; Koenig, B.G.; Struger, J. Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes, Canada. Environ. Toxicol. Chem. 2003, 22, 2881–2889. [Google Scholar] [CrossRef]
- MacLeod, S.L.; Sudhir, P.; Wong, C.S. Stereoisomer analysis of wastewater-derived β-blockers, selective serotonin re-uptake inhibitors, and salbutamol by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1170, 23–33. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Gagnon, C.; Sauvé, S. Determination of basic antidepressants and their N-desmethyl metabolites in raw sewage and wastewater using solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal. Chem. 2008, 80, 5325–5333. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Tracing pharmaceutical residues of different therapeutic classes in environmental waters by using liquid chromatography/quadrupole-linear ion trap mass spectrometry and automated library searching. Anal. Chem. 2009, 81, 898–912. [Google Scholar] [CrossRef]
- Martínez Bueno, M.J.; Agüera, A.; Gómez, M.J.; Hernando, M.D.; García-Reyes, J.F.; Fernández-Alba, A.R.; Bueno, M.J.M.; Agüera, A.; Gómez, M.J.; Hernando, M.D.; et al. Application of liquid chromatography/quadrupole-linear ion trap mass spectrometry and time-of-flight mass spectrometry to the determination of pharmaceuticals and related contaminants in wastewater. Anal. Chem. 2007, 79, 9372–9384. [Google Scholar] [CrossRef]
- Salgado, R.; Marques, R.; Noronha, J.; Mexia, J.; Carvalho, G.; Oehmen, A. Assessing the diurnal variability of pharmaceutical and personal care products in a full-scale activated sludg plant. Environ. Pollut 2011, 159, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.G.; Catalá, M.; Maroto, R.R.; Gil, J.L.R.; de Miguel, Á.G.; Valcárcel, Y. Pollution by psychoactive pharmaceuticals in the Rivers of Madrid metropolitan area (Spain). Environ. Int. 2010, 36, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Schultz, M.M.; Furlong, E.T. Trace analysis of antidepressant pharmaceuticals and their select degradates in aquatic matrixes by LC/ESI/MS/MS. Anal. Chem. 2008, 80, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.; Kolpin, D.; Furlong, E.; Zaugg, S.; Meyer, M.; Barber, L. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States—I) Groundwater. Sci. Total Environ. 2008, 402, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Furlong, E.T.; Kolpin, D.W.; Werner, S.L.; Schoenfuss, H.L.; Barber, L.B.; Blazer, V.S.; Norris, D.O.; Vajda, A.M. Antidepressant pharmaceuticals in two U.S. effluent-impacted streams: Occurrence and fate in water and sediment and selective uptake in fish neural tissue. Environ. Sci. Technol. 2010, 44, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Selective serotonin re-uptake inhibitors (SSRIs) in the aquatic environment: An ecopharmacovigilance approach. Sci. Total Environ. 2012, 437, 185–195. [Google Scholar] [CrossRef]
- Snyder, S.A. Occurrence, treatment, and toxicological relevance of EDCs and pharmaceuticals in water. Ozone Sci. Eng. 2008, 30, 65–69. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Yang, M.; Xu, H.; Xu, B.; Jiang, L.; Wu, M. Tissue bioconcentration and effects of fluoxetine in zebrafish (Danio rerio) and red crucian cap (Carassius auratus) after short-term and long-term exposure. Chemosphere 2018, 205, 8–14. [Google Scholar] [CrossRef]
- Dorelle, L.S.; Da Cuña, R.H.; Rey Vázquez, G.; Höcht, C.; Shimizu, A.; Genovese, G.; Lo Nostro, F.L. The SSRI fluoxetine exhibits mild effects on the reproductive axis in the cichlid fish Cichlasoma dimerus (Teleostei, Cichliformes). Chemosphere 2017, 171, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, X.; Bao, X.; Ling, X.; Yang, H.; Liu, J.; Lu, G.; Ji, Y. Influence of dissolved organic matter on the accumulation, metabolite production and multi-biological effects of environmentally relevant fluoxetine in crucian carp (Carassius auratus). Aquat. Toxicol. 2020, 226, 105581. [Google Scholar] [CrossRef] [PubMed]
- Cunha, V.; Rodrigues, P.; Santos, M.M.; Moradas-Ferreira, P.; Ferreira, M. Danio rerio embryos on Prozac—Effects on the detoxification mechanism and embryo development. Aquat. Toxicol. 2016, 178, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Lu, G.; Li, Y. Interactive effects of selected pharmaceutical mixtures on bioaccumulation and biochemical status in crucian carp (Carassius auratus). Chemosphere 2016, 148, 21–31. [Google Scholar] [CrossRef]
- Duarte, I.A.; Pais, M.P.; Reis-Santos, P.; Cabral, H.N.; Fonseca, V.F. Biomarker and behavioural responses of an estuarine fish following acute exposure to fluoxetine. Mar. Environ. Res. 2019, 147, 24–31. [Google Scholar] [CrossRef]
- Duarte, I.A.; Reis-Santos, P.; Novais, S.C.; Rato, L.D.; Lemos, M.F.L.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Cabral, H.N.; Fonseca, V.F. Depressed, hypertense and sore: Long-term effects of fluoxetine, propranolol and diclofenac exposure in a top predator fish. Sci. Total Environ. 2020, 712, 136564. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, X.; Mu, L.; Hou, L.; Yang, B.; Zhao, J.; Schlenk, D.; Dong, W.; Xie, L.; Zhang, Q. Effects of acute and chronic exposures of fluoxetine on the Chinese fish, topmouth gudgeon Pseudorasbora parva. Ecotoxicol. Environ. Saf. 2018, 160, 104–113. [Google Scholar] [CrossRef]
- Mishra, P.; Gong, Z.; Kelly, B.C. Assessing biological effects of fluoxetine in developing zebrafish embryos using gas chromatography-mass spectrometry based metabolomics. Chemosphere 2017, 188, 157–167. [Google Scholar] [CrossRef]
- Abreu, M.S.; Giacomini, A.C.V.V.; Kalueff, A.V.; Barcellos, L.J.G. The smell of “anxiety”: Behavioral modulation by experimental anosmia in zebrafish. Physiol. Behav. 2016, 157, 67–71. [Google Scholar] [CrossRef]
- Dzieweczynski, T.L.; Campbell, B.A.; Kane, J.L. Dose-dependent fluoxetine effects on boldness in male Siamese fighting fish. J. Exp. Biol. 2016, 219, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Giacomini, A.C.V.V.; Abreu, M.S.; Giacomini, L.V.; Siebel, A.M.; Zimerman, F.F.; Rambo, C.L.; Mocelin, R.; Bonan, C.D.; Piato, A.L.; Barcellos, L.J.G. Fluoxetine and diazepam acutely modulate stress induced-behavior. Behav. Brain Res. 2016, 296, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Greaney, N.E.; Mannion, K.L.; Dzieweczynski, T.L. Signaling on Prozac: Altered audience effects on male-male interactions after fluoxetine exposure in Siamese fighting fish. Behav. Ecol. Sociobiol. 2015, 69, 1925–1932. [Google Scholar] [CrossRef]
- Martin, J.M.; Bertram, M.G.; Saaristo, M.; Ecker, T.E.; Hannington, S.L.; Tanner, J.L.; Michelangeli, M.; O’Bryan, M.K.; Wong, B.B.M. Impact of the widespread pharmaceutical pollutant fluoxetine on behaviour and sperm traits in a freshwater fish. Sci. Total Environ. 2019, 650, 1771–1778. [Google Scholar] [CrossRef]
- McCallum, E.S.; Bose, A.P.H.; Warriner, T.R.; Balshine, S. An evaluation of behavioural endpoints: The pharmaceutical pollutant fluoxetine decreases aggression across multiple contexts in round goby (Neogobius melanostomus). Chemosphere 2017, 175, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Meijide, F.J.; Da Cuña, R.H.; Prieto, J.P.; Dorelle, L.S.; Babay, P.A.; Lo Nostro, F.L. Effects of waterborne exposure to the antidepressant fluoxetine on swimming, shoaling and anxiety behaviours of the mosquitofish Gambusia holbrooki. Ecotoxicol. Environ. Saf. 2018, 163, 646–655. [Google Scholar] [CrossRef]
- Pelli, M.; Connaughton, V.P. Chronic exposure to environmentally-relevant concentrations of fluoxetine (Prozac) decreases survival, increases abnormal behaviors, and delays predator escape responses in guppies. Chemosphere 2015, 139, 202–209. [Google Scholar] [CrossRef]
- Pittman, J.; Hylton, A. Behavioral, endocrine, and neuronal alterations in zebrafish (Danio rerio) following sub-chronic coadministration of fluoxetine and ketamine. Pharmacol. Biochem. Behav. 2015, 139, 158–162. [Google Scholar] [CrossRef]
- Singer, M.L.; Oreschak, K.; Rhinehart, Z.; Robison, B.D. Anxiolytic effects of fluoxetine and nicotine exposure on exploratory behavior in zebrafish. PeerJ 2016, 4, e2352. [Google Scholar] [CrossRef] [Green Version]
- Weinberger, J.; Klaper, R. Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the fish Pimephales promelas (fathead minnow). Aquat. Toxicol. 2014, 151, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Zindler, F.; Stoll, S.; Baumann, L.; Knoll, S.; Huhn, C.; Braunbeck, T. Do environmentally relevant concentrations of fluoxetine and citalopram impair stress-related behavior in zebrafish (Danio rerio) embryos? Chemosphere 2020, 261, 127753. [Google Scholar] [CrossRef]
- Veldman, M.B.; Lin, S. Zebrafish as a Developmental Model Organism for Pediatric Research. Pediatr. Res. 2008, 64, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Saleem, S.; Kannan, R.R. Zebrafish: An emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery. Cell Death Discov. 2018, 4, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleman, C.; Holtzman, N.G. Growth and maturation in the zebrafish, Danio rerio: A staging tool for teaching and research. Zebrafish 2014, 11, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Zhao, G.; Zhou, Y.; Chen, R.; Li, J.; Zha, J. Risks to aquatic environments posed by 14 pharmaceuticals as illustrated by their effects on zebrafish behaviour. Sci. Total Environ. 2021, 771, 145450. [Google Scholar] [CrossRef]
- Blahova, J.; Doubkova, V.; Plhalova, L.; Lakdawala, P.; Medkova, D.; Vecerek, V.; Svobodova, Z.; Faggio, C. Embryotoxicity of selective serotonin reuptake inhibitors—Comparative sensitivity of zebrafish (Danio rerio) and african clawed frog (xenopus laevis) embryos. Appl. Sci. 2021, 11, 10015. [Google Scholar] [CrossRef]
- Correia, D.; Almeida, A.R.A.R.; Santos, J.; Machado, A.L.A.L.; Koba Ucun, O.; Žlábek, V.; Oliveira, M.; Domingues, I. Behavioral effects in adult zebrafish after developmental exposure to carbaryl. Chemosphere 2019, 235, 1022–1029. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Pan, R.; Xu, T.; Zhao, J.; Huang, W.; Liu, Y.; Yin, D. Effects of three different embryonic exposure modes of 2, 2’, 4, 4’-tetrabromodiphenyl ether on the path angle and social activity of zebrafish larvae. Chemosphere 2017, 169, 542–549. [Google Scholar] [CrossRef]
- Wong, K.; Elegante, M.; Bartels, B.; Elkhayat, S.; Tien, D.; Roy, S.; Goodspeed, J.; Suciu, C.; Tan, J.; Grimes, C.; et al. Analyzing Habituation Responses to Novelty in Zebrafish ( Danio Rerio ); Elsevier B.V.: Amsterdam, The Netherlands, 2010; Volume 208. [Google Scholar]
- Champagne, D.L.; Hoefnagels, C.C.M.; de Kloet, R.E.; Richardson, M.K. Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav. Brain Res. 2010, 214, 332–342. [Google Scholar] [CrossRef]
- Maximino, C.; de Brito, T.M.; da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A. Measuring anxiety in zebrafish: A critical review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef]
- Domingues, I.; Oliveira, R.; Soares, A.M.V.M.V.M.; Amorim, M.J.B.B. Effects of ivermectin on Danio rerio: A multiple endpoint approach: Behaviour, weight and subcellular markers. Ecotoxicology 2016, 25, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M.V.M. Inhibition of acetylcholinesterase activity as effect criterion in acute tests with juvenile Daphnia Magna. J. Econ. 1996, 32, 727–738. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Vassault, A. Lactate Dehydrogenase; Acad Press: New York, NY, USA, 1983. [Google Scholar]
- Diamantino, T.C.; Almeida, E.; Soares, A.M.V.M.; Guilhermino, L. Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 2001, 45, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Mohandas, J.; Marshall, J.J.; Duggin, G.G.; Horvath, J.S.; Tiller, D.J. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem. Pharmacol. 1984, 33, 1801–1807. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, H.; Li, K.; Zhao, X.; Liu, Q.; Li, D.; Zhao, G. Occurrence of pharmaceuticals and personal care products, and their associated environmental risks in a large shallow lake in north China. Environ. Geochem. Health 2018, 40, 1525–1539. [Google Scholar] [CrossRef]
- Li, Y.; Ding, J.; Zhang, L.; Liu, X.; Wang, G. Occurrence and ranking of pharmaceuticals in the major rivers of China. Sci. Total Environ. 2019, 696, 133991. [Google Scholar] [CrossRef]
- Burgess, H.A.; Granato, M. Modulation of locomotor activity in larval zebrafish during light adaptation. J. Exp. Biol. 2007, 210, 2526–2539. [Google Scholar] [CrossRef] [Green Version]
- Andrade, T.S.; Henriques, J.F.; Rita, A.; Luísa, A.; Koba, O.; Thai, P.; Soares, A.M.V.M.; Domingues, I. Carbendazim exposure induces developmental, biochemical and behavioural disturbance in zebrafish embryos. Aquat. Toxicol. 2016, 170, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Blaser, R.; Gerlai, R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behav. Res. Methods 2006, 38, 456–469. [Google Scholar] [CrossRef]
- Best, C.; Kurrasch, D.M.; Vijayan, M.M. Maternal cortisol stimulates neurogenesis and affects larval behaviour in zebrafish. Sci. Rep. 2017, 7, 40905. [Google Scholar] [CrossRef] [Green Version]
- Bencan, Z.; Sledge, D.; Levin, E.D. Buspirone, Chlordiazepoxide and Diazepam Effects in a Zebrafish Model of Anxiety. Pharmacol. Biochem. Behav. 2009, 94, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Marcon, M.; Herrmann, A.P.; Mocelin, R.; Rambo, C.L.; Koakoski, G.; Abreu, M.S.; Conterato, G.M.M.; Kist, L.W.; Bogo, M.R.; Zanatta, L.; et al. Prevention of unpredictable chronic stress-related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology 2016, 233, 3815–3824. [Google Scholar] [CrossRef]
- Pittman, J.T.; Ichikawa, K.M. IPhone® applications as versatile video tracking tools to analyze behavior in zebrafish (Danio rerio). Pharmacol. Biochem. Behav. 2013, 106, 137–142. [Google Scholar] [CrossRef]
- Snekser, J.L.; Mcrobert, S.P.; Murphy, C.E.; Clotfelter, E.D. Aggregation Behavior in Wildtype and Transgenic Zebrafish. Ethology 2006, 112, 181–187. [Google Scholar] [CrossRef]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The Behaviour and Ecology of the Zebrafish, Danio rerio. Biol. Rev. Camb. Philos. Soc. 2008, 83, 13–34. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18093234%5Cnhttp://onlinelibrary.wiley.com/store/10.1111/j.1469-185X.2007.00030.x/asset/j.1469-185X.2007.00030.x.pdf?v=1&t=gzie1u8h&s=0a780dc45692a9a4e938b3d2c662 (accessed on 16 November 2021). [CrossRef]
- Lachowicz, J.; Niedziałek, K.; Rostkowska, E.; Szopa, A.; Swiader, K.; Szponar, J.; Serefko, A. Zebrafish as an Animal Model for Testing Agents with Antidepressant Potential. Life 2021, 11, 792. [Google Scholar] [CrossRef]
- Collymore, C.; Tolwani, R.J.; Rasmussen, S. The behavioral effects of single housing and environmental enrichment on adult zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 280–285. [Google Scholar]
- Shams, S.; Chatterjee, D.; Gerlai, R. Chronic social isolation affects thigmotaxis and whole-brain serotonin levels in adult zebrafish. Behav. Brain Res. 2015, 292, 283–287. [Google Scholar] [CrossRef]
- Fasulo, S.; Mauceri, A.; Maisano, M.; Giannetto, A.; Parrino, V.; Gennuso, F.; D’Agata, A. Immunohistochemical and molecular biomarkers in Coris julis exposed to environmental contaminants. Ecotoxicol. Environ. Saf. 2010, 73, 873–882. [Google Scholar] [CrossRef]
- Dalzochio, T.; Rodrigues, G.Z.P.; Petry, I.E.; Gehlen, G.; da Silva, L.B. The use of biomarkers to assess the health of aquatic ecosystems in Brazil: A review. Int. Aquat. Res. 2016, 8, 283–298. [Google Scholar] [CrossRef] [Green Version]
- Trapp, J.; Armengaud, J.; Salvador, A.; Chaumot, A.; Geffard, O. Next-generation proteomics: Toward customized biomarkers for environmental biomonitoring. Environ. Sci. Technol. 2014, 48, 13560–13572. [Google Scholar] [CrossRef]
- He, X.; Nie, X.; Yang, Y.; Liu, X.; Pan, D.; Cheng, Z.; Liang, X. Multi-biomarker responses in fishes from two typical marine aquaculture regions of South China. Mar. Pollut. Bull. 2012, 64, 2317–2324. [Google Scholar] [CrossRef]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P.E.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, D.; Domingues, I.; Faria, M.; Oliveira, M. Chronic Effects of Fluoxetine on Danio rerio: A Biochemical and Behavioral Perspective. Appl. Sci. 2022, 12, 2256. https://doi.org/10.3390/app12042256
Correia D, Domingues I, Faria M, Oliveira M. Chronic Effects of Fluoxetine on Danio rerio: A Biochemical and Behavioral Perspective. Applied Sciences. 2022; 12(4):2256. https://doi.org/10.3390/app12042256
Chicago/Turabian StyleCorreia, Daniela, Inês Domingues, Melissa Faria, and Miguel Oliveira. 2022. "Chronic Effects of Fluoxetine on Danio rerio: A Biochemical and Behavioral Perspective" Applied Sciences 12, no. 4: 2256. https://doi.org/10.3390/app12042256
APA StyleCorreia, D., Domingues, I., Faria, M., & Oliveira, M. (2022). Chronic Effects of Fluoxetine on Danio rerio: A Biochemical and Behavioral Perspective. Applied Sciences, 12(4), 2256. https://doi.org/10.3390/app12042256






