Abstract
Nanoporous carbon is synthesized on the base of phenol-formaldehyde resin and polyolefin wax, a by-product from industrial production of polyethylene at low pressure. The adsrption of phenol derivates from aqueous solutions on obtained carbon material was studied. The adsorption capacity of the carbon is related to the surface area and composition of the synthesized material, as well as to the nature of the adsorbent. The obtained adsorbent is characterized by high surface area and porosity, and it demonstrates high adsorption capacity towards aromatic compounds. All studied phenolic compounds show high affinity towards carbon, confirming that the retention mechanism occurs via non-specific interactions between the electronic density of the adsorbent and molecules of aromatic pollutants. Electrostatic interactions may also appear depending on pH of the solution pH and charge distribution of the carbons; and these effects has a strong influence on the final performance of the carbon.
1. Introduction
Water is a universal solvent used in almost every industrial process-hence, it is expected that wastewaters tcontain a lot of organic and inorganic pollutants. As a result of the extensive development of industrial activities in recent decades, large amounts of various pollutants have been released into waters. The chemical pollution of surface waters presents a threat to the aquatic environment with negative effects on ecosystems, as well as to human health. The presence of large concentrations of toxic pollutants in wastewater has become an important environmental problem, and very often small amounts of different organic compounds, including phenol and its derivatives, pesticides, aliphatic and aromatic hydrocarbons, dyes, surfactants, etc., are detected in waters [1].
Phenolic compounds are prevalent in many industrial wastewaters from pharmaceutical, rubber, herbicide, pulp and paper industry, etc. [2,3]. Along with phenol, nitro-phenols, amino-phenols and chloro-phenols are the most abundant phenolic compounds in industrial waste waters [4,5,6]. Due to their toxicity, high mobility in the environment and low biodegradability, the US and European Environmental Protection Agencies (EPA and EEA) have included these compounds in their lists of priority pollutants to be monitored in industrial effluents.
In this regard, adsorption technology has become a well-established and powerful technique for water treatment, as activated carbons are the most effectively used adsorbents, due to their suitable porous and chemical features [7].
Carbon adsorbents are considered to be effective, but their high cost has stimulated interest in examining the feasibility of using cheaper waste materials as potential adsorbents. In recent years, there is a special attention towards the production of low-cost acti-vated carbons from different waste materials [8]. In this connection, a number of works report the thermo-chemical conversion of different waste materials from biomass (agricultural by-products, different sources of plants, etc.), coal by-products, and polymer wastes as alternative precursors for the preparation of carbon-based adsorbents [9,10,11]. Plastic by-products as a side materials from the production of different polymer materials are very promising raw materials for synthesis of activated carbons due to their availability at a low price [12,13,14]. Recently the thermo-chemical conversion of plastic by-products has become popular due to their availability and potential to produce energy as well as activated carbon with very good properties. Our earlier investigations [14,15], which are focused on the synthesis of porous carbons, show that the chemical composition of the precursor strongly influences the structure and properties of the obtained adsorbent. For instance, the carbonizate—before activation—obtained from pyrolysis of a specific raw material containing dominantly aromatic compounds (such as biomass derived tar), is characterized by a dense structure and weak reactivity towards steam activation. In turn, when polymer material is used as a precursor, the final carbon material is less dense and more reactive, (due to the insertion of oxygen in the carbon skeleton), which favors pore formation during the activation process.
There are various nanomaterials with huge potential to treat polluted water [1]. The aim of our research is to investigate the adsorptive performance of nanoporous synthetic carbon, obtained by high-temperature hydro-pyrolysis of a mixture of phenol-formaldehyde resin and polyolefine wax, towards p-nitrophenol, pentachlorophenol and m-aminophenol in aqueous solutions. m-aminophenol, p-nitrophenol and pentachlorophenol were chosen as representative phenols, due to the presence of electron-donating and withdrawing groups, respectively. The precursor is prepared by thermo-oxidation treatment of a mixture of polyolefin wax (which is used as binder, and it is a by-product from polyethylene production at low pressure) and high-reactive phenol-formaldehyde resin (filler), thus ensuring intensive polymerization and polycondensation reactions, leading to easier solidification and pore formation. Sulfuric acid acts as oxidizer and catalyst of polymerizarion and polymerization reactions. A novelty is using new precursor mixture and new synthesis method. Since industrial by-products are used, this ensures contribution to waste valorization and sustainable development.
2. Materials and Methods
2.1. Preparation of Nanoporous Carbon
The precursor mixture was oxidized by treatment with sulfuric acid, and then activated with water vapor in order to incorporate further oxygen functionalities of different nature. The initial materials for producing polymer-based nanoporous carbon are polyolefine wax and phenol-formaldehyde resin. The polyeolefin wax is waste material, obtained as a by-product from the production of polyethylene (Burgas, Bulgaria). Phenol-formaldehyde resin (type Novolac Z with molecular mass of around 570 g mol−1) was obtained from the chemical plants in Nowa Sarzyna (Poland). A mixture of polyolefin wax and phenol-formaldehyde resin (50:50%) is heated to a melting temperature (150–200 °C) and then 98% sulfuric acid is added by drops to obtain solid product. This solid product was cooled down and washed with water, and then dried at 150 °C and carbonized at 600 °C in a covered silica crucible with a heating rate of 10 °C min−1 under nitrogen atmosphere. The obtained carbonizate product was subjected to high temperature hydro-pyrolysis at 800 °C in a stainless-steel vertical reactor for 1 h (labeled as AC).
2.2. Textural Measurements
The texture of the synthesized carbon material was characterized by N2 adsorption at −196 °C, carried out in an automatic volumetric apparatus Quantachrome Autosorb iQ-C-XR/MP.
Before the experiments, the sample was outgassed under vacuum at 350 °C overnight. The isotherms were used to calculate the specific surface area SBET, total pore volume VT, and micropore volume using the Dubinin—Radushkevich method.
2.3. Oxygen-Containing Functional Groups
The amount of oxygen-containing functional groups with increasing acidity was determined by Boehm’s method of titration with basic solutions of different base strength (NaHCO3, Na2CO3, NaOH, C2H5ONa). For this purpose the samples were agitated for at least 16 h with 0.05 N solutions of four bases. The amount of OH− anions remaining in the solution is determined by adding an excess of standard HCl solution and back-titration. The basic surface groups content of the samples is determined with 0.05 N HCl. Every measurement is performed three times and the average was taken.
2.4. IR Spectroscopy
The infrared spectra are recorded on Bruker Tensor 27 FTIR Spectrometer in the range of 4000–400 cm−1 using 64 scans (64 spectra were performed in 1 min, and the final spectrum is obtained by intergration of these 64 spectra, which is carried out automatically by the apparatus via Fourier Transformation, thus increasing the accuracy of the measurement) and a resolution of 0.5 cm−1.
2.5. Adsorption Measurements in Water Solution
m-aminophenol, p-nitrophenol and pentachlorophenol were obtained from Merck (99% purity). The phenolic solutions were prepared in non-buffered distilled water in the concentration range of 100–250 mg L−1 for adsorption of p-aminophenol and p-nitrophenol, and 10–30 mg L−1 for pentachlorophenol. Phenol in a concentration ranging from 50 to 2000 mg L−1 has been reported by many researchers in industrial wastes [16]. Around 100 mg of activated carbons were mixed with 50 mL of phenolic solution of the desired concentration, and the suspensions were shaken for 2 h. The samples were filtered and the equilibrium concentrations of the phenolic compounds remaining in solution were determined spectrophotometrically (286 nm for m-aminophenol, 305 nm for penthachlorophenol, and 316 nm for m-nitrophenol) by Pfaro 300 UV spectrometer, whereas every point was measured three times and the average was taken. The pH of the solutions during adsorption is measured triplicate in the pH range 5–6 and the average value is taken into account. The effect of pH of the solution on the adsorption capacity of the studied carbons at pollutant concentration 100 mg L−1 was investigated. The pH was adjusted by adding NaOH or HCl to the stock solution.
3. Results and Discussion
3.1. Adsorbent Characterization
In this work, polyolefine wax and phenol-formaldehyde resin were selected as the carbon precursors. The addition of phenol-formaldehyde resin to polyolefine wax, used in the precursor mixture and treatment with sulfuric acid, aims to incorporate a reactive structure in the carbon matrix, capable of inducing polymerization and polycondensation reactions, that will facilitate the solidification of the mixture. The resulting solid product was carbonized and submitted to steam activation to produce a suitable porous adsorbent. Inserting oxygen in the carbon precursor leads to the formation of oxygen containing structures on the surface of the final product, confirmed by elemental analysis (Table 1). The analysis is performed three times and the average was taken.

Table 1.
Chemical characterization of activated carbon (wt.%, accuracy 0.01%).
The X-ray diffraction pattern of the synthesized nanoporous carbon is shown in Figure 1. Two intense and considerably narrow peaks located at 24.5° and 43.5° are detected, corresponding to the (0 0 2) and (1 0 0) reflexes of crystalline graphite. These peaks corroborate the presence of porous network, associated with the typical microcrystal turbostratic structure of carbon materials.
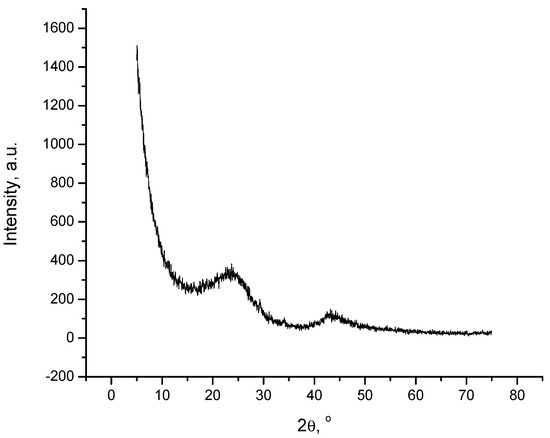
Figure 1.
XRD pattern of obtained activated carbon (accuracy 0.5%).
Porosity has strong effect on the adsorption properties of activated carbon. The pore structure of obtained activated carbon was investigated by N2 gas adsorption. Textural characterization was carried out by measuring the N2 adsorption isotherms at −196 °C. The nitrogen adsorption isotherms of the activated carbon are presented in Figure 2a. Analysis of nitrogen adsorption data indicates that the activated carbon obtained from polymer waste and phenol-formaldehyde resin has a high surface area (SBET) and a well-developed pore structure (Figure 2b). The part of the isotherms in the range of the relatively lower pressures has a steep increase with a tendency for saturation, which is typical for microporous adsorbents. The N2 adsorption isotherms obtained correspond to IV type according to Brunauer classification at low pressure. The main textural parameters of the prepared carbon, obtained from the analysis of the nitrogen adsorption isotherms, are compiled in Table 2.
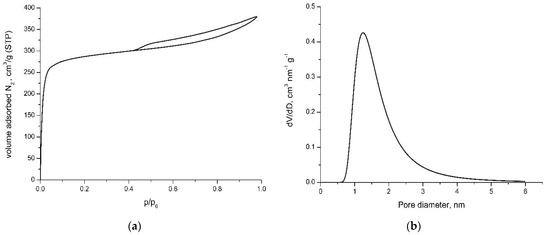
Figure 2.
N2 adsorption isotherm (a) and pore size distribution (b) of activated carbon at −196 °C.

Table 2.
Textural characteristics of activated carbon.
According to IUPAC classification, the isotherm is of IV type, with hysteresis of H4 type, characteristic for micro-mesoporous material. Type H4 hysteresis is also often associated with narrow slit pores.
Our results show that using the precursor mixture of polyolefin wax and phenol-formaldehyde resin (containing oxygen) leads to activated carbon with a high surface area. The pore volume analysis shows that this activated carbon has prevailing content of micropores.
The surface functionalities are characterized by the Boehm titration (Table 3). Data shows that the surface chemistry of carbon comprises carbonyl and small amount of carboxyl, carboxyl in lactone like structures and phenolic functionalities, and a considerably high content of carbonyl groups, which render a basic character to the adsorbent. The synthesized carbon also displays strong alkaline character, as inferred from the pH value.

Table 3.
Quantification of oxygen-containing groups on carbon surface (mmol/g, accuracy 0.01 mmol/g).
Along with the incorporation of acidic surface groups (due to the treatment with a sulfuric acid), a moderate amount of basic groups is detected, which determine the basic character of the surface. This is attributed to the incorporation of the surface groups at the edges of the basal planes in the graphene layers, thereby reducing the capacity of these sites to accept H+ ions (acting as a Lewis base). These porous features should be ideally adapted for the removal of aromatics from aqueous phase. The IR spectroscopy results confirm the presence of oxygen-containing groups on the surface, which increase the adsorption properties of activated carbon. IR spectra of the activated carbon sample are presented in Figure 3. The band at 1709 cm−1 could be related to the stretching of C=O in linear aliphatic aldehydes, ketones and carboxyls [17]. The bands around 1660 cm−1 could be due to the aromatic ring stretching of carbonyl groups C=O, C=C bonds or OH groups. The bands in the region of 1200–1000 cm−1 are due to C-O in ethers structures.
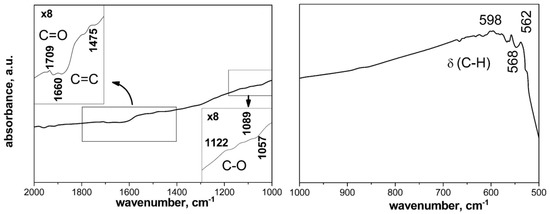
Figure 3.
IR spectrum of activated carbon.
3.2. Adsorption from Water Solution
Initially, the adsorption kinetics of three aromatics on the studied carbons were evaluated, in order to assess the time, needed for equilibrium before determining the maximal adsorption capacity of each adsorbent. The kinetics of adsorption of pentachlorophenol, m-aminophenol and p-nitrophenol from aqueous solutions were studied for various concentrations ranging from 100 to 250 mg L−1 for carbon. The obtained kinetics of adsorption shows the well-known pattern of aromatic compounds described in the literature [18,19], with a fast uptake of three compounds at the initial stage of the adsorption process (short times), and equilibrium is attained within 40 min in all cases. Moreover, the amounts adsorbed show smooth continuous curves in all the cases, with a well-defined saturation plateau. This behavior is attributed to the decrease in the number of available adsorption sites on the carbon surface as the adsorption proceeds and the first molecules of adsorbate are retained.
Since m-aminophenol and p-nitrophenol show rather close solubility in water, 2.6 g and 1.7 g per 100 g H2O solution, respectively, the similarities in the rate of adsorption on activated carbon are somewhat expected. Pentachlorophenol shows a small solubility in water—0.03 g per 100 g water solution—which could explain why the adsorption is lower in comparison with the pollutants. The adsorption isotherms of m-aminophenol, pentachlorophenol and m-aminophenol on activated carbon are presented in Figure 4.
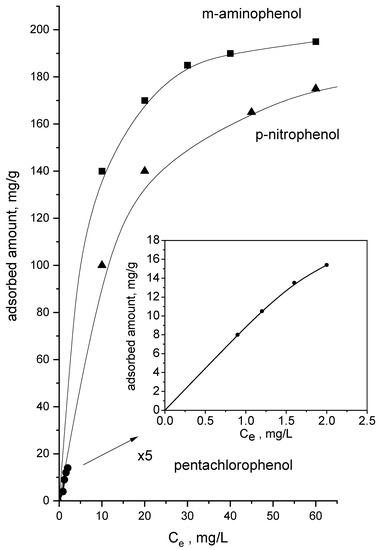
Figure 4.
Equilibrium adsorption isotherms of pentachlorophenol, m-aminophenol and p-nitrophenol on carbon.
All of them belong to type L of Giles classification, indicating that adsorption proceeds by the formation of a monolayer in the range of concentrations used [20]. Our results show that the adsorption mechanism of pentachlorophenol, m-aminophenol and p-nitrophenol is similar. The experimental data fits well with the Langmuir equation [21].
where Ce /relative error of the measurement 1.0%/ is the equilibrium pollutant concentration remaining in solution after adsorption (mg L−1), Qe /relative error 2.01%/ is the amount of pollutant bound to the adsorbent (mg g−1), Qo /relative error 0.01%/ is the maximum amount of the pollutant per unit weight of adsorbent (mg g−1), and b /relative error 1.0%/ is a constant related to the affinity of binding sites (L mg−1).
Qe = QobCe/(1 + bCe),
These parameters, together with the relative affinity of the adsorbates towards the surface of the adsorbents—Parameter bQo, and the correlation coefficients, are compiled in Table 4. The very good fittings, obtained for the sample, indicate that the Langmuir model is capable of predicting the adsorptive behavior of these carbons towards pentachlorophenol, m-aminophenol and p-nitrophenol. The adsorption capacities of obtained carbon towards the three pollutants studied carbons are high, probably due to the hydrophobic character of the carbon surface. The maximal adsorption capacities calculated from the Langmuir equation are 22 mg/g, 165 mg/g, and 152 mg/g, for pentachlorophenol, m-aminophenol and p-nitrophenol, respectively. These values are higher in comparison to those reported in the literature for activated carbons with higher surface areas [22,23,24].

Table 4.
Results obtained from the Langmuir equation applied to the adsorption isotherms of phenolic compounds on the studied activated carbons.
The differences in the uptake of the three pollutants, p-nitrophenol, m-aminophenol and pentachlorophenol, confirm that the adsorptive performance depends on the porosity and the chemical nature of the activated carbon. The slightly lower uptake of p-nitrophenol and lower uptake of pentachlorophenol by the carbon is somewhat expected, implying that these compounds displays a lower water solubility (1.7 g and 2.6 g per 100 g water solution, for p-nitrophenol and m-aminophenol, respectively, and 0.003 g per 100 g water solution for pentachlorophenol).
On the other hand, the trend observed for the affinity parameter seem to be related to the electron-withdrawing or electron-donating properties of the moieties present in the phenolic compound (i.e., nitro-, amino- and Cl− groups). The NH2− moiety is a powerful electron-donating group, which increases the π-electron density of the aromatic ring; in contrast, the NO2− moiety is a strong deactivator of the aromatic ring.
It is well known that oxygen functionalities withdraw the π-electron density of the basal planes of the carbons [18]; therefore, it seems reasonable to expect that the affinity of both aromatics would be higher in carbons with a rich electron density, and stronger for m-aminophenol, which aromatic ring is not deactivated by the amino group.
Whereas previous studies reported in the literature [2,25,26] deal exclusively with hydrophobic carbon adsorbents, disregarding the complexity of the adsorption process, our studies reveal the essential importance of the carbon surface chemistry and porous structure.
In this regard, carbon exhibits a strong interaction with p-nitrophenol, m-aminophenol and pentachlorophenol, despite its relatively high oxygen content (11.52 wt.%), which is often associated with a large deactivation effect of the π-electron density of graphene layers. Our results suggest that oxygen content should not be considered as the key factor in the removal of aromatics; rather the basic/acidic nature of the surface functionalities and porous structure should be taken into account.
3.3. Effect of pH
The pH of the water solution is one of the key factors that control the adsorption process, since it influences the electrostatic interactions between the adsorbent and the adsorbate. The adsorption of m-amonophenol, p-nitrophenol and pentachlorophenol as a function of pH was studied on carbon over a pH range 2–12. The adsorption of pentachlorophenol depending on pH is presented in Figure 5. For all three phenolic substances these results are similar. The desired pH was obtained by adding adequate amounts of NaOH or HCl diluted solutions to the initial non-buffered solution containing the phenolic compound. The maximal uptake of all three pollutant is found to be maximal in the range pH 6–7. As expected, these results show that m-aminophenol and p-nitrophenol are preferentially adsorbed from their neutral form. The effect of pH depends to a large extent on the nature of the adsorbent.
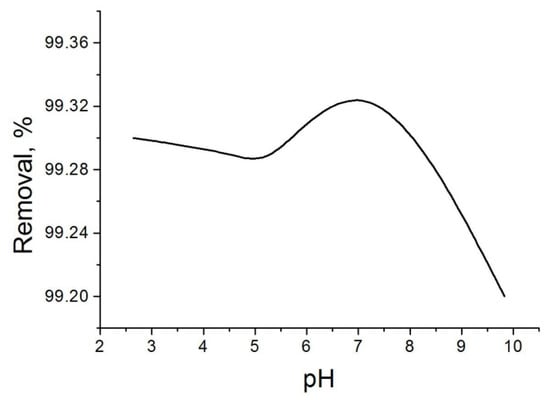
Figure 5.
Effect of pH on penthachlorophenol removal. Conditions: time of treatment—60 min; activated carbon amount—0.1 g per 50 mL solution; penthachlorophenol concentration—0.03 g/L.
The amount adsorbed does not alter too much at acidic pH < 6, whereas the gradual shift at basic media results in a sharp uptake fall of the uptake. As the pH increases, the surface of the activated carbons is being negatively charged until pH > pHPZC, (PZC—Point zero charge is the pH value, corresponding to zero net surface charge of adsorbent surface), where the number of negative charges becomes predominant on the carbon surface. At this point, the uptake fall is due to repulsive interactions, that appear between the anionic form of the adsorbates and the charges on the carbon surfaces. For hydrophobic carbon, the occurrence of negative electrostatic interactions starts at pH > 8.2.
4. Conclusions and Future Perspectives
Polymer waste—a mixture of by product polyolefine wax from the production of polyethylene at low pressure and phenol-formaldehyde resin—is an appropriate precursor for the synthesis of nanoporous carbons with negligible ash content and well-developed porosity. The synthetic activated carbon demonstrated high adsorption activity towards phenolic derivates—pentachlorophenol, m-aminophenol and p-nitrophenol—comparable to those of other carbon materials reported in the literature.
The adsorption capacities of activated carbon towards phenolic compounds (pentachlorophenol, m-aminophenol and p-nitrophenol) depend on porous parameters and surface chemistry (basic/acidic nature of the surface functionalities). High oxygen content is only associated with the withdrawal effect of π-electron density of graphene layers present in carbon materials.
This work contributes to waste valorization. On the base of polymer waste, a carbon adsorbent with a high surface area was prepared. The obtained carbon material has very good adsorption properties towards organic pollutants and could be successfully applied for water purification.
Further investigations are planned, including the modification of the synthesis procedure, searching for new precursors, testing adsorption properties towards different pollutants in waters and air, and adsorption studies of waste-waters in real conditions, to name a few.
Author Contributions
Conceptualization, I.S., B.T. (Boyko Tsyntsarski), T.B., B.T. (Barbara Trzebicka) and N.P.; methodology, I.S., B.T. (Boyko Tsyntsarski), T.B., B.T. (Barbara Trzebicka), B.K., S.P., U.S., G.G., B.P. and N.P.; formal analysis, B.T. (Boyko Tsyntsarski), I.S., T.B., B.K., U.S., S.P., G.G. and B.P.; investigation, B.T. (Boyko Tsyntsarski), I.S., T.B., B.K., U.S., S.P., G.G. and B.P.; writing—original draft preparation, I.S., B.T. (Boyko Tsyntsarski), T.B., B.T. (Barbara Trzebicka), U.S., S.P. and N.P.; writing—review and editing, I.S., B.T. (Boyko Tsyntsarski), T.B., B.T. (Barbara Trzebicka), U.S., S.P. and N.P.; funding acquisition, B.T. (Boyko Tsyntsarski), I.S., U.S. and B.T. (Barbara Trzebicka). All authors have read and agreed to the published version of the manuscript.
Funding
This work was co-financed by Projects KP-06-27/9, KP-06-29/2 and KP-06-37/3. The authors also gratefully appreciate the funding from Polish-Bulgarian joint project.
Institutional Review Board Statement
The study was conducted in accordance with the The European Code of Conduct for Research Integrity and the Declaration of Helsinki, and approved by the Ethics Committee of INSTITUTE OF ORGANIC CHEMISTRY WITH CENTRE OF PHYTOCHEMISTRY (protocol code IOCCP-005 and date of approval 23 April 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yaqoob, A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Dutta, R.K.; Naik, D.V.; Ray, A.; Kanaujia, P.K. High surface area Eucalyptus wood biochar for the removal of phenol from petroleum refinery wastewater. Environ. Chall. 2021, 5, 100353. [Google Scholar] [CrossRef]
- Kakavandi, B.; Jahangiri-rad, M.; Rafiee, M.; Ramazanpour Esfahani, A.; Akbar Babaei, A. Development of response surface methodology for optimization of phenol and p-chlorophenol adsorption on magnetic recoverable carbon. Microporous Mesoporous Mater. 2016, 231, 192–206. [Google Scholar] [CrossRef]
- Han, Y.; Wang, N.; Guo, X.; Jiao, T.; Ding, H. Influence of ultrasound on the adsorption of single-walled carbon nanotubes to phenol: A study by molecular dynamics simulation and experiment. Chem. Eng. J. 2022, 427, 131819. [Google Scholar] [CrossRef]
- Chaghaganooj, Z.D.; Asasian-Kolur, N.; Sharifian, S.; Sillanpää, M. Ce and Mn/bio-waste-based activated carbon composite: Characterization, phenol adsorption and regeneration. J. Environ. Chem. Eng. 2021, 9, 105788. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Netto, M.S.; Allasia, D.; Oliveira, M.L.S.; Foletto, E.L.; Dotto, G.L. Highly effective adsorption of synthetic phenol effluent by a novel activated carbon prepared from fruit wastes of the Ceiba speciosa forest species. J. Environ. Chem. Eng. 2021, 9, 105927. [Google Scholar] [CrossRef]
- Ahmad, A.; Setapar, S.H.M.; Yaqoob, A.A.; Ibrahim, M.N.M. Synthesis and characterization of GO-Ag nanocomposite for removal of malachite dye from aqueous solution. Mater. Today Proc. 2021, 47, 1359–1365. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Ahmad, A.; Reddy, A.V.B. Toxicology and Environmental Application of Carbon Nanocomposite. In Environmental Remediation through Carbon Based Nano Composites; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–18. [Google Scholar]
- Petrov, N.; Budinova, T.; Razvigorova, M.; Ekinci, E.; Ferhat Yardim, M.; Minkova, V. Preparation and characterization of carbon adsorbents from furfural. Carbon 2000, 38, 2069–2075. [Google Scholar] [CrossRef]
- Savova, D.; Apak, E.; Ekinci, E.; Ferhat Yardim, M.; Petrov, N.; Budinova, T.; Razvigorova, M.; Minkova, V. Biomass conversion to carbon adsorbents and gas. Biomass Bioenergy 2001, 21, 133–142. [Google Scholar] [CrossRef]
- Petrova, B.; Tsyntsarski, B.; Budinova, T.; Petrov, N.; Ania, C.O.; Parra, J.B.; Mladenov, M.; Tzvetkov, P. Synthesis of nanoporous carbons from mixtures of coal tar pitch and furfural and their application as electrode materials. Fuel Process. Technol. 2010, 91, 1710–1716. [Google Scholar] [CrossRef] [Green Version]
- Conde-Rivera, L.R.; Suarez-Escobar, A.F.; Marin-Perez, J.J.; Junco-Rodriguez, M.J.; Lopez-Suarez, F.E. TiO2 supported on activated carbon from tire waste for ibuprofen removal. Mater. Lett. 2021, 291, 129590. [Google Scholar] [CrossRef]
- Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M.; Parveen, T.; Safian, M.T. Environmental applications of smart polymer composites. Smart Polym. Nanocompos. Biomed. Environ. Appl. 2020, 15, 295–320. [Google Scholar]
- Racheva, I.G.; Tsyntsarski, B.G.; Petrova, B.N.; Budinova, T.; Petrov, N.V.; Nagel, B.; Pusz, S.; Szegula, U. Conversion of polyolefine wax to carbon adsorbent by thermooxidatyion treatment. Bulg. Chem. Commun. 2014, 46, 129–133. [Google Scholar]
- Tsyntsarski, B.; Petrova, B.; Budinova, T.; Petrov, N.; Teodosiev, D.K.; Sarbu, A.; Sandu, T.; Ferhat Yardim, M.; Sirkecioglu, A. Removal of detergents from water by adsorption on activated carbons obtained from various precursors. Desalin. Water Treat. 2014, 52, 3445–3452. [Google Scholar] [CrossRef]
- Hussain, A.; Dubey, S.K.; Kumar, V. Kinetic study for aerobic treatment of phenolic wastewater. Water Resour. Ind. 2015, 11, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Zawadzki, J.; Wisniewski, M.; Weber, J.; Heintz, O.; Azambre, B. IR study of adsorption and decomposition of propan-2-ol on carbon and carbon-supported catalysts. Carbon 2001, 39, 187–192. [Google Scholar] [CrossRef]
- Cabal, B.; Ania, C.O.; Parra, J.B.; Pis, J.J. Kinetics of naphthalene adsorption on an activated carbon: Comparison between aqueous and organic media. Chemosphere 2009, 76, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J. Hazard. Mater. 2009, 164, 473–482. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solutions adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 60, 3973–3993. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surgaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Troca-Torrado, C.; Dominguez, M.; Gуmez-Serrano, V. Development of adsorbents from used tire rubber. Their use in the adsorption of organic and inorganic solutes in aqueous solution. Fuel Process. Technol. 2011, 92, 206–212. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Rivera-Utrilla, J.; Lopez-Ramon, M.V.; Carrasco-Marin, F. Adsorption of some substituted phenols on activated carbons from a bituminous coal. Carbon 1995, 33, 845–851. [Google Scholar] [CrossRef]
- Qi, L.; Jiang, H.; Lin, T.; Chang, X.; Jiang, B. Fabrication of MIL-53(Al) based composites from biomass activated carbon (AC) for efficient p-nitrophenol adsorption from aqueous solution. J. Taiwan Inst. Chem. Eng. 2021, 127, 220–227. [Google Scholar] [CrossRef]
- Jadoon, T.; Ahsin, A.; Ullah, F.; Mahmood, T.; Ayub, K. Adsorption mechanism of p- aminophenol over silver-graphene composite: A first principles study. J. Mol. Liq. 2021, 341, 117415. [Google Scholar] [CrossRef]
- Wasilewska, M.; Marczewskim, A.W.; Derylo-Marczewska, A.; Dariusz Sternik, D. Nitrophenols removal from aqueous solutions by activated carbon—Temperature effect of adsorption kinetics and equilibrium. J. Environ. Chem. Eng. 2021, 9, 105459. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).