Abstract
Background: Oxidative stress, lipid profile and renal functions are well-known conventional risk factors for diabetes mellitus (DM). Metformin and gliclazide are popularly used monotherapy drugs for the treatment of DM. Aims: This study aims to assess the short-term treatment of single and dual therapy of glipizide/metformin on oxidative stress, glycemic control, serum lipid profiles and renal function in diabetic rats. Methods: DM was induced in rats with streptozotocin (STZ), then five different treatments were applied, including group I (untreated healthy control), group II (diabetic and untreated), group III (diabetic and treated with metformin), group IVI (diabetic and treated with glipizide) and group V (diabetic and treated with a combination of metformin and glipizide. Lipid peroxidation (LPO), nitric oxide (NO), total antioxidant capacity (TAC), fasting blood glucose (FBG), glycated hemoglobin (HbA1c), total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), creatinine and urea were measured. Results: Compared to the untreated DM group, FBG and HbA1c were significantly reduced in the DM groups (p < 0.01) treated with metformin (159.7 mg/dL & 6.7%), glipizide (184.3 mg/dL & 7.3%) and dual therapy (118 mg/dL & 5.2%), respectively. Treatment with dual therapy and metformin significantly decreased LPO and NO levels but increased TAC in diabetic rats more than glipizide compared to untreated diabetic rats. Furthermore, metformin (19.8 mg/dL, p < 0.001), glipizide (22.7 mg/dL, p < 0.001), and dual therapy (25.7 mg/dL, p < 0.001) significantly decreased urea levels in the treated rats compared to untreated DM rats (32.2 mg/dL). Both drugs and their combination exhibited a substantial effect on total cholesterol, HDL, LDL and atherogenic index. Conclusions: These results suggest that the therapeutic benefits of metformin and glipizide are complementary. Metformin exhibited superior performance in improving glycemic control and decreasing oxidative stress, while glipizide was more effective against dyslipidemia. These findings could be helpful for the treatment of future vascular patients, antilipidemic medicines and antioxidant therapy to improve the quality of life.
1. Introduction
Diabetes mellitus (DM) is a chronic disorder associated with a group of metabolic derangements characterized by elevated blood sugar levels, frequent urination, increased hunger and increased appetite. DM can lead to several untreated problems, such as diabetic ketoacidosis, hyperosmolar hyperglycemic disorders or death [1]. Severe, long-lasting risks include heart failure, chronic kidney disease, foot ulcers, nerve damage, eye injury and cognitive disability [2,3]. DM has three major types. Type 1 diabetes is attributed to the pancreas’ inability to produce adequate insulin associated with beta-cell loss [4]. Type 2 diabetes begins with insulin resistance, where cells respond incorrectly to insulin. Gestational diabetes is the third primary type that occurs when pregnant women experience elevated blood sugar levels without a prior history of DM [5].
Oxidative stress (OS) is caused by an imbalance between oxygen-derived radicals and the organism’s antioxidant potential. This disrupts the natural cellular balance between radical production and defense. This causes oxidative damage to proteins, lipids and nucleic acids [6]. OS leads to an enormous number of diseases over time, including DM, atherosclerosis, inflammatory conditions, hypertension, heart disease, neurodegenerative diseases and cancer [7,8]. Oxidative stress is enhanced in both insulin-dependent and non-insulin-dependent diabetes. In addition, increasing the generation of free radicals and oxidative stress is a critical event in the development of the micro-and macrovascular complications of diabetes [3]. This hypothesis has been supported by the demonstration of elevated levels of oxidative stress biomarkers in patients with type 2 diabetes who are suffering from complications of their disease [9]. As a result, it appears plausible to assume that antioxidants can play a significant role in the improvement of diabetic symptoms. Various studies have linked diabetes to increased free radical formation and decreased antioxidant capacity [9]. Furthermore, numerous studies have been conducted on the impact of antioxidants in the management of diabetes [9].
Dyslipidemia is an abnormal level of lipids in the blood (e.g., triglycerides, fat phospholipids and cholesterol). Dyslipidemia is one of the most significant risk factors for DM and cardiovascular disease [10]. Atherosclerosis is accelerated in both type 1 and type 2 and is induced in diabetic patients by dyslipidemia [11]. Dyslipidemia in diabetic patients suggests the presence of what is known as the lipid triad: hypertriglyceridemia, low levels of high-density lipoprotein (HDL) and high levels of low-density lipoprotein (LDL) [10,12]. However, other studies have reported normal or minimally elevated total cholesterol levels in diabetic patients despite the risk of cardiovascular disease [13].
Hyperglycemia due to DM stimulates glucose self-oxidation to produce free radicals, which contribute to macro and microvascular dysfunction [14]. DM can lower antioxidant protection by reducing plasma/serum status or free radical scavenging activity, and increasing plasma-oxidization levels of specific antioxidant agents like ascorbic acid and vitamin E. Furthermore, people with type 2 diabetes showed a decrease in the endothelial synthesis of nitric oxide (NO) [15]. Current clinical trials consider the therapeutic potential of medications for DM based on the present understanding of the pathogenesis of diabetic complications. However, studies on antidiabetic drug efficacy have raised significant concerns, such as the effects of the medications on antioxidant properties [16], serum lipid profiles [17], blood glucose levels and overall safety [18].
Renal disease is a severe and recurrent DM complication. Kidney damage in DM may occur due to arterial (renovascular) and microvascular (classic diabetic renal disease) factors [19]. Furthermore, DM is the major reason for end-stage renal disease (ESRD). Controlling the glucose level is the best method to avoid diabetic kidney diseases [20]. Diabetic kidney disease affects about 35% of the diabetic population. Moreover, there is a growing risk of cardiovascular mortality at each successive stage of diabetic kidney disease. The number of patients expected to progress to a chronic renal disorder may increase with the younger onset of diabetes among patients with diabetes [21,22]. Kidney disease is typically divided into five stages by glomerular filtration rate (GFR). The first stage presents with albuminuria or another abnormal feature. Then, the next four stages are measured under the 90, 60, 30 and 15 mL/minute/1.73 m2 threshold deterioration. The GFR is measured in normal clinical practice from serum creatinine, age and sex using a diabetes test [21].
Metformin monotherapy has been shown to improve metabolic disorders [23] and reduce oxidative stress [24] in diabetic patients. Many DM patients used metformin alone to regulate their blood glucose levels. In contrast, metformin is used in combination with glipizide to effectively lower blood glucose levels, although they function in different ways. In addition to decreasing the quantity of glucose produced in the liver, metformin also increases the amount of glucose absorbed by muscle tissue; gliclazide increases the amount of insulin produced by the pancreas [23].
Gliclazide (Diamicron®, Servier (Ireland) Industries Ltd, Ireland, 160–320 mg daily) and Glipizide (Minodiab®, Pfizer (USA), New York, NY, USA, 2.5–5 mg daily) belong to a class of drugs known as second-generation sulfonylureas. Second-generation sulfonylureas have a more non-polar side chain than first-generation sulfonylureas, increasing their hypoglycemic potency. There are differences between gliclazide and glipizide in the dose, absorption rate, duration of action, elimination route and binding site on the target pancreatic cell receptor. These tablets work by stimulating the pancreas gland to produce more insulin hormone and lower blood glucose. They are frequently used in combination with Metformin. It has been proven that combining gliclazide with metformin synergistically lowers glycosylated hemoglobin (HbA1c) level [25]. Nevertheless, the combined metformin/gliclazide therapy has a therapeutic effect on oxidative stress, lipid profile and hepatorenal functions [26].
The effects of the combination of gliclazide and metformin on oxidative stress, lipid profile and renal dysfunction have been studied in diabetic patients. However, the combination of metformin and glipizide medication and its effect on oxidative stress, lipid profile and renal function has not been well studied. Furthermore, examining the effectiveness of the metformin/glipizide as a combination therapy in treating type 2 diabetes needs to be further investigated.
Thus, the present study aims to evaluate the effect of metformin and/or glipizide on oxidative stress, lipid profile and renal dysfunction by measuring urea and creatine level, readjusting glycemic control, correcting dyslipidemia and achieving therapeutic benefits without significant toxic effects in streptozotocin-induced diabetic rats. It is also the aim of this study to compare the efficacy of metformin with glipizide in treating type 2 diabetes (T2DM).
2. Materials and Methods
2.1. Materials
Streptozotocin (STZ), metformin hydrochloride, total glycated hemoglobin kit and glipizide were purchased from Sigma Aldrich (St. Louis, MO, USA). Lipid peroxidation (LPO) assay kit, total serum nitrate/nitrite (NO(x)) kit, total antioxidant capacity (TAC) kit, serum creatinine, and urea concentration kits were purchased from Biodiagnostic (Cat. Tahrir, Cairo, Egypt). Triglycerides assay kit, total cholesterol assay kit, HDL& LDL precipitating reagent kit were purchased from United Diagnostics Industry (UDI, Makka, KSA). All chemicals used were of analytical grade and were used as received without any further purification. All solutions were prepared with Millipore water.
2.2. Ethics
The Health Research Ethics, Deanship of Scientific Research, Qassim University (Approval No: 18-02-07), following the National Research Council (US) Guide for the Care and Use of Laboratory Animals [27].
2.3. Animals
The present study used 30 male Sprague Dawley rats with an age range of between 5 and 6 months, weighing 200–250 g, obtained from the animal house at the College of Pharmacy, Qassim University, Saudi Arabia. The rats were maintained in a room at a temperature of 25–27 °C, a humidity of 50–80%, and a 12:12-h light: dark cycle. The animals had free access to standard pulverized rat pellets and water throughout the experiments.
2.4. Experimental Groups
The rats were divided into five groups of equal size (N = 6 rats per group): group I (untreated healthy control), group II (diabetic and untreated), group III (diabetic and treated with metformin), group IV (diabetic and treated with glipizide) and group V (diabetic and treated with a combination of metformin and glipizide). Then, the rats were maintained in a room at a temperature of 25–27 °C, a humidity of 50–80%, and a 12:12-h light: dark cycle with free access to water and food [28,29]. A two-tail option provided effect size d as 4.27 on G Power V.3.1.9.4 software, Neu-Isenburg, Germany [30], while to obtain the statistical power (1-β err prob) of 80% and a specific α error probability of 0.05, the least animal size per group was n > 3.
2.5. Induction of Diabetes
DM was induced in all groups by administering a single dose (65 mg/kg body weight) of streptozotocin (STZ). STZ was freshly dissolved in 5 mM citrate buffer, pH 4.5, and was then injected into rats by the intraperitoneal route of administration (i.p.) [31,32]. STZ injections were preceded by administering nicotinamide at 230 mg/kg i.p. to partially protect the β-cells against STZ [32,33]. Two days after administering STZ, fasting blood glucose levels (BGLs) were measured with an Accu-Chek glucometer (Roche, Germany) using blood samples taken from the tip of the tail. DM was successfully induced in all rats, as indicated by high BGLs (i.e., BGL > 200 mg/L in all cases) [34].
2.6. Medications
Rats in group I and group II (diabetic and untreated) received distilled water through oral gavage twice daily with no medications. Metformin was dissolved in distilled water and orally administered b.i.d. to rats in group III at a dose of 60 mg/kg of body weight [35] via an intragastric tube. Glipizide was dissolved in distilled water and orally administered to rats in group IV at a dose of 2.5 mg/kg of body weight/day once daily [36] via oral gavage. Finally, group V was administered both drugs. All groups were treated for three weeks following the onset of DM. For each of the three groups, random BGL was obtained daily using blood samples taken from the tip of the tail. At the end of the experiment, the rats were anesthetized and sacrificed using the ‘open-drop’ method with an ether-impregnated cotton ball in a bell jar for 5–10 min. Blood samples were collected from the medial canthus of the eye and a heart puncture, then stored in sterilized tubes for serum separation and total glycated hemoglobin (HbA1c) from was measured from whole blood [37].
2.7. Biochemical Assays
2.7.1. Glycemic Control for Total Glycated HbA1c Assay
Total HbA1c was estimated according to a standard technique by Bunn & Gallop et al. [38], using an entire glycated hemoglobin kit.
2.7.2. Oxidative Stress
The extent of (Lipid peroxidation (LPO) assay) was determined calorimetrically (expressed in nmol/L) according to Ohkawa et al. [39] using an LPO assay kit (Cat. No. MD 2529). Moreover, the total serum nitrate/nitrite (NO(x)) was measured calorimetrically (expressed in nmol/mL) according to Miranda et al. [40] through the acidic Griess reaction using total nitric oxide and nitrate/nitrite assay kit. Furthermore, the total antioxidant capacity (TAC) was measured calorimetrically (expressed in mM/L) according to Kovacevic et al. [41] using a TAC kit, Sigma-Aldrich, St. Louis, MO, USA, (Cat. No. TA2513).
2.7.3. Lipid Profile Assays
Serum triglycerides were measured via enzymatic colorimetric detection according to Fossati & Prencipe et al. [42] using the glycerol-3-phosphatase oxidase (GPO) method (expressed in mg/dL) with a Serum Triglyceride Determination Kit (Cat. No. EL59L-1000; KSA). Moreover, the total cholesterol (expressed in mg/dL) was measured using an enzymatic liquid-phase colorimetric test. The cholesterol oxidase phenol-4-aminophenazone-peroxidase (CHOD-PAP) method was proceeded according to Allain et al. [43] using a cholesterol assay kit from UDI (Cat. No. EL24-1200; KSA). The HDL (expressed in mg/dL) was measured via an enzymatic colorimetric test using the HDL-precipitating reagent phosphotungstate in the presence of divalent cation (Mg+2) according to the procedure reported by Warnick et al. [44] using the HDL kit (Cat. No. EL41-360; KSA). Furthermore, an indirect measurement of LDL (expressed in mg/dL) was performed using the Friedewald equation [45] based on the levels of total cholesterol, HDL and triglycerides as follows:
VLDL-C was calculated based on the formula [46]
The atherogenic index was calculated on the formula [47]
2.7.4. Renal Function Assay
Serum creatinine and urea concentrations were measured spectrophotometrically (expressed in mg/dL) according to Fabiny and Ertingshausen et al. [48] and Tabacco et al. [49], using creatinine assay and urea kits, respectively.
2.8. Statistics
Statistical analyses were performed using SPSS (SPSS for Windows version 26; IBM Corp., Armonk, NY, USA). GraphPad Prism Version 9 was used to draw the figures. Data are expressed as the mean (M) ± standard deviation (SD) and presented using bar charts. The Shapiro—Wilk test was used to verify that data were normally distributed. One-way analysis of variance (ANOVA) followed by either Tukey’s HSD or Kruskal Wallis posthoc tests were performed to compare the study groups based on variance equality. Pearson (average) and Spearman’s (not standard) correlation coefficient (r-value) were calculated using the GraphPad Prisms. Significance was considered when the p-value was <0.05.
3. Results
3.1. Glycaemic Control Assays
FBG levels were monitored in the different groups studied. The metformin combination with glipizide had the most effective hypoglycemic effects. Compared to the untreated DM group (362.7 mg/dL), FBG levels were significantly reduced in DM groups treated with metformin (159.7 mg/dL, p < 0.01), glipizide (184.3 mg/dL, p < 0.01) and dual therapy (118 mg/dL, p < 0.01).
HbA1c is glycated hemoglobin glucose mainly bound to the hemoglobin β chain N-terminal valine. HbA1c can be easily precipitated depending on the differences in net charge. The enzyme method currently available measures HbA1c by explicitly applying an enzyme that sticks to the N-terminal valine [49].
The results showed that the level of HbA1c decreased markedly in diabetic rats treated with metformin compared to untreated diabetic rats (6.73 ± 0.4% vs. ~9.0 ± 0.7%, p < 0.001) (Figure 1).
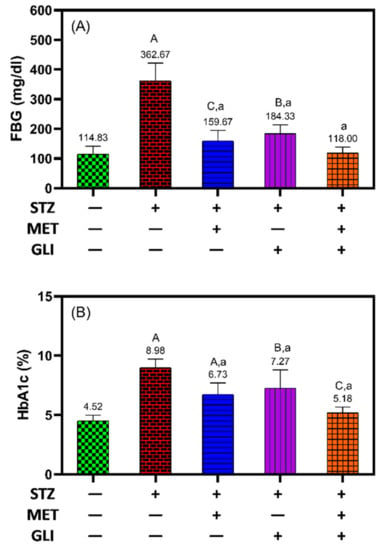
Figure 1.
Effects of metformin, glipizide and their combination on (A) fasting blood glucose levels (FBG) and (B) glycated hemoglobin (HbA1c) levels in diabetic rats. The data is expressed as the Mean ± SD. A, B, and C notation (in capital letters) indicate the significant difference levels p < 0.001, p < 0.01, and p < 0.05 between studied groups and healthy controls, respectively. While a, b and c notation (in small letters) indicate the significant difference levels p < 0.001, p < 0.01, and p < 0.05 between studied groups and untreated DM groups, respectively.
Similarly, the HbA1c level was significantly lower in diabetic rats following treatment with glipizide than in untreated diabetic rats (7.3 ± 1.5% vs. 9.0 ± 0.7%, p = 0.01), and the combination of glipizide and metformin was the most effective at decreasing the level of HbA1c and FBG to the expected levels of the untreated healthy control (5.2 ± 0.4%) (Figure 1A,B). The results suggest that metformin more effectively controls blood glucose levels in diabetic rats than glipizide. The combination of both drugs showed synergistic interaction and is more effective than single therapy in terms of glycemic control.
3.2. Oxidative Stress Assays
Remarkable changes in the LPO, NO and TAC levels were detected in the positive control group, signifying the induction of oxidative stress due to DM induced by STZ [50].
In Figure 2, in relation to healthy rats, the diabetic rats have a significantly high level of LPO (17.5 nmol/L vs. 9.5 nmol/L, p < 0.001) and NO (18.3 nmol/L vs. 8.8 nmol/L, p < 0.001) and significantly low levels of TAC (0.2 mmol/L vs. 0.8 mmol/L, p < 0.001) (Figure 2A–C).
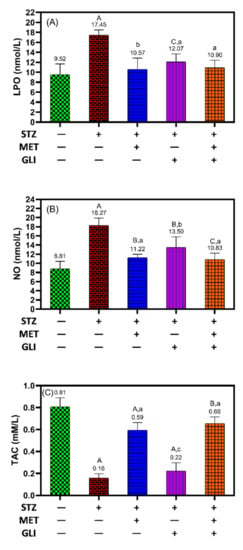
Figure 2.
Effects of metformin, glipizide and their combination on the levels of (A) lipid peroxidation (LPO), (B) nitric oxide (NO) and (C) total antioxidant capacity (TAC) in diabetic rats. The data is expressed as the Mean ± SD. A, B, and C notation (in capital letters) indicate the significant difference levels p < 0.001, p < 0.01, and p < 0.05 between studied groups and healthy controls, respectively. While a, b and c notation (in small letters) indicate the significant difference levels p < 0.001, p < 0.01, and p < 0.05 between studied groups and untreated DM groups, respectively.
The results showed treating diabetic rats with metformin significantly decreased LPO (10.6 nmol/L vs 17.5 nmol/L, p < 0.01) and NO (11.2 nmol/L vs 18.3 nmol/L, p = 0.01), and increased TAC (0.6 mM/L vs 0.2 mM/L, p < 0.001) in comparison to levels in untreated diabetic rats (Figure 2A–C). In contrast, treating diabetic rats with glipizide showed a significant reduction in LPO (12.11 nmol/L) and NO (13.5 1 nmol/L) compared to the diabetic rat; an increase in TAC was comparable to those of untreated diabetic rats (Figure 2C). Interestingly, the combination of metformin and glipizide significantly decreased the levels of LPO and NO and significantly increased the levels of TAC compared to the untreated diabetes rats (Figure 2A–C).
3.3. Lipid Profile Assays
Figure 3 showed an increase in the levels of lipid profile parameters except for HDL in diabetic rats compared to the health control (p < 0.001, p < 0.05 for TG). The group treated with metformin, glipizide and combination showed no remarkable change in total cholesterol (Figure 3A), TG (Figure 3B) and VLDL (Figure 3E) compared to the healthy control. The results showed that HDL increased significantly following treatment with metformin (46.5 mg/dL) and the combination (51.7 mg/dL) more than glipizide rats (40.1 mg/dL) in comparison to levels in the untreated diabetic group (32.1 mg/dL, p = 0.001) (Figure 3C). With the same pattern, LDL levels were markedly lower in diabetic rats treated with combination (33.8 mg/dL), metformin (43.7 mg/dL), and glipizide (46.2 mg/dL) than untreated diabetic rats (72.2 mg/dL, p = 0.001) (Figure 3D). Furthermore, there were significant changes in AIP from 0.60 in the diabetes control group to 0.39 mg/dL (p < 0.001), 0.48 mg/dL (p < 0.01) and 0.38 mg/dL (p < 0.001) in the cases of metformin, glipizide and combination groups, respectively (Figure 3F).
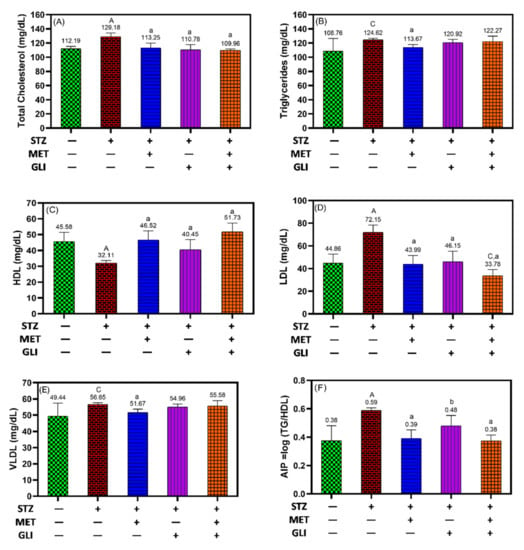
Figure 3.
Effects of metformin, glipizide and their combination on lipid profiles in diabetic rats; (A) total cholesterol, (B) triglycerides, (C) HDL, (D) LDL, (E) VLDL, and (F) AIP. Each data is expressed as the Mean ± SD. A, B, and C notation (in capital letters) indicate the significant difference levels p < 0.001, p < 0.01, and p < 0.05 between studied groups and healthy controls, respectively. While a, b and c notation (in small letters) indicate the significant difference levels p < 0.001, p < 0.01, and p < 0.05 between studied groups and untreated DM groups, respectively.
3.4. Renal Function Assay
Creatine as a parameter of renal function was comparable between all treatment groups with no considerable difference, meaning no change due to induction of DM by STZ (Figure 4A). At the same time, urea levels were significantly decreased in all treated groups compared to untreated diabetic rats and untreated health control (p < 0.001) (Figure 4B).
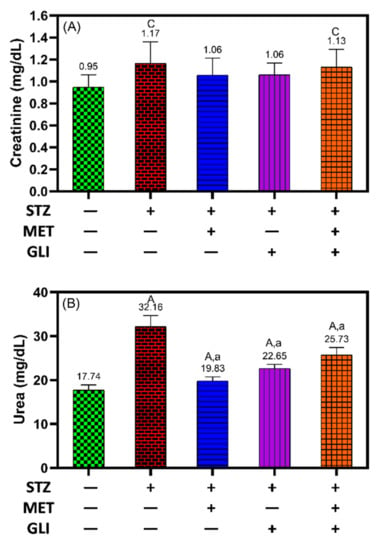
Figure 4.
Effects of metformin, glipizide, and their combination on kidney function tests (A) creatinine and (B) urea in diabetic rats. Each data is expressed as the Mean ± SD. A, B and C notation (in capital letters) indicate the significant difference levels p < 0.001, p < 0.01, and p < 0.05 between studied groups and healthy controls, respectively. While a, b, and c notation (in small letters) indicate the significant difference levels p < 0.001, p < 0.01, and p < 0.05 between studied groups and untreated DM groups, respectively.
3.5. The Association between Oxidative Stress Markers and Atherogenic Index
To find the association and relation between the tested samples, a correlation coefficient (r-value) was calculated. This correlation measures the linear relationship between the tested samples. It is the ratio between the covariance of two variables such that the result always has a value between −1 and 1.
Positive correlations were observed between AIP and both LPO (r = 0.77, p < 0.001, Figure 5A) and NO (r = 0.74, p < 0.001, Figure 5B) and between LPO and NO (r = 0.84, p < 0.001) (Figure 5D). While negative correlations were observed between TAC and AIP (r = −0.72, p < 0.001, Figure 5C), LPO (r = −0.72, p < 0.001, Figure 5E), and NO (r = −0.78, p < 0.001, Figure 5F).
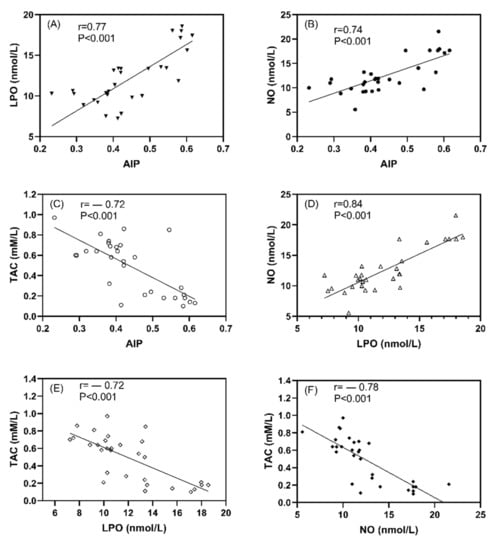
Figure 5.
The correlation coefficient between studied oxidative stress markers and atherogenic index plasma in all studied rats; (A) LPO vs AIP, (B) NO vs AIP, (C) TAC vs AIP, (D) NO vs LPO, (E) TAC vs LPO, and (F) TAC vs NO. Data expressed as correlation coefficient (r) values and p < 0.001 indicates the significant difference between studied parameters.
3.6. The Association between Oxidative Stress Markers and FBG and Cholesterol Levels
Positive correlations were observed between NO and Cholesterol (r = 0.68, p < 0.001, Figure 6A) and LPO and cholesterol (r = 0.67, p < 0.001, Figure 6B), as well as between LPO and FBG (r = 0.76, p < 0.001, Figure 6D) and NO and FBG (r = 0.73, p < 0.001, Figure 6E). While negative corrleations were observed between TAC and cholesterol (r = −0.49, p < 0.01, Figure 6C) and FBG (r = −0.53, p < 0.001, Figure 6F).
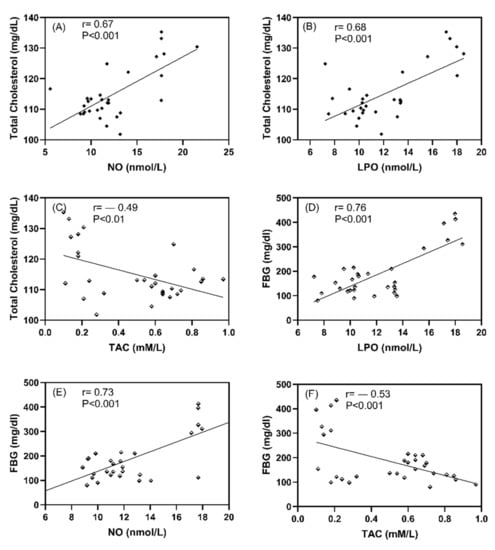
Figure 6.
The correlation coefficient between studied oxidative stress markers and FBG and cholesterol in all studied rats; (A) Total cholesterol vs NO, (B) Total cholesterol vs LPO, (C) Total cholesterol vs TAC, (D) Total FBG vs LPO, (E) FBG vs NO, and (F) FBG vs TAC. Data expressed as correlation coefficient (r) values and p < 0.001 indicates the significant difference between studied parameters.
4. Discussion
In the present study, we compared the beneficial effects of metformin, glipizide, and their combination in diabetic rats and found that: (i) the treatment with the combination of metformin and glipizide was more potent than single therapy in improving glycemic control and attenuating oxidative stress, (ii) metformin was non-inferior to glipizide in improving glycemic control and attenuating oxidative stress; (iii) glipizide exhibited a more powerful effect against atherogenic dyslipidemia than metformin (only by increasing HDL levels but most notably by lowering LDL); and (iv) neither drug significantly affected creatinine levels or renal function, and metformin decreased urea levels further than glipizide or the combination, indicating that both drugs were safe at the prearranged doses. The study flowchart (Figure 7) represents all divided groups in the methodology. The rats were anesthetized and sacrificed after three months, and blood samples were collected from the medial canthus of the eye and a heart puncture. The HbA1C percent was determined using whole blood, while the lipid profile, renal function tests and oxidative stress biomarkers were determined using serum lipid peroxide, nitric oxide and total antioxidant capacity.
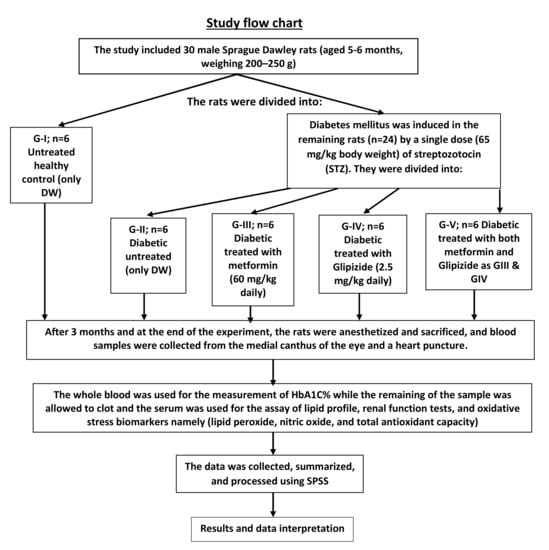
Figure 7.
Scheme summarizes all the experimental groups, treatment and administration.
Lipid peroxidation represents oxidative lipid degradation. Meanwhile, oxidative stress plays a significant role in DM. One of the common treatments for type 2 diabetes is the combination of metformin and glipizide; however, the therapeutic benefits of this combination on oxidative stress, lipid profile and renal functions have not been thoroughly investigated. The antioxidant indices, including LPO, NO and TAC, were measured in this study. It is clear from the current study results that the parameters mentioned above were comparable in all studied populations. When comparing diabetic patients receiving combined therapy to those receiving metformin or glipizide monotherapy, the results were more efficient for the diabetic rats. Nowadays, oxidative stress in diabetes is considered the basis for the pathogenesis of all diabetes-related complications [51]. Glycemic control, lipoprotein profile and hepatorenal function in diabetic patients are all adversely affected by increased free radical production.
The findings of this study showed that the administration of a combination of metformin and glipizide significantly improved oxidative stress status in patients with type 2 diabetes. The synergistic interaction between both drugs with different targets and mechanisms of action leads to an increase in the antioxidant capacity, insulin secretion and glucose uptake. This synergistic interaction results in control of oxidative stress damage effects, the lipid profile and glycemic status. The conclusive evidence of such a combination prevents the vascular complications of hyperglycemia (Figure 8).
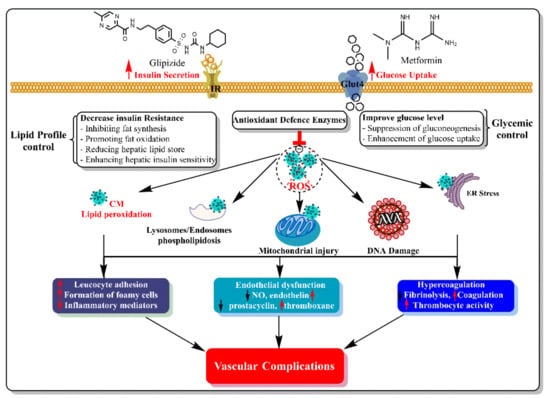
Figure 8.
The synergistic interactions between metformin and Glipizide in diabetes with different targets and mechanisms of action lead to decreased oxidative stress damages and prevented vascular complications of hyperglycemia.
A lipid profile assay is a blood analysis that checks fatty lipids and fatty substances in the body used as energy sources. Lipids include cholesterol, triglycerides, HDL, LDL, VLDL and AIP. Lipid profile evaluation is a significant tool in aiding the diagnosis and prognosis of DM [52]. This agrees with previous studies, including on the combinations of metformin and gliclazide (same class) [53,54]. Similarly to the findings of this study, Hassan and Abd-Allah demonstrated that combined metformin/gliclazide treatment significantly improved lipid profile, as evidenced by decreased total cholesterol and significantly increased HDL levels compared to the control group, when compared the study participants were compared [55]. The similar effects of monotherapy versus combination therapy on lipid profile, as demonstrated in this study, are consistent with a previous study that found similar efficacy of both drugs on lipid profile in the same patient [56].
Like most sulfonylureas, metformin has been shown to decrease oxidative stress [15,23] effectively. Studies that have investigated the effect of glipizide on oxidative stress are scarce [57]. The studies showed reduced oxidant stress in diabetic rats, as in the present study, while the influence of metformin on oxidative stress was even more pronounced. Furthermore, the TAC of diabetic rats treated with metformin was significantly higher than that of rats treated with glipizide, highlighting the increased capacity of metformin to minimize oxidative stress compared to glipizide.
Further, recent studies confirmed the valuable role of metformin in controlling oxidative stress [23,58]. Moreover, the effect of metformin on oxidative stress was investigated in a study by Esteghamati et al. [58], demonstrating that metformin is more effective than lifestyle change alone in reducing oxidative stress in patients with type II DM. Similarly, a study by Chakraborty et al. [23] with a larger sample size of diabetic patients randomly assigned to metformin or a placebo, showed that treatment with metformin restored antioxidant status in patients with type II diabetes and suggested a cardioprotective role for metformin. This conclusion was later supported by SPREAD-DIMCAD investigators, who reported that treatment with metformin for three years substantially reduced major cardiovascular events compared to therapy with glipizide over a median follow-up time of 5 years [17].
Our findings suggest that glipizide is more effective at treating dyslipidemia than metformin, which appears to contradict earlier clinical studies. A very preliminary investigation conducted by Zhang et al. [59] attempted to confirm the potential benefits of metformin over glipizide, as previously established in the SPREAD-DIMCAD study. In a 3-year study, Zhang and his group evaluated lipid profiles in serum samples obtained from diabetic patients treated with either metformin or glipizide. They confirmed that metformin was non-inferior to glipizide in lowering atherogenic lipids. In contrast, the present study showed that glipizide exerted a more powerful effect than metformin against atherogenic dyslipidemia, with markedly lower LDL levels in diabetic rats treated with glipizide compared to those treated with metformin. Likewise, we only observed a significant increase in HDL following treatment with glipizide, and not after treatment with metformin.
Similarly, other studies have demonstrated similar patterns of changes in serum lipid profiles due to glipizide treatment [60,61]. These results explain the mechanism of type 2 diabetes dyslipidemias, which was defined based on insulin resistance that distorts lipoprotein lipase to hepatic lipase ratio, resulting in decreased levels of HDL-cholesterol. The decline of HDL cholesterol esters is mainly due to increased cholesterol ester transfer protein (CETP) activity that eventually results in lower HDL cholesterol [10]. The mechanism for increasing triglyceride levels in hyperglycemic individuals also includes decreasing lipoprotein lipase activity (LPL). LPL activity has been lower in Type II DM patients [62]. However, apart from the study by Zhang et al. [59], there are few studies regarding the effects of glipizide and metformin on serum lipid profiles. Future investigations should further explore this topic.
The kidneys play a vital role in the excretion of waste products and toxins such as urea, creatinine and uric acid. Assessment of renal function is essential in managing patients with kidney disease, pathologies affecting renal function, hypertension and DM [63]. Our results indicated that the level of creatine in all treatment groups was similar, indicating no change owing to STZ-induced DM. Urea levels were also reduced in all treatment groups compared to the untreated diabetic rats and healthy controls.
In addition, it is worth mentioning that a recent study conducted by Alsharidah et al. (2018) [26] failed to demonstrate the therapeutic benefits of a combined metformin/gliclazide treatment on oxidative stress, lipid profiles and hepatorenal function in comparison to metformin monotherapy. Furthermore, glycaemic control was poor in diabetic patients undergoing the combined therapy. In contrast, metformin and glipizide successfully exerted these therapeutic benefits in STZ-induced diabetic rats. The contradictions between Alsharidah et al. (2018) [26] and the present study are exciting, as the individual therapy was superior in STZ-induced diabetic rats than in diabetic patients. Further research is necessary to explain the physiological variations in responses to metformin and glipizide and to explore the likely confounders that could augment or weaken the therapeutic potential of these drugs.
5. Conclusions
The therapeutic benefits of metformin and glipizide seemed to be complementary in the rats’ model: metformin was superior in improving glycaemic control and mitigating oxidative stress, while glipizide exhibited a more powerful effect against atherogenic dyslipidemia. The combination of both drugs showed the most potent products in glycemic, lipid profile and oxidative stress. The complementary nature of the effects of metformin and glipizide explains the growing evidence for the synergistic effects of these two drugs. Further research is needed to extend these conclusions to human patients.
Author Contributions
Conceptualization, A.-M.H.A.-M., O.A.R., S.Y.E., M.Z.E.-R. and M.A.; Data curation, M.F.L., G.S., S.Y.E., M.Z.E.-R. and A.A.H.A.; Formal analysis, A.-M.H.A.-M., M.F.L., A.S.A., O.A.R. and M.A.; Investigation, W.F., M.Z.E.-R., A.A.H.A. and M.A.; Methodology, A.-M.H.A.-M., M.F.L., A.S.A. and G.S.; Project administration, S.Y.E., M.Z.E.-R., M.A.; Resources, M.F.L., A.S.A., W.F. and M.A.; Software, A.A.H.A.; Supervision, A.S.A. and M.A.; Validation, A.-M.H.A.-M., A.A.H.A. and M.A.; Visualization, M.F.L., S.Y.E., M.Z.E.-R., G.S. and K.M.M.; Writing—original draft, W.F., A.A.H.A., S.Y.E., M.Z.E.-R. and M.A.; Writing—review & editing, A.A.H.A., S.Y.E., M.Z.E.-R., O.A.R., M.A. and K.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funding was supported for this study.
Institutional Review Board Statement
The Health Research Ethics, Deanship of Scientific Research, Qassim University (Approval No: 18-02-07), following the National Research Council (US) Guide for the Care and Use of Laboratory Animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The researcher(s) would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kitabchi, A.E.; Umpierrez, G.E.; Miles, J.M.; Fisher, J.N. Hyperglycemic Crises in Adult Patients with Diabetes. Diabetes Care 2009, 32, 1335–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grindel, A.; Guggenberger, B.; Eichberger, L.; Pöppelmeyer, C.; Gschaider, M.; Tosevska, A.; Mare, G.; Briskey, D.; Brath, H.; Wagner, K.-H. Oxidative Stress, DNA Damage and DNA Repair in Female Patients with Diabetes Mellitus Type 2. PLoS ONE 2016, 11, e0162082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groeneveld, O.N.; Kappelle, L.J.; Biessels, G.J. Potentials of incretin-based therapies in dementia and stroke in type 2 diabetes mellitus. J. Diabetes Investig. 2016, 7, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.H.L. Hormones; Elsevier Science Publishing Company: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Li, Z.; Cheng, Y.; Wang, D.; Chen, H.; Chen, H.; Ming, W.-K.; Wang, Z. Incidence Rate of Type 2 Diabetes Mellitus after Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of 170,139 Women. J. Diabetes Res. 2020, 2020, 3076463. [Google Scholar] [CrossRef]
- Birnboim, H.C. DNA strand breaks in human leukocytes induced by superoxide anion, hydrogen peroxide and tumor promoters are repaired slowly compared to breaks induced by ionizing radiation. Carcinogenesis 1986, 7, 1511–1517. [Google Scholar] [CrossRef]
- Hueper, K.; Hartung, D.; Gutberlet, M.; Gueler, F.; Sann, H.; Husen, B.; Wacker, F.; Reiche, D. Assessment of impaired vascular reactivity in a rat model of diabetic nephropathy: Effect of nitric oxide synthesis inhibition on intrarenal diffusion and oxygenation measured by magnetic resonance imaging. Am. J. Physiol. Physiol. 2013, 305, F1428–F1435. [Google Scholar] [CrossRef] [Green Version]
- Small, D.M.; Morais, C.; Coombes, J.S.; Bennett, N.C.; Johnson, D.W.; Gobe, G.C. Oxidative stress-induced alterations in PPAR-gamma and associated mitochondrial destabilization contribute to kidney cell apoptosis. Am. J. Physiol. Renal. Physiol. 2014, 307, F814–F822. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef]
- Mooradian, A.D. Dyslipidemia in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 150–159. [Google Scholar] [CrossRef]
- Yuan, C.; Lai, C.W.K.; Chan, L.W.C.; Chow, M.; Law, H.K.W.; Ying, M. Cumulative Effects of Hypertension, Dyslipidemia, and Chronic Kidney Disease on Carotid Atherosclerosis in Chinese Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2014, 2014, 179686. [Google Scholar] [CrossRef]
- Jani, Y.; Kamberi, A.; Ferati, F.; Rexhepi, A.; Pocesta, B.; Orovcanec, N.; Lala, D.; Polisi, G.; Iseni, M.; Mirto, A.; et al. Influence of dyslipidemia in control of arterial hypertension among type-2 diabetics in the western region of the Republic of Macedonia. Am. J. Cardiovasc. Dis. 2014, 4, 58–69. [Google Scholar] [PubMed]
- Farmer, J.A. Diabetic dyslipidemia and atherosclerosis: Evidence from clinical trials. Curr. Atheroscler. Rep. 2007, 9, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Indian J. Endocrinol. Metab 2012, 16 (Suppl. S2), S267–S271. [Google Scholar]
- Laight, D.; Carrier, M.; Änggård, E. Antioxidants, diabetes and endothelial dysfunction. Cardiovasc. Res. 2000, 47, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Diwan, V.; Gobe, G.; Brown, L. Glibenclamide improves kidney and heart structure and function in the adenine-diet model of chronic kidney disease. Pharmacol. Res. 2014, 79, 104–110. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, Y.; Lai, S.; Lv, A.; Su, Q.; Dong, Y.; Zhou, Z.; Tang, W.; Zhao, J.; Cui, L.; et al. Effects of Metformin Versus Glipizide on Cardiovascular Outcomes in Patients with Type 2 Diabetes and Coronary Artery Disease. Diabetes Care 2013, 36, 1304–1311. [Google Scholar] [CrossRef] [Green Version]
- Fararh, K.M.; Ibrahim, A.K.; Elsonosy, Y.A. Thymoquinone enhances the activities of enzymes related to energy metabolism in peripheral leukocytes of diabetic rats. Res. Vet. Sci. 2010, 88, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Drury, P.L.; Ting, R.; Zannino, D.; Ehnholm, C.; Flack, J.R.; Whiting, M.J.; Fassett, R.G.; Ansquer, J.-C.; Dixon, P.H.; E Davis, T.M.; et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 2011, 54, 32–43. [Google Scholar] [CrossRef]
- Tsai, S.-F.; Chen, C.-H. Management of Diabetes Mellitus in Normal Renal Function, Renal Dysfunction and Renal Transplant Recipients, Focusing on Glucagon-Like Peptide-1 Agonist: A Review Based upon Current Evidence. Int. J. Mol. Sci. 2019, 20, 3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmer, A.J.; Stevens, R.; Hirst, J.; Lung, T.; Oke, J.; Clarke, P.; Glasziou, P.; Neil, A.; Dunger, P.D.; Colhoun, H.; et al. Optimal strategies for identifying kidney disease in diabetes: Properties of screening tests, progression of renal dysfunction and impact of treatment—Systematic review and modelling of progression and cost-effectiveness. Heal. Technol. Assess. 2014, 18, 1–128. [Google Scholar] [CrossRef] [Green Version]
- de Boer, I.H.; Rue, T.C.; Cleary, P.A.; Lachin, J.M.; Molitch, M.E.; Steffes, M.W.; Sun, W.; Zinman, B.; Brunzell, J.D.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: An analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch. Intern. Med. 2011, 171, 412–420. [Google Scholar]
- Chakraborty, A.; Chowdhury, S.; Bhattacharyya, M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res. Clin. Pr. 2011, 93, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Shin, J.-A.; Lee, S.-H.; Kim, E.-S.; Cho, J.-H.; Son, H.-Y.; Yoon, K.-H. A Comparative Study of the Effects of a Dipeptidyl Peptidase-IV Inhibitor and Sulfonylurea on Glucose Variability in Patients with Type 2 Diabetes with Inadequate Glycemic Control on Metformin. Diabetes Technol. Ther. 2013, 15, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Al-Gareeb, A.I.; Alrubai, H.F.; Suliaman, S.M. Effects of gliclazide add on metformin on serum omentin-1 levels in patients with type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2016, 20, 195–198. [Google Scholar] [CrossRef]
- Alsharidah, M.; Algeffari, M.; Abdel-Moneim, A.-M.H.; Lutfi, M.F.; Alshelowi, H. Effect of combined gliclazide/metformin treatment on oxidative stress, lipid profile, and hepatorenal functions in type 2 diabetic patients. Saudi Pharm. J. 2018, 26, 1–6. [Google Scholar] [CrossRef]
- Sutter, J. Maget M-Guide d’étude directe des comportements culturels. Population 1953, 8, 805. [Google Scholar]
- Futfi, M.F.; Abdel-Moneim, A.-M.; Alsharidah, A.; Mobark, M.; Abdellatif, A.; Saleem, I.; Al Rugaie, O.; Mohany, K.; Alsharidah, M. Thymoquinone Lowers Blood Glucose and Reduces Oxidative Stress in a Rat Model of Diabetes. Molecules 2021, 26, 2348. [Google Scholar]
- Alsharidah, M.; Abdel-Moneim, A.-M.; Alsharidah, A.; Mobark, M.; Rahmani, A.; Shata, A.; Abdellatif, A.; El-Readi, M.; Mohany, K.; Al Rugaie, O. Thymoquinone, but Not Metformin, Protects against Gentamicin-Induced Nephrotoxicity and Renal Dysfunction in Rats. Appl. Sci. 2021, 11, 3981. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Molehin, O.R.; Oloyede, O.I.; Adefegha, S.A. Streptozotocin-induced diabetes in rats: Effects of White Butterfly (Clerodendrum volubile) leaves on blood glucose levels, lipid profile and antioxidant status. Toxicol. Mech. Methods 2018, 28, 573–586. [Google Scholar] [CrossRef]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillaire-Buys, D.; Novelli, M.; Ribes, G. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Tochigi, M.; Moriwaki, A.; Takeuchi, S.; Takahashi, S. Insulin-like Growth Factor 1 mRNA Expression in the Uterus of Streptozotocin-treated Diabetic Mice. J. Reprod. Dev. 2013, 59, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Uchino, H.; Shimizu, T.; Yoshii, H.; Niwa, M.; Ohmura, C.; Mitsuhashi, N.; Onuma, T.; Kawamori, R. Effect of metformin on advanced glycation endproduct formation and peripheral nerve function in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 1999, 376, 17–22. [Google Scholar] [CrossRef]
- Dagchi, C.; Das, S.; Mitra, A.; De Pati, A.; Tripathi, S.K.; Datta, A. Antidiabetic and antihyperlipidemic activity of hydroalcoholic extract of Withania coagulans Dunal dried fruit in experimental rat models. J. Ayurveda Integr. Med. 2013, 4, 99–106. [Google Scholar]
- Riley, V. Adaptation of Orbital Bleeding Technic to Rapid Serial Blood Studies. Exp. Biol. Med. 1960, 104, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Bunn, H.F.; Haney, D.N.; Kamin, S.; Gabbay, K.H.; Gallop, P.M. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J. Clin. Investig. 1976, 57, 1652–1659. [Google Scholar] [CrossRef] [Green Version]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Koracevic, D.; Harris, G.; Rayner, A.; Blair, J.; Watt, B. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Warnick, G.R.; Albers, J.J. A comprehensive evaluation of the heparin–manganese precipitation procedure for estimating high density lipoprotein cholesterol. J. Lipid Res. 1978, 19, 65–76. [Google Scholar] [CrossRef]
- Nauck, M.; Warnick, G.R.; Rifai, N. Methods for measurement of LDL-cholesterol: A critical assessment of direct measurement by homogeneous assays versus calculation. Clin. Chem. 2002, 48, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Crook, M.A. Plasma Lipids and Lipoproteins; Medicine, C.C.a.M., Ed.; Edwarld Arnold publishers Ltd.: London, UK, 2006. [Google Scholar]
- Dobiasova, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Fabiny, D.L.; Ertingshausen, G. Automated Reaction-Rate Method for Determination of Serum Creatinine with the CentrifiChem. Clin. Chem. 1971, 17, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Tabacco, A.; Meiattini, F.; Moda, E.; Tarli, P. Simplified enzymic/colorimetric serum urea nitrogen determination. Clin. Chem. 1979, 25, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Gili-Ahmadabadi, A.; Ghobadi, S.; Dastan, D.; Soleimani, M. Hepatoprotective potential and antioxidant activity of Allium tripedale in acetaminophen-induced oxidative damage. Res. Pharm. Sci. 2019, 14, 488–495. [Google Scholar] [CrossRef]
- Valente, T.; Arbex, A.K. Glycemic Variability, Oxidative Stress, and Impact on Complications Related to Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2021, 17, e071620183816. [Google Scholar] [CrossRef] [PubMed]
- França, C.N.; Mendes, C.; Ferreira, C. Time collection and storage conditions of lipid profile. Braz. J. Med. Biol. Res. 2018, 51, e6955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erem, C.İ.; Ozbas, H.M.; Nuhoglu, I.; Deger, O.R.; Civan, N.A.; Ersoz, H.O. Comparison of effects of gliclazide, metformin and pioglitazone monotherapies on glycemic control and cardiovascular risk factors in patients with newly diagnosed uncontrolled type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes. 2014, 122, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.; Hossain, M.S.; Bhatta, R.; Akter, M. Attenuation of lipid peroxidation and atherogenic factors in diabetic patients treated with gliclazide and metformin. J. Res. Med. Sci. 2018, 23, 77. [Google Scholar] [CrossRef]
- Hassan, M.; Abd-Allah, G.M. Effects of metformin plus gliclazide versus metformin plus glimepiride on cardiovascular risk factors in patients with type 2 diabetes mellitus. Pak. J. Pharm. Sci. 2015, 28, 1723–1730. [Google Scholar] [PubMed]
- Tessier, D.; Maheux, P.; Khalil, A.; Fülöp, T. Effects of gliclazide versus metformin on the clinical profile and lipid peroxidation markers in type 2 diabetes. Metabolism 1999, 48, 897–903. [Google Scholar] [CrossRef]
- Agarwal, R. Anti-inflammatory effects of short-term pioglitazone therapy in men with advanced diabetic nephropathy. Am. J. Physiol. Physiol. 2006, 290, F600–F605. [Google Scholar] [CrossRef] [PubMed]
- Esteghamati, A.; Eskandari, D.; Mirmiranpour, H.; Noshad, S.; Mousavizadeh, K.; Hedayati, M.; Nakhjavani, M. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: A randomized clinical trial. Clin. Nutr. 2013, 32, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, C.; Hong, J.; Zeng, J.; Lai, S.; Lv, A.; Su, Q.; Dong, Y.; Zhou, Z.; Tang, W.; et al. Lipid Profiling Reveals Different Therapeutic Effects of Metformin and Glipizide in Patients With Type 2 Diabetes and Coronary Artery Disease. Diabetes Care 2014, 37, 2804–2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-H.; Li, X.-M.; Han, C.-C.; Fang, X.-F.; Ma, L. Effects of combined therapy with glipizide and Aralia root bark extract on glycemic control and lipid profiles in patients with type 2 diabetes mellitus. J. Sci. Food Agric. 2015, 95, 739–744. [Google Scholar] [CrossRef]
- Rosenstock, J.; Wilson, C.; Fleck, P. Alogliptin versus glipizide monotherapy in elderly type 2 diabetes mellitus patients with mild hyperglycaemia: A prospective, double-blind, randomized, 1-year study. Diabetes Obes. Metab. 2013, 15, 906–914. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Nikkilä, E.A. Lipoprotein lipase of adipose tissue and skeletal muscle in human obesity: Response to glucose and to semistarvation. Metabolism 1981, 30, 810–817. [Google Scholar] [CrossRef]
- Okoro, R.N.; Farate, V.T. The use of nephrotoxic drugs in patients with chronic kidney disease. Int. J. Clin. Pharm. 2019, 41, 767–775. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).