Abstract
To improve the mechanical strength and oil-loading performances of egg white protein (EWP) aerogel, the effects of different grafting degrees on the modification of EWP by sodium carboxymethylcellulose (CMC-Na) were investigated. After different dry-heat treatment durations (0, 12, 24, 36, and 48 h), the EWP/CMC-Na conjugates with different grafting degrees (noted as EC0, EC12, EC24, EC36, and EC48, respectively) were obtained. Subsequently, the physicochemical properties of the conjugates, as well as the microstructure, mechanical properties, pore parameters, emulsification properties and oil-carrying properties of the conjugated aerogels, were characterized. The results showed that EC12 (with a grafting degree of 8.35%) aerogel possessed a uniform structure, the largest specific surface area, and the best emulsification performance. This facilitated a more robust aerogel (2.05 MPa) with nearly three times the mechanical strength of EWP aerogel. Moreover, this had a positive influence on the efficient loading and stable retention of oil. EC12 aerogel thus achieved an oil absorption capacity of 5.46 g/g aerogel and an oil holding capacity of 31.95%, and both values were nearly 1.7 times higher than those of EWP aerogel. In general, the EWP-based aerogel with a grafting degree of 8.35% had the best mechanical and oil-loading properties.
1. Introduction
Aerogel is a traditional material with excellent structural properties such as a high porosity and large specific surface area, and is mainly used in the fields of biomedicine [,,,], food [,,], etc. At present, a variety of precursor materials, including some traditional materials such as silicon and its oxides [,], metals and their oxides [,], and organic synthetics such as phenolic resins [], are used to prepare aerogels. Although these materials exhibit high application values in some fields, they are not in line with today’s green and sustainable development strategies because of serious pollution generated during the preparation process. Interestingly, the development of innovative precursor materials from renewable and abundant biological resources is becoming a key aspect of aerogel preparation because these materials show high molecular designability and cause less environmental pollution.
Increasing attention has been drawn to a variety of biopolymers, mainly including polysaccharides and proteins. Food-grade polysaccharides, such as starch, chitosan, pectin, and cellulose, have already been employed for the preparation of aerogels [,,,,]. Although there have been many studies on polysaccharide-based aerogels, with quite remarkable results, investigations have focused on protein-based precursor materials in recent years. The unique gel properties of proteins and the greater number of functional groups they carry on their molecules (e.g., thiol, hydroxy, amine, amide, and carboxyl groups) endow aerogels with the potential for extended chemical functionalities and easier functionally oriented customization. Certain proteins, including whey and silk protein, have been used as aerogel precursors in related fields [,]. Egg white protein (EWP), an important component of poultry egg protein, is also known for its excellent gelation properties. Its consistency and texture can especially be improved by gelation processes, which also provide corresponding nutrition and flavor benefits []. To further expand the application of EWP in different fields and to enhance its added value, many efforts have been made regarding gels and aerogels [,,]. In recent years, oleogelation has been widely used as a novel and efficient strategy to study the structuring of liquid oils and fats. Oleogels are considered to be semi-solid systems that consist of a network structure formed by a polymer with a hydrophobic liquid (e.g., oil). Thus far, several techniques have been developed for the preparation of oleogels. On the one hand, gelators, including natural waxes and cellulose derivatives [,], have been used to self-assemble into continuous three-dimensional networks and to immobilize liquid oils and fats by means of their surface tension and capillary forces. However, they mostly have a “waxy” texture and some gelators such as 12-hydroxystearic acid are not allowed to be used in food. On the other hand, the techniques of indirect oleogelation using emulsions and hydrogels as templates have been exploited [,], whereas further high-temperature drying or solvent exchange treatments limit their far-reaching development.
However, the single EWP gel network structure and its texture do not meet current demands for the formation of protein-based aerogels. For desirable oil-loading matrices, EWP aerogels are required to achieve high mechanical properties and loading efficiency to broaden their applicability [,]. Therefore, improving the mechanical performance and loading efficiency of EWP aerogels are urgent problems to be solved. Excitingly, considerable efforts have been made to improve the textural and functional properties of protein-based aerogels, mainly by changing the hydrogel conditions such as protein concentration [], pH [,], and ionic strength [,], and also by introducing chemical cross-linking agents [,,]. Nevertheless, simply changing the gel conditions is not sufficient to address the current demand for protein-based aerogels in actual production processes. Certainly, a better aerogel structure can be achieved by introducing chemical cross-linking agents, although we cannot deny that the presence of these cross-linking agents is usually a great threat to people’s health [,]. Recently, researchers have investigated the combinations of different proteins, such as whey [,], soy [,], and collagen [], with various polysaccharides to improve the functional and mechanical properties of aerogels. Sodium carboxymethylcellulose (CMC-Na) is a food additive which contains COO−, an anionic polymer, which enables electrostatic interactions with some proteins in a positively charged state and contributes to a certain thickening and gelation effect. Moreover, cross-linking between CMC-Na and proteins could be enhanced by the dry/wet-heat glycosylation process, and the proteins would be activated to improve their emulsification properties []. This may be a positive effect on the mechanical strength of protein-based aerogels and the loading of active substances. However, limited work has been carried out on the Maillard reaction with polysaccharides to improve the functional properties of EWP aerogels.
In this study, EWP-based aerogels were prepared from the heat-set gels of EWP/CMC-Na conjugates with different grafting degrees via a freeze-drying process. The effects of grafting degrees on the characteristics of conjugated aerogels, such as emulsion properties, mechanical strength, porous parameters, morphology, and oil adsorption/holding capacity were investigated. This research demonstrated a positive influence on EWP-based aerogels as a carrier matrix for hydrophobic substances and facilitated an expansion of their applications in food and pharmaceuticals, such as the embedding and transport of fat-soluble nutrients and drugs.
2. Materials and Methods
2.1. Materials
Egg white protein (EWP) powder was obtained from Kang De Egg Industry Co., LTD (Jiangsu, China). Vegetable blend oil (soybean oil, 59.0%; canola oil, 23.5%; sunflower seed oil, 10.0%; other oil, 7.5%) was purchased from the local supermarket. Sodium carboxymethylcellulose (CMC-Na), glucose oxidase (10 kU/30.5 mg), and potassium bromide were obtained from Solarbio Co., LTD (Beijing, China). Unless additionally described, the reagents used in this study were of analytical grade.
2.2. Fabrication of EWP/CMC-Na Conjugates
A 15% (w/w) EWP solution was prepared, magnetically stirred for 1 h, and then placed in a refrigerator at 4 °C overnight. After centrifugation (5000× g, 20 min),the supernatant was filtered through gauze to obtain the stock protein solution. Then, the concentration of the protein solution was adjusted to 10% (w/w, measured by the Kjeldahl method) with ultrapure water; subsequently, the pH of the protein solution was adjusted to 7.0 with 1 M HCl solution. Glucose oxidase (GOD) was added to the diluted EWP solution at a ratio of 100 mg/kg and heated in a water bath at 30 °C for 2 h. During the heating process, a 0.35% (w/w) mass fraction of H2O2 solution (7%, w/w)was added to the EWP solution per hour. After the sugar had been removed, the solution was prepared to contain CMC-Na at a mass fraction of 0.25% and then freeze-dried to obtain the composite EWP powder sample. The lyophilized samples were placed in a desiccator at a relative humidity of 79% (with saturated KBr solution at the bottom) and heated at 60 °C for 0, 12, 24, 36, and 48 h. The resulting samples were noted as EC0, EC12, EC24, EC36, and EC48, respectively.
2.3. Aerogel Preparation
EWP and graft powders were dissolved in ultrapure water at a ratio of 1:9 (w/w). Then, the solution was filled into a 10 mL syringe (with the inside of the syringe coated with a small amount of paraffin oil) and heated at 85 °C for 30 min to form a thermally induced EWP-based gel []. Immediately after heating, the syringes were cooled in ice water for 5 min and subsequently placed in a 4 °C refrigerator overnight. The gels were cut into cylinders of 15 mm in diameter and 8 mm in height using a slicer and then placed in a lyophilizer (SCIENTZ-10N, Ningbo Xinzhi Biotechnology Co., Ningbo, China) under vacuum (<3 Pa) for 48 h after freezing at −80 °C for over 24 h.
2.4. Grafting Degree (DG) of the Conjugates
The free amino acid content of the conjugates was determined following the O-phthalaldehyde (OPA) approach described by Rao et al. [] with slight modifications: 200 μL of the sample was blended with 4 mL of OPA solution and ultrapure water was employed as a control, and the absorbance value was determined at 340 nm. The DG values of the original EWP and the grafts were calculated by the following equation
where A0 and Ac represent the free amino contents of the original EWP and the grafts, respectively.
DG (%) = (A0 − Ac)/A0
2.5. Determination of Absorbance for the Maillard Reaction Products
The conjugates were diluted to a concentration of 5 mg/mL with ultrapure water to obtain an optical density of less than 1.5. Then, the absorbance was measured at 420 nm using a visible spectrophotometer (V-5600, Yuanxi Instruments Co., Shanghai, China) based on the reported method of Tan et al. [] with slight modifications.
2.6. Color Difference Analysis
The L*, a*, and b* values of the samples were measured according to the method of Cai et al. [] with a colorimeter instrument (ColorQuest XE, HunterLab, Reston, VA, USA). The sample was loaded in a transparent quartz cuvette after the calibration of a high-precision spectrophotometer with a standard black and white background plate. Three points on the cuvette were taken for each sample, and the average value of each sample was obtained. L*: luminance value (0−100 value ranged from black to white); a*: red (+) or green (−); b*: yellow (+) or blue (−).
2.7. Determination by Fourier Transform Infrared Spectroscopy (FTIR)
FTIR analysis of the conjugates was performed following the method described by Tan et al. []. In summary, the samples were thoroughly lyophilized, then mixed with KBr at a ratio of approximately 1:100 and well ground to ensure a uniform distribution. Homogeneous transparent flakes were prepared using a tablet press held at a pressure of 30 MPa for 30 s, and scanning was performed with the spectrometer (Nicolet 380, Nicolet Co., Madison, WI, USA) in the wavelength range of 4000–400 cm−1, with 32 scans at a resolution of 4 cm−1.
2.8. Microstructure of Aerogels
Microstructural observation of the aerogels was performed following the method described by Ahmadi et al. with slight modifications []. The aerogel samples were divided into pea-sized particles to allow for better observation of the internal microstructure through the fracture surface. These particles were adhered to a metal sheet with conductive tape and sprayed with gold. The surface morphology of the samples was visualized using a HITACHIS-3500N scanning electron microscope (SEM, Hitachi, Mito, Japan). The accelerating voltage of the instrument was 26 kV.
2.9. Mechanical Strength of Aerogels
The textural strength of the aerogels was measured using a physical property analyzer (TA.XT Plus, Stable Micro Systems Co., Surrey, UK) based on the following parameters: P50 probe type, pre-test speed, 1 mm/s, mid-test speed, 1 mm/s, post-test speed, 2 mm/s, and 75% strain of the original sample height. The slope of the strain ranged from 8% to 15%, and the linear region on the stress–strain curve was calculated and recorded as the Young’s modulus of the aerogels []. The Young’s modulus was calculated with the formula below
where E, σ, and γ represent the Young’s modulus, stress (maximum force on the unit area in a linear stage of stress–strain curve), and strain (changes of height to initial height), respectively.
E = σ/γ
2.10. Analysis of Specific Surface Area and Pore Parameters for Aerogels
The specific surface area and pore parameters of aerogels were determined according to the method reported by Kleemann et al. [] with slight modifications. The adsorption–desorption isotherms of EWP-based aerogels were measured and obtained with a specific surface area and porosity analyzer (JW-BK132F, Jing Wei Gao Bo Co., Beijing, China), using nitrogen as the adsorption medium. Moreover, the specific surface area of the samples was calculated by the Brunauer–Emmett–Teller (BET) theory, and the pore size distribution of mesopores (2–50 nm) was obtained by the Barrett–Joyner–Halenda (BJH) theory, resulting in the calculation of the average pore size and total pore volume of the samples. All samples were dried under vacuum at 50 °C for 24 h before the measurement. These calculations were performed using the software ASAP2020 to process and analyze the graphs, as well as the data, accordingly.
2.11. Emulsifying Properties of Aerogels
The emulsification activity and emulsion stability were measured following the approach described by Chen et al. [] with minor modifications. In brief, 5 mL of oil was mixed with 20 mL of 0.1% (w/v) sample solution and then homogenized for 3 min at 10,000 rpm using a homogenizer (Ultra-Turrax T25, IKA Co., Staufen, Germany). Then, it was diluted with 9.9 mL of sodium dodecyl sulfate (SDS) solution (0.1%, w/v) for 100 µL of the emulsion. Emulsification activity (EA) and emulsion stability (ES) were obtained using the formula below
where A0 and A15 represent the absorbances of the emulsion measured at 500 nm after 0 and 15 min, respectively, c is the concentration of protein (g/mL), DF represents the dilution factor (DF = 100), ϕ is the optical path width (ϕ = 1 cm), θ is the proportion of oil in the emulsion (θ = 0.2), and Δt is the interval between two sampling times (Δt =15 min).
EA (m2/g) = (2 × 2.303 × A0 × DF)/(c⋅ϕ⋅θ × 104)
ES (min) = (A0⋅Δt)/(A0 − A15)
2.12. Oil Absorption/Holding Capacity of the Aerogels
The aerogels were precisely weighed and subsequently dipped into the soybean oil. The absorption process achieved a balance, and oleogels were formed during the 6 h of dipping. As soon as the absorption procedure was completed, the oleogels were collected and the oil was sucked out of the surface using filter paper. The oleogels were instantly weighed. The oil absorption capacity (OAC) of the aerogel templates was determined by the formula below []
where M0 represents the original weight of the aerogel and M represents the weight of the prepared oleogel.
OAC (g/g aerogel) = (M − M0)/M0 × 100
The oil holding capacity (OHC) of oleogels prepared from saturated aerogels containing oil, was determined using the approach of Manzocco et al. [] with some modifications. About 0.1 g of the oleogel sample was wrapped in filter paper, placed in a 10 mL centrifuge tube, and then centrifuged at 10,000 rpm for 30 min. All measurements were performed in triplicate. The OHC was calculated according to the following equation
where M0 represents the total weight of oil before centrifugation, and M1 represents the weight of oil that remained in the oleogel after centrifugation.
OHC (%) = M1/M0 × 100
2.13. Statistical Analysis
All measurements were taken in triplicate. Data are reported as the mean ± standard deviation. Statistical analyses of the results were performed using SPSS 25 (SPSS Corp., Chicago, IL, USA) software. One-way analysis of variance (ANOVA) was employed in the analysis of data, and Duncan’s multiple range test was applied to identify differences between means.
3. Results and Discussion
3.1. Effects of the Grafting Degrees on the Physicochemical Properties of EWP
3.1.1. Basic Characteristics of the Conjugates
We used the dry-heat Maillard reaction method at 60 °C for 0–48 h to obtain EWP with different grafting degrees. The degree of grafting determined using the OPA method could characterize the progression of the preliminary stage of the Maillard reaction, where an increase in DG represents a decrease in free amino groups. As shown in Table 1, compared with the original EWP, the DG value of the graft increased significantly with the increasing incubation time, indicating that the sugar chains were covalently cross-linked with the protein molecules as the grafting reaction proceeded.

Table 1.
The grafting degree (DG), color differences, and absorbance at 420 nm (A420) of EWP and its conjugates.
The absorbance values of the conjugates at 420 nm are closely related to the magnitude of their grafting degree, which can also indirectly reflect the grafting degree of the grafts. The final stage of the Maillard reaction is considerably more complex, where many kinds of brown polymers are formed that can be used to indicate the browning reaction. The formation of brown products in later stages of the Maillard reaction can be indicated using absorbance at 420 nm (A420). The results of the absorbance of the grafts at 420 nm as a function of dry-heat time are shown in Table 1. A420 also gradually increased with the increase in the grafting degrees, indicating the gradual transition to the final stage of the Maillard reaction with the generation of browning.
The Maillard reaction, also known as a non-enzymatic browning reaction, is a reaction between carbonyl compounds and amino compounds, resulting in brown or even black macromolecules. Hence, the presence of a reddish-brown color was a strong indicator of the progress of the Maillard reaction and the grafting degree of the grafts []. We thus observed the apparent color variation in the grafts with different grafting degrees. As shown in Table 1, the L* of EWP was 90.35, a* was −0.08, and b* was 14.04, indicating a high whiteness, as well as a yellow and slight green color. The color of EC0 was not significantly different from that of the original EWP. Moreover, the color of the EWP did not change significantly after the dry-heat treatment because it contained almost no reducing sugars, whereas the color of the EWP with CMC-Na changed significantly. With the increase in the grafting degree, the L* value gradually decreased, the a* value gradually increased from negative to positive, and the b* value (positive) gradually became larger, which indicated that with the increase in grafting degree, the red and yellow color of EWP gradually deepened, leading to the overall darker color. Furthermore, this fact revealed that the higher the grafting degree, the more pronounced the color change, which was in agreement with the findings of A420 in Table 1.
3.1.2. The Grafting Degrees Changed the Structural Properties of EWP
To further investigate the effects of the grafting degrees on the structure of EWP and the conjugate, samples were analyzed with FTIR spectroscopy. Figure 1 shows the FTIR spectra of the analyzed samples. The hydroxyl stretching band was represented by 3306 cm−1, which was one of the characteristic structures of the sugar chains. Compared with EWP, EC0 and the conjugated samples displayed higher absorption peaks here, indicating that the introduction of CMC-Na increased the hydroxyl content of EWP. The most distinctive spectral features of the protein are the amide I and II bands, which are distributed at approximately 1650 cm−1 and 1540 cm−1, respectively. When the protein was grafted by the grafting reaction, the incorporation of polysaccharides could increase the hydroxyl content of the protein, which was also accompanied by the consumption of amino acid residues during the Maillard reaction. That altered the protein structure and tended to follow with changes in its functional properties, such as gel properties, emulsification properties, etc. Meanwhile, these changes were reflected in the spectrum as the migration, appearance, or disappearance of the bands []. The absorption peaks of the EWP amide I and II bands were at 1651 cm−1 and 1531 cm−1, respectively. These two bands of EC0 hardly migrated and just increased in absorption intensity, which indicated that the CMC-Na at this time did not undergo a Maillard reaction with EWP, but rather, non-covalent binding. Meanwhile, the absorption peaks of samples EC12, EC24, EC36, and EC48 at the amide II band shifted from 1531 cm−1 to 1541 cm−1 as the grafting reaction proceeded, indicating the consumption of amino acid residues during the Maillard reaction []. The amide I band at 1661 cm−1 represented the spectral overlap of C=O groups and was accompanied by in-plane –NH bending and the formation of C=N bonds []. Moreover, the absorption peak of their amide I band shifted from 1651 cm−1 to 1661 cm−1, a result that indicated the formation of Schiff bases containing C=N bonded structures []. Additionally, as the reaction time increased, we found that the intensity of the bands of the conjugates increased, which was consistent with the change in their grafting degree.
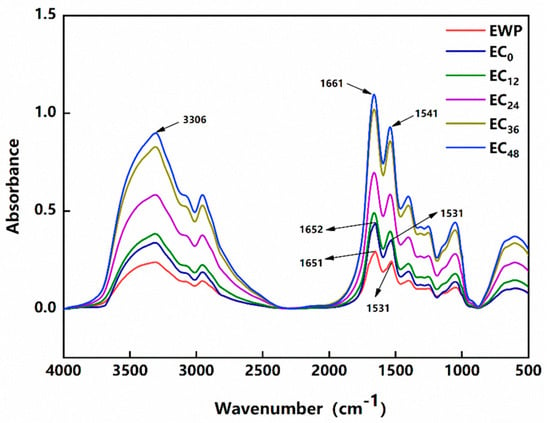
Figure 1.
FTIR spectra of EWP and EWP/CMC-Na conjugates.
3.2. Effects of Grafting Degrees on the Physicochemical Properties of EWP Aerogels
3.2.1. Effects of Grafting Degrees on the Morphology of EWP Aerogels
In Figure 2, the morphologies of the resulting aerogels possessing porous structures are shown. As seen in the figure, EWP aerogel exhibited a larger pore-size distribution and thicker pore walls, although the network structure was not uniformly distributed, which could be unfavorable as an active substance transport carrier matrix. Interestingly, due to the introduction of CMC-Na, compared with EWP aerogels, EC0 aerogels exhibited a well-developed network with significantly smaller pore size and uniform pore distribution. However, this network was not a true encapsulated network and mainly lacked a semi-encapsulated structure supported by some backbone structures []. After grafting, the EC12 aerogel was characterized by a fine network distribution, smaller pore size, tightly connected pore walls, and semi-enveloped honeycomb pore structures. It is generally acknowledged that high-porosity systems with honeycomb structures facilitate the effective immobilization of oils, drugs, and active agents, which is ideal in regard to numerous applications relating to oil absorption, antimicrobial packaging, and nutrient delivery [,,]. However, with the increase in grafting degrees, a large number of compact and non macroporous inhomogeneities, rather than uniformly distributed fine pore structures, were found in the internal network of aerogels. This was probably due to the higher degree of grafting of conjugates with more hydrophilic groups on the molecules, which would reduce the exposure of hydrophobic residues during the heating process of the protein, thus leading to the formation of a sparser structure and larger pores rather than a tight, homogeneous network structure [].
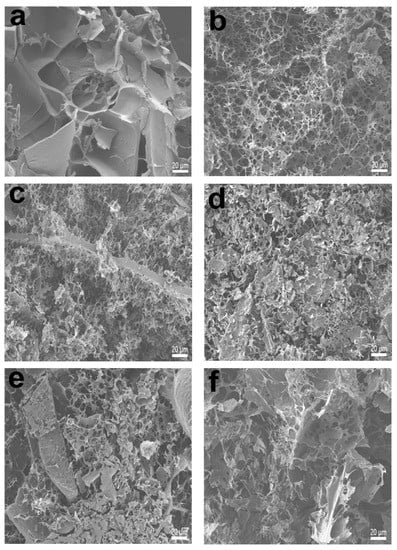
Figure 2.
Scanning electron micrographs of the aerogels. Graphs (a–f) show the microscopic morphology of the EWP, EC0, EC12, EC24, EC36, and EC48 at ×500 magnification, respectively.
3.2.2. The Mechanical Strength of EWP Aerogels Changed with Grafting Degrees
Generally, aerogels with higher mechanical strength reflect better mechanical stability and are therefore recommended for practical applications []. The typical stress–strain curves and compressive strength of the aerogels are shown in Figure 3a. In Figure 3a, it can be seen that the stress–strain curves during compression were nearly linear and fractured at about a 25% strain, indicating that these samples formed a rigid structure. The slope of the compression curve can be used to evaluate the stiffness of the aerogels: the higher the slope, the stiffer the aerogel. Therefore, the grafted aerogel samples possessed higher stiffness compared with the EWP aerogel. At the same time, with the increasing degree of grafting, a stress “plateau” appeared in the curve, indicating a stable state, which may have been due to the elimination of heterogeneous pores and cavities in the internal structure of the aerogels during the compression process []. The compressive moduli of aerogels with different grafting degrees are shown in Figure 3. Clearly, the compressive moduli of the aerogel samples were significantly increased by adding CMC-Na to the EWP with or without the grafting process, which suggested that the introduction of CMC-Na had a positive and significant effect on the mechanical strength of EWP aerogels. Moreover, the compressive moduli of all aerogel samples showed a trend of increasing and then decreasing, and peaked with the EC12 aerogel, reaching 2.05 MPa, which was more than three times the compressive modulus of the EWP aerogel and significantly higher than the EC0 aerogel (1.78 MPa). It has been shown in the literature that appropriate cross-linking reactions contribute to the enhancement of covalent interactions in the network, which, in turn, reinforces the mechanical strength of the entire aerogel network []. Meanwhile, the higher the degree of grafting, the more flexible the network structure, which result in the weaker mechanical properties. Based on that, we believe that the grafting of EWP with CMC-Na could significantly improve its mechanical properties and obtain more mechanically stable composite aerogels.
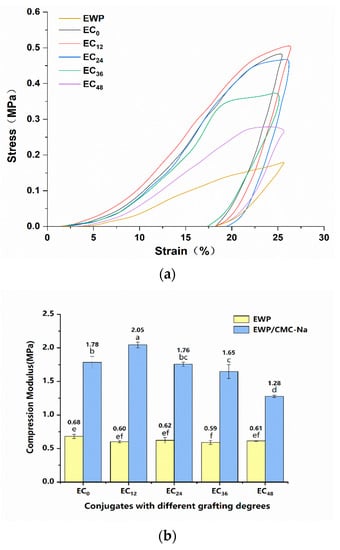
Figure 3.
Mechanical properties of the aerogels in dry conditions: (a) stress−strain curves and (b) compressive strength.
3.2.3. The Porous Properties of EWP Aerogels Changed with Grafting Degrees
The adsorption–desorption isotherms of the samples were finally plotted by first determining the adsorption efficiency of the samples exposed to the inert gas stream, and subsequently measuring the amount of gas desorbed due to heating the samples. As shown in Figure 4a, all of the isotherms belonged to type IV, following the categorization of the International Union of Pure and Applied Chemistry (IUPAC). These materials were inherently mesoporous and the hysteresis loops were a feature of the isotherms, which did correlate with the presence of capillary condensation in the mesopores [,].
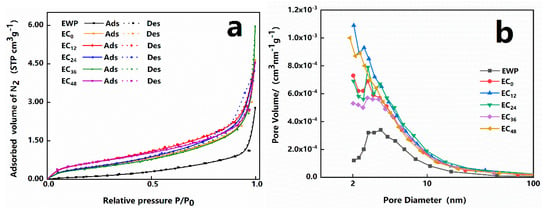
Figure 4.
Nitrogen adsorption–desorption isotherms (a) and BJH plot (b) for the aerogels with different grafting degrees.
The pore parameters of all samples are listed in Table 2. The BET specific surface areas (SBET) of EWP, EC0, EC12, EC24, EC36, and EC48 were 0.54, 1.87, 2.59, 1.96, 1.75, and 2.43 m2/g, respectively, as determined by the adsorption–desorption isotherms. In general, the fine-structured network contains a large number of smaller pores, which implies a higher specific surface area []. The graft-modified EWP aerogels network became more porous and fine, which resulted in a general trend of increasing and then decreasing the SBET of EWP aerogels with increasing grafting degree. This is also reflected in the column of DA in Table 2. Notably, the EC48 aerogel was second to the EC12 aerogel in SBET, which may be because the higher grafting degree could significantly improve the hydrophilic performance of the molecular surface of EWP and inhibit the heating aggregation behavior of EWP, which reduces the generation of water-insoluble aggregates []. Meanwhile, we found that the values of BET specific surface area (SBET) were significantly correlated with the values of average pore diameters (DA); in other words, the lower the DA values, the higher the SBET values, which was also reflected in the study by Betz et al. []. As is well known, in a porous material, a lower pore size distribution means higher porosity and, in general, a higher pore volume. However, in our research, there was no evident relationship between the values of SBET and the pore volumes (VP). As reported by Selmer et al. [], the only possible explanation would be the existence of micropores or macropores, which could not be detected in the nitrogen adsorption measurement technique used here. The corresponding distribution of pore sizes obtained by the BJH method is shown in Figure 4b. Although all samples showed a fairly broad distribution of pore sizes in the range of 2–100 nm, their peaks were concentrated between 2 and 10 nm. Moreover, we found that the microporosity of the graft-treated aerogel samples seemed to have a tendency to increase, which may be due to the increased grafting degree of the aerogel, contributing further to the cross-linking interaction []. The average mesopore diameter was calculated according to the simple formula 4 Vm/SBET, which led to values within the range of 12–36 nm.

Table 2.
Pore parameters of the sample aerogels: BET specific surface area (SBET), pore volumes (VP), and average pores diameter (DA).
3.2.4. The Emulsifying Properties of EWP Aerogels Changed with Grafting Degrees
The emulsification characteristics of the aerogels with different grafting degrees are presented in Table 3. After blending with CMC-Na, the emulsifying activity and emulsion stability of EWP aerogel showed a significant increase (p < 0.05) in general, probably due to the increased solubility of the conjugates, which was in agreement with the study by Chen et al. [], who investigated the functional behavior of soy protein–sodium alginate conjugates and ascribed the enhanced emulsification performance to their increased solubility. Notably, the emulsifying activity of EC24 aerogels reached the maximum, whereas the emulsion stability continuously kept improving with the increase in grafting degree.

Table 3.
Emulsifying activity and emulsion stability of the aerogels.
3.2.5. Effects of Grafting Degrees on the Oil Absorption Capacity (OAC) and Oil Holding Capacity (OHC) of EWP Aerogels
The OAC and OHC of the aerogels are shown in Figure 5. All aerogels presented favorable oil-absorbing behavior, indicating that the aerogels maintained a well-structured network during the freeze-drying process without significant structural collapse. Moreover, the EC12 aerogel had the significantly (p < 0.05) highest OAC (5.46 g/g aerogel), suggesting a better binding to soybean oil. In addition, conjugated aerogels with grafting degrees higher than 8.35% had relatively low oil absorption capacity, which was in agreement with the trend in the specific surface area values of aerogels that we measured previously. The higher OAC values in the presence of the EC0 aerogels were probably based on the fact that the entrapment of oil within the aerogel would be determined not only by the physical structure of the polymer network, but also by its internal functional groups, which would influence the ability to bind the oil []. Overall, in comparison with EWP aerogel, the conjugated aerogels possessed remarkably higher oil holding capacity (~30%) values. This could be explained by the exposure of more hydrophobic groups of protein due to chemical cross-linking through the Maillard reaction with polysaccharide chains [], which also made sense with the trend in aerogel emulsification properties that we investigated earlier. Theoretically, conjugated aerogels were exposed to more hydrophobic groups with increasing grafting degrees. However, the conjugated aerogels, especially EC48 aerogels, presented lower OHCs compared with the EC12 aerogels. This could be attributed to the fact that a looser structure with sheet-like aggregates could not encapsulate oil well within them [], which can be observed in Figure 2.
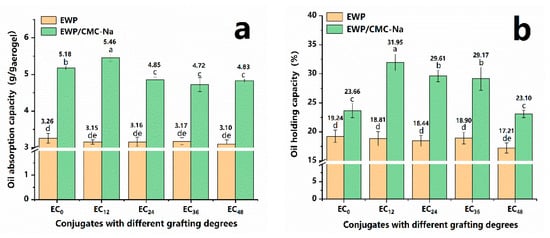
Figure 5.
Oil absorption (a)/holding (b) capacity of the aerogels with different grafting degrees.
4. Conclusions
The grafting degree had significant effects on the mechanical strength and oil-loading properties of EWP-based aerogels. When the grafting degree of EWP treated with CMC-Na reached 8.35%, the mechanical properties and oil-loading properties of egg white aerogels were significantly improved. At the same time, the grafting reaction resulted in a more homogeneous microscopic network structure, smaller average pore-size distribution, and larger overall pore specific surface area of the EWP aerogel, which provided a suitable microcapsule environment for subsequent oil-loading. In addition, the significant improvements in the emulsification performance of the EWP aerogels could induce synergistic effects with the previous microencapsulation environment, which not only improved the oil absorption performance of the EWP aerogel, but also enhanced its ability to bind to oil. Based on that, they are expected to be further applied in the food industry, such as being carrier matrices for fat-soluble substances. Our current study suggests that EWP-based aerogels are suitable for encapsulating fat-soluble active substances and provides an insight into their suitability for different food applications.
Author Contributions
Conceptualization, methodology, investigation, data curation and writing—original draft preparation, S.T.; writing—review and editing, Y.J.; methodology, T.T.; formal analysis, H.D.; resources, Y.T.; funding acquisition, project administration, and writing—review and editing, M.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (Grant No. 2018YFD0400303).
Acknowledgments
The authors are grateful to Jingru Hui and Ji’en Tan for their valuable assistance with the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ulker, Z.; Erkey, C. An Emerging Platform for Drug Delivery: Aerogel Based Systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-Based Aerogel Microspheres for Oral Drug Delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafari, R.; Jonoobi, M.; Amirabad, L.M.; Oksman, K.; Taheri, A.R. Fabrication and Characterization of Novel Bilayer Scaffold from Nanocellulose Based Aerogel for Skin Tissue Engineering Applications. Int. J. Biol. Macromol. 2019, 136, 796–803. [Google Scholar] [CrossRef]
- Zeinali, K.; Khorasani, M.T.; Rashidi, A.; Joupari, M.D. Preparation and Characterization of Graphene Oxide Aerogel/Gelatin as a Hybrid Scaffold for Application in Nerve Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 674–683. [Google Scholar] [CrossRef]
- Guo, L.C.; Xia, J.; Yu, S.H.; Yan, J.A.; He, F.; Zhang, M.Q.; Fan, Q.L.; Yang, R.J.; Zhao, W. Natural Edible Materials Made of Protein-Functionalized Aerogel Particles for Postprandial Hyperglycemia Management. Int. J. Biol. Macromol. 2021, 167, 279–288. [Google Scholar] [CrossRef]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Structural Characterization of Oleogels from Whey Protein Aerogel Particles. Food Res. Int. 2020, 132, 109099. [Google Scholar] [CrossRef]
- Selvasekaran, P.; Chidambaram, R. Food-Grade Aerogels Obtained from Polysaccharides, Proteins, and Seed Mucilages: Role as a Carrier Matrix of Functional Food Ingredients. Trends Food Sci. Technol. 2021, 112, 455–470. [Google Scholar] [CrossRef]
- Jung, S.B.; Park, S.W.; Yang, J.K.; Park, H.H.; Kim, H. Application of Sio2 Aerogel Film for Interlayer Dielectric on Gaas with a Barrier of Si3n4. Thin Solid Film. 2004, 447, 580–585. [Google Scholar] [CrossRef]
- Rao, A.V.; Bhagat, S.D.; Hirashima, H.; Pajonk, G.M. Synthesis of Flexible Silica Aerogels Using Methyltrimethoxysilane (Mtms) Precursor. J. Colloid Interface Sci. 2006, 300, 279–285. [Google Scholar] [CrossRef]
- Baumann, T.F.; Gash, A.E.; Chinn, S.C.; Sawvel, A.M.; Maxwell, R.S.; Satcher, J.H. Synthesis of High-Surface-Area Alumina Aerogels without the Use of Alkoxide Precursors. Chem. Mater. 2005, 17, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Demydov, D.; Klabunde, K.J. Characterization of Mixed Metal Oxides (Srtio3 and Batio3) Synthesized by a Modified Aerogel Procedure. J. Non-Cryst. Solids 2004, 350, 165–172. [Google Scholar] [CrossRef]
- Lorjai, P.; Chaisuwan, T.; Wongkasemjit, S. Porous Structure of Polybenzoxazine-Based Organic Aerogel Prepared by Sol–Gel Process and Their Carbon Aerogels. J. Sol-Gel Sci. Technol. 2009, 52, 56–64. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Wang, W.; Fang, Y.; Riffat, S.B.; Jiang, F. The Advances of Polysaccharide-Based Aerogels: Preparation and Potential Application. Carbohydr. Polym. 2019, 226, 115242. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H. Alginate/Pectin Aerogel Microspheres for Controlled Release of Proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Sakamoto, T.; Ohe, K.; Baba, Y. Cellulose Aerogel Regenerated from Ionic Liquid Solution for Immobilized Metal Affinity Adsorption. Carbohydr. Polym. 2014, 103, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Zhao, S.; Malfait, W.J.; Koebel, M.M. Chemistry of Chitosan Aerogels: Three-Dimensional Pore Control for Tailored Applications. Angew. Chem. Int. Ed. Engl. 2020, 60, 9828–9851. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, R. Starch Aerogel Loaded with Poorly Water-Soluble Vitamins through Supercritical Co 2 Adsorption. Chem. Eng. Res. Des. 2017, 119, 221–230. [Google Scholar] [CrossRef]
- Marin, M.A.; Mallepally, R.R.; McHugh, M.A. Silk Fibroin Aerogels for Drug Delivery Applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Betz, M.; García-González, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of Novel Whey Protein-Based Aerogels as Drug Carriers for Life Science Applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Mine, Y. Recent Advances in the Understanding of Egg White Protein Functionality. Trends Food Sci. Technol. 1995, 6, 225–232. [Google Scholar] [CrossRef]
- Somaratne, G.; Nau, F.; Ferrua, M.J.; Singh, J.; Ye, A.; Dupont, D.; Singh, R.P.; Floury, J. Characterization of Egg White Gel Microstructure and Its Relationship with Pepsin Diffusivity. Food Hydrocoll. 2020, 98, 105258. [Google Scholar] [CrossRef]
- Chang, C.; Meikle, T.G.; Su, Y.; Wang, X.; Dekiwadia, C.; Drummond, C.J.; Conn, C.E.; Yang, Y. Encapsulation in Egg White Protein Nanoparticles Protects Anti-Oxidant Activity of Curcumin. Food Chem. 2019, 280, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Selmer, I.; Kleemann, C.; Kulozik, U.; Heinrich, S.; Smirnova, I. Development of Egg White Protein Aerogels as New Matrix Material for Microencapsulation in Food. J. Supercrit. Fluids 2015, 106, 42–49. [Google Scholar] [CrossRef]
- Liu, L.; Ramirez, I.S.A.; Yang, J.; Ciftci, O.N. Evaluation of Oil-Gelling Properties and Crystallization Behavior of Sorghum Wax in Fish Oil. Food Chem. 2020, 309, 125567. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, W.; Wang, X.; Cheng, S.; Zhou, J.; Wu, Z.; Li, Y. Fabrication and Physicochemical and Antibacterial Properties of Ethyl Cellulose-Structured Cinnamon Oil Oleogel: Relation between Ethyl Cellulose Viscosity and Oleogel Performance. J. Sci. Food Agric. 2019, 99, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Rajarethinem, P.S.; Cludts, N.; Lewille, B.; De Vos, W.H.; Lesaffer, A.; Dewettinck, K. Biopolymer-Based Structuring of Liquid Oil into Soft Solids and Oleogels Using Water-Continuous Emulsions as Templates. Langmuir 2015, 31, 2065–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, A.; Hendriks, J.; Van Der Linden, E.; Scholten, E. Protein Oleogels from Protein Hydrogels Via a Stepwise Solvent Exchange Route. Langmuir 2015, 31, 13850–13859. [Google Scholar] [CrossRef]
- Selmer, I.; Karnetzke, J.; Kleemann, C.; Lehtonen, M.; Mikkonen, K.S.; Kulozik, U.; Smirnova, I. Encapsulation of Fish Oil in Protein Aerogel Micro-Particles. J. Food Eng. 2019, 260, 1–11. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Marin, M.A.; Surampudi, V.; Subia, B.; Rao, R.R.; Kundu, S.C.; McHugh, M.A. Silk Fibroin Aerogels: Potential Scaffolds for Tissue Engineering Applications. Biomed. Mater. 2015, 10, 035002. [Google Scholar] [CrossRef]
- Kleemann, C.; Selmer, I.; Smirnova, I.; Kulozik, U. Tailor Made Protein Based Aerogel Particles from Egg White Protein, Whey Protein Isolate and Sodium Caseinate: Influence of the Preceding Hydrogel Characteristics. Food Hydrocoll. 2018, 83, 365–374. [Google Scholar] [CrossRef]
- Andlinger, D.J.; Bornkeßel, A.C.; Jung, I.; Schroeter, B.; Smirnova, I.; Kulozik, U. Microstructures of Potato Protein Hydrogels and Aerogels Produced by Thermal Crosslinking and Supercritical Drying. Food Hydrocoll. 2021, 112, 106305. [Google Scholar] [CrossRef]
- Pekala, R.W.; Kong, F.M. A Synthetic Route to Organic Aerogels—Mechanism, Structure, and Properties. J. Phys. Colloq. 1989, 24, C4-33–C4-40. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Grishechko, L.; Szczurek, A.; Fierro, V.; Pizzi, A.; Kuznetsov, B.; Celzard, A. Highly Mesoporous Organic Aerogels Derived from Soy and Tannin. Green Chem. 2012, 14, 3099–3106. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite Aerogels Based on Dialdehyde Nanocellulose and Collagen for Potential Applications as Wound Dressing and Tissue Engineering Scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Pierron, D.; Harmad, M.F. Crosslinked Fibrous Composites Based on Cellulose Nanofibers and Collagen with in Situ Ph Induced Fibrillation. Cellulose 2011, 19, 139–150. [Google Scholar] [CrossRef]
- Chen, H.B.; Wang, Y.Z.; Schiraldi, D.A. Foam-Like Materials Based on Whey Protein Isolate. Eur. Polym. J. 2013, 49, 3387–3391. [Google Scholar] [CrossRef]
- Ahmadi, M.; Madadlou, A.; Saboury, A.A. Whey Protein Aerogel as Blended with Cellulose Crystalline Particles or Loaded with Fish Oil. Food Chem. 2016, 196, 1016–1022. [Google Scholar] [CrossRef]
- Arboleda, J.C.; Hughes, M.; Lucia, L.A.; Laine, J.; Ekman, K.; Rojas, O.J. Soy Protein–Nanocellulose Composite Aerogels. Cellulose 2013, 20, 2417–2426. [Google Scholar] [CrossRef]
- Chen, K.L.; Zhang, H. Fabrication of Oleogels Via a Facile Method by Oil Absorption in the Aerogel Templates of Protein-Polysaccharide Conjugates. ACS Appl. Mater. Interfaces 2020, 12, 7795–7804. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Li, J.; Wang, L. Magnetic N-Doped Carbon Aerogel from Sodium Carboxymethyl Cellulose/Collagen Composite Aerogel for Dye Adsorption and Electrochemical Supercapacitor. Int. J. Biol. Macromol. 2018, 115, 185–193. [Google Scholar] [CrossRef]
- An, Y.; Cui, B.; Wang, Y.; Jin, W.; Geng, X.; Yan, X.; Li, B. Functional Properties of Ovalbumin Glycosylated with Carboxymethyl Cellulose of Different Substitution Degree. Food Hydrocoll. 2014, 40, 1–8. [Google Scholar] [CrossRef]
- Xiong, W.; Li, Y.; Li, B.; Geng, F. Relationship between Gel Properties and Water Holding of Ovalbumin-Carboxymethylcellulose Electrostatic Complex Hydrogels. Int. J. Biol. Macromol. 2021, 167, 1230–1240. [Google Scholar] [CrossRef]
- Rao, Q.; Rocca-Smith, J.R.; Schoenfuss, T.C.; Labuza, T.P. Accelerated Shelf-Life Testing of Quality Loss for a Commercial Hydrolysed Hen Egg White Powder. Food Chem. 2012, 135, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.E.; Liu, T.; Yao, Y.; Wu, N.; Du, H.; Xu, M.; Liao, M.; Zhao, Y.; Tu, Y. Changes in Physicochemical and Antioxidant Properties of Egg White During the Maillard Reaction Induced by Alkali. LWT 2021, 143, 111151. [Google Scholar] [CrossRef]
- Cai, L.; Li, D.; Dong, Z.; Cao, A.; Lin, H.; Li, J. Change Regularity of the Characteristics of Maillard Reaction Products Derived from Xylose and Chinese Shrimp Waste Hydrolysates. LWT Food Sci. Technol. 2016, 65, 908–916. [Google Scholar] [CrossRef]
- Kleemann, C.; Schuster, R.; Rosenecker, E.; Selmer, I.; Smirnova, I.; Kulozik, U. In-Vitro-Digestion and Swelling Kinetics of Whey Protein, Egg White Protein and Sodium Caseinate Aerogels. Food Hydrocoll. 2020, 101, 105534. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.; Shang, K.; Wang, Y.T.; Ye, D.D.; Kang, A.H.; Liao, W.; Wang, Y.Z. Ultrasoft Gelatin Aerogels for Oil Contaminant Removal. J. Mater. Chem. A 2016, 4, 9381–9389. [Google Scholar] [CrossRef]
- Manzocco, L.; Valoppi, F.; Calligaris, S.; Andreatta, F.; Spilimbergo, S.; Nicoli, M.C. Exploitation of Kappa-Carrageenan Aerogels as Template for Edible Oleogel Preparation. Food Hydrocoll. 2017, 71, 68–75. [Google Scholar] [CrossRef]
- Spotti, M.J.; Perduca, M.J.; Piagentini, A.; Santiago, L.G.; Rubiolo, A.C.; Carrara, C.R. Gel Mechanical Properties of Milk Whey Protein–Dextran Conjugates Obtained by Maillard Reaction. Food Hydrocoll. 2013, 31, 26–32. [Google Scholar] [CrossRef]
- Deygen, I.M.; Kudryashova, E.V. New Versatile Approach for Analysis of Peg Content in Conjugates and Complexes with Biomacromolecules Based on Ftir Spectroscopy. Colloids Surf. B Biointerfaces 2016, 141, 36–43. [Google Scholar] [CrossRef]
- Umemura, K.; Kawai, S. Preparation and Characterization of Maillard Reacted Chitosan Films with Hemicellulose Model Compounds. J. Appl. Polym. Sci. 2008, 108, 2481–2487. [Google Scholar] [CrossRef]
- Affes, S.; Nasri, R.; Li, S.; Thami, T.; Van Der Lee, A.; Nasri, M.; Maalej, H. Effect of Glucose-Induced Maillard Reaction on Physical, Structural and Antioxidant Properties of Chitosan Derivatives-Based Films. Carbohydr. Polym. 2021, 255, 117341. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, S.L.; Weerakkody, R.; Augustin, M.A. Chitosan—Glucose Conjugates: Influence of Extent of Maillard Reaction on Antioxidant Properties. J. Agric. Food Chem. 2010, 58, 12449–12455. [Google Scholar] [CrossRef] [PubMed]
- Nešić, A.; Gordić, M.; Davidović, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-Based Nanocomposite Aerogels for Potential Insulated Food Packaging Application. Carbohydr. Polym. 2018, 195, 128–135. [Google Scholar] [CrossRef]
- Chengbin, Z.; Jiannan, Y.; Jingsheng, L.; Xiuying, X.; Yuzhu, W.; Hao, Z.; Yong, C.; Baokun, Q. Effect of Glycation Reaction on Rheological Property and Microstructure of Acid-Induced Gels Formed by Soybean Protein Isolate/Maltodextrin Systems. J. Chin. Inst. Food Sci. Technol. 2019, 19, 35–41. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; Del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and Biomedical Applications of Aerogels: Possibilities and Challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Geng, X.; Cui, B.; Li, Y.; Jin, W.; An, Y.; Zhou, B.; Ye, T.; He, L.; Liang, H.; Wang, L.; et al. Preparation and Characterization of Ovalbumin and Carboxymethyl Cellulose Conjugates Via Glycosylation. Food Hydrocoll. 2014, 37, 86–92. [Google Scholar] [CrossRef]
- Castaldo, R.; Gentile, G.; Avella, M.; Carfagna, C.; Ambrogi, V. Microporous Hyper-Crosslinked Polystyrenes and Nanocomposites with High Adsorption Properties: A Review. Polymers 2017, 9, 651. [Google Scholar] [CrossRef] [Green Version]
- Spotti, M.J.; Loyeau, P.A.; Marangón, A.; Noir, H.; Rubiolo, A.C.; Carrara, C.R. Influence of Maillard Reaction Extent on Acid Induced Gels of Whey Proteins and Dextrans. Food Hydrocoll. 2019, 91, 224–231. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).