Abstract
The growth-promoting effects of Gluconacetobacter diazotrophicus inoculation on the leaf lettuce (Lactuca sativa L.) cultivars “Black Seeded Simpson” and “Bibb/Limestone” were investigated. Plants of each cultivar were grown hydroponically in Kratky jars in a growth chamber-controlled environment in a completely randomized factorial design with three or four replications. Each experiment was repeated once. Factors were (1) with or without inoculant and (2) seven levels of nitrogen (N) fertilization ranging from deficient (37.5 mg L−1 N) to excessive (172.5 mg L−1 N). The shoot, root, and total biomass accumulation, nitrogen density, and carbon/nitrogen (C/N) ratios were measured for each variety. Black Seeded Simpson demonstrated a shifting of production towards aerial tissues, with significantly greater shoot production and reduced root production. The observed increase in shoot biomass was greatest at the slightly deficient N rate of 105 mg L−1 N where inoculated plants produced 14.8% more than uninoculated plants. Lower N density and higher C/N ratios in inoculated shoot tissues indicate greater N use efficiency. Bibb/Limestone responded to inoculation with an average increase of 10.9% in shoot production and with greater root biomass. Bibb/Limestone also exhibited lower N density in inoculated shoot tissues with a corresponding increase in the C/N ratio. For growers looking to maximize lettuce yields, G. diaz inoculation may present a beneficial additive to the growing system by increasing leaf yields while not increasing N fertilizer requirements.
1. Introduction
Lettuce (Lactuca sativa L.) is one of the most important vegetable crops sown in the United States, ranking third in total acreage (106,027 hectares) and first in economic value (USD 3.493 billion) in 2019 [1]. Over 97% of domestic production is concentrated in California and Arizona where environmental conditions allow multiple crops per season and year-round production [1,2]. Several impediments to the intensification of field-grown lettuce production have arisen in recent years, including limited acreage of ideally suited farmland, high nitrogen (N) requirements for optimal production, risk of fecal bacterial contamination when manures are used as an N source for organic lettuce production, and significant irrigation demands. These water demands are increasingly placing a burden on lettuce production, with chronic drought and increased salinity hindering yields [2,3].
Exogenous N applications exacerbate production concerns as leaching and run-off impairs water quality, reduces yields, and increases costs. Large-scale operations in these states have also been impacted by food safety recalls over contaminated produce, leading to millions of dollars of losses and erosion of public trust [1]. The sustainability of such geographically constrained production can be questioned, especially when coupled with the negative environmental impacts of cross-country transportation. Unfortunately, environmental conditions outside of these growing areas are unsuitable for year-round production.
Controlled environmental agriculture in the form of hydroponic cultivation has arisen to localize and intensify lettuce production in areas otherwise unsuitable for continual, field-based sowing. In hydroponic production, light, temperature, and nutrient fertilization are all monitored and adjusted to maximize the crop output and can be implemented regardless of climate or latitude. Turnaround time from planting to harvest can be reduced by half under these conditions and yields can increase 11-fold per unit area, increasing the yields per annum and helping to offset the cost of hydroponic production [4,5].
Additionally, hydroponic systems can be located near centers of consumption to minimize transportation costs, environmental impacts, and loss of nutritive composition during shipping and transport. Hydroponic production also tightly controls water salinity and can recycle and/or reuse irrigation water, thereby, improving their water use efficiency. One costly consideration of hydroponic lettuce production is the need to provide 100% of the fertilization requirements in the form of nutrient salts.
Nitrogen salts are often provided in the form of synthetically sourced fertilizers whose production has significant energy and environmental costs. If microbe-assisted crop production strategies [6] can be incorporated into the lettuce production system to promote increased yield and/or fix atmospheric N, it may help alleviate some of the need for exogenous fertilizer additions.
Gluconacetobacter diazotrophicus (G. diaz.) is an obligate endophyte that was originally identified in sugarcane [7,8]. The significance of this discovery is that, in sugarcane, G. diaz can fix up to 200 kg N ha−1 year−1 meeting nearly half of the crop’s N requirement [9,10]. G. diaz was subsequently found to be naturally associated with coffee [11], finger millet [12], banana, pineapple [13,14], tea, mango [8,15], and wetland rice [16]. Research has shown that inoculation with G. diaz. can result in non-pathogenically infected crop species across numerous plant families [17], including sweet potato, sugarcane, maize [18,19], tomato [20], sweet sorghum [21,22], carrot, beet [23,24], snap bean [25], and casava [26].
Unlike most N-fixing bacteria, G. diaz’s nitrogenase is not wholly suppressed by agricultural levels of environmental N and can tolerate especially high nitrate levels as it lacks nitrate reductase genes [9,27]. However, the colonization of sugarcane by G. diaz. is partially inhibited by high N-fertilization [28], and the effect was shown to be related to the plant physiology [29]. In addition to its N-fixing capability, G. diaz. produces indole-3-acetic acid (IAA) and gibberellins A1 and A3, which are important plant growth-regulating hormones [30,31].
G. diaz. also produces a bacteriocin against Xanthomonas albilineans, a sugar cane pathogen [32,33]. Drought stress has been shown to be mitigated by G. diaz. colonization in sugarcane and rice [34,35]. In a survey of 50 tropical and subtropical plants in southern India, G. diaz was found in association with the rhizosphere in ten plants [16]. This is of potential benefit as G. diaz. has been shown to solubilize and enhance the availability and uptake of zinc and iron [36,37].
The objective of this study was to determine the influence of G. diaz inoculation on root and leaf plant growth as well as the variance between cultivars in two hydroponically grown lettuces.
2. Materials and Methods
2.1. Experimental Design and Randomization
Two cultivars of lettuce were chosen for the study based on similar days to maturity, compact stature, and disease resistance. The plants were grown in Kratky hydroponic jars under temperature-, light-, and humidity-controlled conditions in growth chambers (Conviron MTR30, Conviron Environmentals Limited) that housed a maximum of 44 jars for the duration of the experiment [38]. The experiment was conducted as a two-factor fixed-effect completely randomized design (CRD) with three or four replicates.
The first factor was the presence/absence of G. diaz from the initial seed soaking solution. The second factor was different levels of nitrogen fertilization ranging from deficient (37.5, 60, 82.5, and 105 mg kg−1 N) to sufficient (127.5 and 150 mg kg−1 N) and hyper-sufficient (172.5 mg kg−1 N). Placement of the Kratky jars was randomized via the “RAND” function in Microsoft Excel (2013), with re-randomization occurring once per week. The experiment was conducted two times for each of the two cultivars in the study.
2.2. Plant and Bacterial Material
The two cultivars of lettuce selected for this trial, Black Seeded Simpson (BSS) and Bibb/Limestone (BIB), exhibit different leaf growth patterns but similar overall structure and suitability for hydroponic production. Both varieties had an estimated days to maturity of 43–45 days after planting and were sourced from commercial seed companies. G. diaz was provided by Azotic Technologies, Ltd. (Nottingham, United Kingdom) and is an intracellular, endophytic strain of the bacterium developed for delivery through their commercialized ‘N-Fix’ product (Azotic-na.com 2019). Pure strain stocks of the bacteria were maintained in 25% glycerol at −80 °C until inoculum preparation.
2.3. Inoculant Preparation
G. diaz was cultured from glycerol preserved stock in a modified MESMA/ATGUS medium (2.7 g L−1 glucose; 4.8 g L−1 dipotassium phosphate (K2HPO4); 0.65 g L−1 monopotassium phosphate (KH2PO4); 1.8 g L−1 mannitol; 4.4 g L−1 2-(N-morpholino) ethanesulfonic acid (MES hydrate); 2.7 g L−1 yeast extract) [39]. Nutrients were combined in ASTM Type II, deionized water (>1 MΩ·cm) and autoclaved prior to aseptic bacterial introduction to ensure a pure, single-strain culture.
Inoculated medium and bacteria-free controls were incubated in a positive-pressure, enclosed shaker table (30 °C at 120 rpm) for five days. Colony forming units (CFU) per milliliter solution were determined by serial dilution and standard plate counts on semi-solid ATGUS medium (same as above, plus 0.8% wt/vol agar) in triplicate, corroborated with OD600 based estimation (NanoDrop One, Thermo Scientific) against standard curves. The achieved CFUs in concentrate were 4.0 × 108 mL−1 for BSS trial #1, BSS trial #2, and BIB trial #1, and 1.8 × 109 mL−1 for BIB trial #2. Differences in CFU were attributed to random variations inherent in bacterial culturing.
Liquid medium for the seed soak inoculation was prepared from a proprietary blend of nutritive and adhesive adjuvants in deionized water, as described by an operating protocol provided by Azotic Technologies, Ltd. (Internal document dated 2016) and reported on the safety data sheet (Azotic Technologies, Ltd.; Lancashire, United Kingdom; 2017). For each trial, 50 mL portions of medium were aseptically aliquoted into each of two sterilized flasks. One flask then received a 50 mL portion of cultured bacterial concentrate, and the second flask received a 50 mL portion of deionized water.
The portion containing bacterium, now at ½ cultured CFU mL−1, became the inoculation treatment (INOC), while the adjuvant and water only solution became the un-inoculated control (NON). Both treatment stocks were assessed for equal pH and EC to ensure that the seeds soaked would experience identical conditions save for the bacterial presence. Thus, BSS trials #1 and #2 (BSS1, BSS2), and BIB trial #1 (BIB1) received 2.0 × 108 CFU mL−1 on seed, and BIB trial #2 (BIB2) received 9.0 × 108 CFU mL−1 on seeds at inoculation.
2.4. Seed Inoculation, Planting, and Growing Conditions
For seed treatment application, 130 seeds each were distributed to two 60 × 15 mm sterile petri dishes, and 250 μL of solution (INOC or NON) was added via pipette to each dish. Dishes were immediately sealed and gently agitated. After five minutes, the solutions were pipetted out, and a second 250 μL portion of the same treatment was applied as before to ensure saturation. After a second five-minute soak, all residual solution was removed. Seeds were then stored for 10–30 min at 4 °C and 65% relative humidity until planting.
Three seeds were sown in 4 cm rockwool cubes (Grodan A036/40; Roxul Inc., Milton, ON, Canada) that were nested into 5 cm circular net cups (Hummert International) and placed into foil-wrapped Kratky hydroponic jars (Figure 1) [38]. Jars were prefilled with 800 mL of nutrient solution formulated to provide a full and balanced measure of all essential nutrients required for growth of a lettuce plant to maturity except for N, which was added according to the N treatments described above.
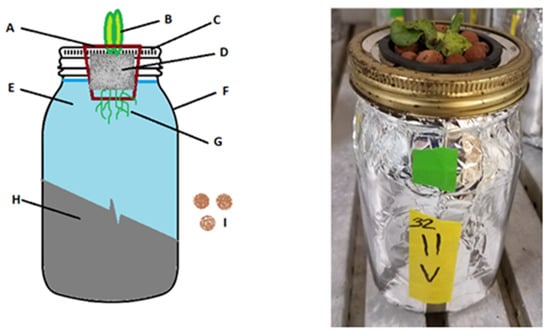
Figure 1.
The Kratky soilless hydroponic growing method [38].
For all nutrients other than N, the nutrient content in mg L−1 matched that of the Jack’s Hydroponic (5-12-26) + calcium nitrate recipe [40]. The bottom of the rockwool was immersed to allow for nutrient solution wicking onto the seeds. Extruded horticultural clay pebbles (Hydroton 8–16 mm, Hummert International) were placed on top of the rockwool to reduce evaporation and limit algal growth. No further nutrient solution was added after the beginning of the trial.
- A.
- 2” circular net cup used to suspend the plant above the nutrient solution through a hole cut in the center of the Mason jar lid.
- B.
- Growing lettuce shoots.
- C.
- Wide-mouth quart jar metal lid and collar. Pre-drilled 7/16” hole in lid center allows net cup to rest inside on the top lip.
- D.
- A036/40 rockwool used as seedling and plant support media. Inert, pH neutral, high wicking growth media with no nutrient content. Bottom 1/4” of rockwool extends into the nutrient solution to allow wicking.
- E.
- Nutrient solution, 800 mL in a 946 mL (1 qt) jar.
- F.
- 946 mL (1 qt) wide-mouth Mason jar.
- G.
- Roots growing into the media solution.
- H.
- Aluminum foil completely wrapping the sides and bottom of jar to prevent exposure to light.
- I.
- Hydroton clay pebbles placed atop the rockwool to limit evaporation and block light.
Growth chamber conditions were a 16/8 h day/night cycle under metal halide and high-pressure sodium lamps providing a photosynthetic photon flux density of 210 μmol m−2 s−1 and 23/20 °C. Relative humidity was maintained at 70%. Plants were re-randomized once per week by operator-blind removal and redistribution back into the chamber concurrent with jars being topped off with deionized water to a minimum of 400 mL solution. Each jar had 0.11 m2 of growing area and was thinned to a single plant in trials BSS1, BSS2, and BIB1. Due to access restrictions stemming from the COVID-19 pandemic, BIB2 seedlings were not thinned.
2.5. Harvesting and Analysis
After 30/31 days, when the lettuce was still in a rapid growth stage, all plant material was harvested, sectioned, and dried at 60 °C until stable weights were achieved to yield shoot dry matter (SDM) and root dry matter (RDM). The dry weight of root material that could not be removed from the rockwool cubes was determined by subtracting the pre-recorded mass of the dried cubes. After all dry matter masses were recorded, the roots and shoots were finely ground to 0.5 mm, and elemental combustion analysis was performed (Elementar, Thermo Fisher Scientific) to determine the nitrogen and carbon content. The N content in tissues was calculated by multiplying the total tissue mass by the determined N%.
2.6. Statistical Analysis
Data were analyzed using SAS 9.4 software for Windows (Copyright 2016 by SAS Institute Inc., Cary, NC, USA). Observations across all N levels for INOC were pooled and statistically compared to the pooled observations for NON allowing for 28 degrees of freedom. All significance tests were performed at alpha < 0.05 using PROC MIXED and PROC GLM for multi-factor ANOVA with type 3 tests of fixed effects and Tukey–Kramer HSD for the least squares mean differences. For dependent variables data exhibiting violations of Studentized and/or Pearson residual plots, PROC TRANSREG was used to generate BOX-COX lambdas for data normalization. For BIB2, where jars were not thinned, the number of plants present was included in the analysis as a discrete covariate in ANCOVA.
3. Results
3.1. Black Seeded Simpson (BSS) Trials
3.1.1. Biomass Accumulation
For the BSS cultivar, plant biomass responded curvilinearly to nitrogen fertilization in the shoot dry matter (SDM), root dry matter (RDM), and total dry matter (TDM) across both trials in accordance with typical luxury consumption trends (Table 1). In BSS1, inoculation affected a significant increase (INOC; 235 ± 106 mg, p ≤ 0.0345) in the shoot biomass, an increase of 8.19%, and a significant decrease (INOC; −425 ± 137 mg, p ≤ 0.0140) in the root biomass. The growth response did not vary by N-level, and the total plant biomass was not significantly affected in BSS1. In BSS2, there were no significant differences for any of the biomass measures.

Table 1.
Effects of inoculation and nitrogen fertilization on Black Seeded Simpson Biomass.
3.1.2. Carbon Nitrogen Ratios and Nitrogen Accumulation
For the BSS cultivar, the N content responded curvilinearly to nitrogen fertilization in SDM and RDM across both trials in accordance with typical luxury consumption trends (Table 2). In BSS1, inoculation affected a significant decrease in SDM nitrogen density from 2.209% to 1.977% (INOC; −0.233 ± 0.659 %N, p ≤ 0.0015), but there was no difference in root tissues. C/N ratios followed a downward curvilinear trend with INOC treatment in shoot tissue higher than NON (INOC; 2.223 ± 0.730, p ≤ 0.005). Root tissue exhibited no differences. In BSS2, no significant differences emerged for C/N ratios.

Table 2.
Effects of inoculation and nitrogen fertilization on the Black Seeded Simpson N density and C/N ratios.
3.2. Bibb/Limestone (BIB) Trials
3.2.1. Biomass Accumulation
For the BIB cultivar, the plant biomass responded curvilinearly to nitrogen fertilization across both trials in accordance with typical luxury consumption trends (Table 3). Inoculation affected a significant increase in SDM (10.9%) and TDM in BIB1 (10.0%), and a significant increase in SDM (16.2%) and RDM (18.2%) in BIB2. TDM in BIB2, therefore, also significantly increased in response to inoculation (INOC; 906 ± 294 mg, p ≤ 0.0041).

Table 3.
Effects of inoculation and nitrogen fertilization on the Bib/Limestone biomass.
3.2.2. Carbon Nitrogen Ratios and Nitrogen Accumulation
For the BIB cultivar, the N content responded curvilinearly to nitrogen fertilization in SDM and RDM across both trials in accordance with typical luxury consumption trends (Table 4). In BIB1, inoculation had no effect on the N density or C/N ratio. In BIB2, inoculation affected a 20.6% decrease in nitrogen density in SDM and a corresponding increase in the C/N ratio, but RDM was not affected.

Table 4.
Effects of inoculation and nitrogen fertilization on the Bib/Limestone N density and C/N ratios.
4. Discussion
4.1. Black Seeded Simpson (BSS) Trials
For BSS1, the observed increase in shoot biomass was greatest at the slightly deficient N rate of 105 mg L−1 N. At this N rate, INOC outperformed NON by 456 mg dry matter, an increase of 14.8% (Figure 2a). This corresponds to the best conservative fertilization rate, because the harvested aerial biomass was greater at this combination of G. diaz and fertilization than that for all NON plants fertilized at an equivalent or higher rate. By inoculating with G. diaz, a grower could decrease the amount of N fertilizer use by 42.8% (150 mgL−1 reduced to 105 mgL−1) for the same biomass yield, which would represent a considerable savings on the cost of fertilizer input.

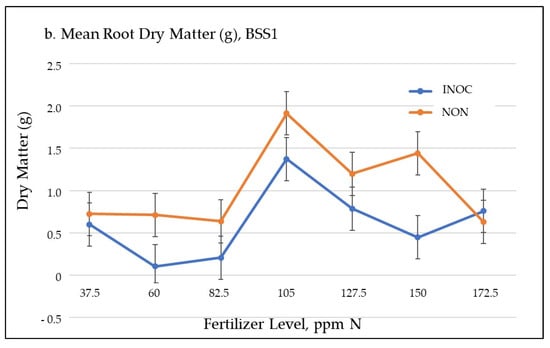
Figure 2.
The mean shoot (a) and root (b) dry matter, Black Seeded Simpson1.
At the same time, the root biomass decreased by 425 mg dry weight, or 41.0% (Figure 2b), with no significant difference in total biomass accumulation in the whole plant (TDM). Inoculated plants shifted biomass synthesis away from root tissues and into shoot tissues. Since root material is of no value in hydroponic lettuce production, there is no detriment due to reduced root biomass production under hydroponic growth conditions. A previously observed response of lettuce to high levels of IAA in aerial tissues [41] possibly indicates G. diaz contributions to the plant available IAA. Exogenous gibberellins at moderate levels can also produce this phenotype response [42], which would be another potential explanation for the differences but this would require quantitative testing.
The total amount of N contained in the INOC shoot tissue was not significantly different from NON plants. Therefore, the plants were producing more shoot biomass with an equivalent amount of N incorporation as in the control. This indicates increased nitrogen use efficiency (NUE) [43,44]. The result also results in significantly lower N density in INOC SDM tissues compared with NON (1.97% INOC vs. 2.21% NON) and a higher C/N ratio in inoculated SDM (21.04 INOC vs. 18.82 NON). Both hormone families produced by G. diaz have been implicated in increased NUE in other plant-microbe synergisms [45]. Increased NUE was most significant at the lower levels of N fertilization (Figure 3). At these lower levels, N would be the limiting growth factor. The greater biomass, greater carbon sequestration, and static N presence indicates considerable added value to producers.
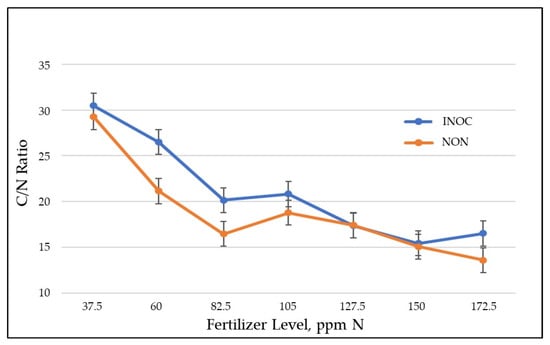
Figure 3.
The C/N ratio in shoot dry matter, Black Seeded Simpson 1.
The reason for a lack of response in the second trial is not clear. Conditions were consistent between trials, and seed treatments contained the same bacterial CFU concentration. Seeds for the second trial were held over from the first trial but maintained in the dark at 4 °C and 70% relative humidity for the five months between BSS1 and BSS2. Storage conditions exclude the possibility of heat-induced thermal dormancy. The seeds were commercially sourced, and thus the age could not be determined prior to receipt. Therefore, the age of the seeds may still have been a factor affecting the observed plant response.
4.2. Bibb/Limestone (BIB) Trials
For Bibb lettuce, both trials exhibited similar responses to inoculation, with greater SDM and TDM in both trials and an increase in RDM in trial 2. As has been exhibited in other multi-plant cultivar G. diaz experiments [18,46,47], the response to inoculation was not consistent between even closely related varieties, as is seen here in comparing BSS to BIB. Both cultivars exhibit an improvement in aerial tissue production under inoculation, but root response was reversed (i.e., lower RDM in BSS1 and higher RDM in BIB2).
For the BIB trials, inoculated BIB1 saw an average SDM increase of 10.86% (5.407g INOC vs. 4.877 g NON), and for BIB2 this was +16.16% (4.690 g INOC vs. 4.038 g NON) compared to +8.19% for BSS1 (3.105 g INOC vs. 2.870 g NON). Even with the significant reduction in root mass in BSS1, this was not able to match the percent increases in either BIB trial. BIB2 outperformed both its replicate and BSS1 in that it also significantly increased in root biomass by 18.73% (1.606 g INOC vs. 1.353 g NON) over the controls. Whereas the total biomass for BIB1 and BIB2 was also significantly improved by inoculation, Bibb/Limestone lettuce biomass responded to inoculation with a greater total biomass and did not employ any root/shoot tradeoffs as seen in BSS.
This may be important under non-hydroponic growing conditions, such as smallholder or low-input systems, where inconsistent irrigation would favor the survival of plants with more prolific root systems. In BIB1, the nitrogen density and C/N ratios were not affected by inoculation. BIB2 did exhibit a lower N density in INOC shoot tissues with a corresponding increase in the C/N ratio. As in BSS1, this indicates increased NUE in aerial tissues.
4.3. Cross Experiment Observations and Implications
Although determination of the causes and effects of the plant growth response to inoculation is beyond the scope of this study, certain observations suggest possible mechanisms. At high N levels, lettuce often exhibits luxury consumption with an accumulation of nitrate that is categorized as an anti-nutrient [48]. Previous studies on Poa pratensis, which shares a similar luxury consumption trait, demonstrated that, concurrent with inoculation, high levels of N fertilization can still increase crop physiological responses [49].
Conversely, under nitrogen deprivation conditions where lettuce yields are significantly reduced, bacterial nitrogen fixation may alleviate some of the nutrient deficiency impairing growth, as has been demonstrated in aquaponic lettuce inoculated with N-fixing Rahnella [50]. Endophyte-sourced plant growth-regulating hormones can increase the production of both above ground and below ground biomass as has been shown in carrot leaf and taproot [23] as well as beetroot [24]. Specific to lettuce, IAA imbalances have been implicated in altering root and shoot formation, with increased concentrations in aerial tissues reducing root formation [42].
Gibberellins at low concentrations have been shown to increase lettuce leaf production; however, at high concentrations, they impede growth [42]. In this study, the shift from RDM growth to SDM growth in BSS with no overall increase in TDM suggests a plant growth-regulating hormone effect. However, greater SDM and TDM in both BIB trials and an increase in RDM in BIB trial 2 suggests a possible N-fixation effect with or without a possible plant growth-regulating hormone effect in combination.
5. Conclusions
For growers looking to maximize lettuce yields on a static agricultural footprint, G. diaz inoculation may present a beneficial additive to the growing system. The ability to thrive under oxygenated conditions and avoid suppression in a high nitrogen presence make it especially suitable for hydroponic and/or aeroponic setups. For the two cultivars tested in this study, the harvestable biomass was increased in all but one of the trials, with increases from +8.19% in Black Seeded Simpson to +16.16% in Bibb/Limestone. For BIB lettuce, the highest biomass yield was realized with inoculation and an N fertilization level well below the established requirements, potentially allowing growers to reduce their N fertilizer requirements and still maintain production.
There is seemingly a significant bacterium x cultivar interaction when it comes to root production, allowing growers to tailor their use of G. diaz or their selection of lettuce variety to match their cultivation needs. Luxury consumption trends were not seen in this study, partly because of the non-replenishment fertilizer regime employed in Kratky jars. As this is the first reported case of G. diaz inoculation of lettuce, further research is needed to elucidate the exact mechanisms behind the plant responses. Whether from PGRH production, N fixation, NUE, or alteration in the shuttling of free N in planta, G. diaz has the potential to increase the yield of this valuable crop.
Author Contributions
Conceptualization: R.L.S., S.W.D. and R.B.B.; methodology: R.L.S.; validation: S.W.D., R.D.B., J.M.R., J.D.L. and R.B.B.; formal analysis: R.L.S.; resources: S.W.D. and R.B.B.; writing—original draft preparation: R.L.S., S.W.D. and R.B.B.; writing—review and editing: R.D.B., J.M.R. and J.D.L.; supervision: S.W.D. and R.B.B.; project administration: R.B.B.; funding acquisition: R.B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sustainable Agriculture Research and Education program, the United States Department of Agriculture, The Pennsylvania State University, Azotic Technologies LLC, and the Jeanne and Charles Rider Endowment. The Pennsylvania State University and the U.S. Department of Agriculture are equal opportunity providers and employers.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Azotic Technologies, LLC for providing the strain of Gluconacetobacter diazotrophicus that was used in this research and technical advice for preparing the inoculum.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- USDA National Agricultural Statistics Service. 2020; Vegetables 2019 Summary. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/vegean20.pdf (accessed on 22 September 2021).
- Agricultural Marketing Resource Center. 2018. Lettuce. Available online: https://www.agmrc.org/commodities-products/vegetables/lettuce (accessed on 19 April 2021).
- Smith, R.; Cahn, M.; Daugovish, O.; Koike, S.; Natwick, E.; Smith, H.; Subbarao, K.; Takele, E.; Turini, T. Leaf Lettuce production in California. UC Davis Vegetable Research and Information Center. Publication 7216. 2011. Available online: https://anrcatalog.ucanr.edu/pdf/7216.pdf (accessed on 13 April 2021).
- Cometti, N.N.; Matias, G.C.S.; Zonta, E.; Mary, W.; Fernandes, M.S. Efeito da concentração da solução nutritiva no crescimento da alface em cultivo hidropônico-sistema NFT. Hortic. Bras. 2008, 26, 262–267. [Google Scholar] [CrossRef][Green Version]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, P.; Schlaeppi, K.; Sessitsch, A. miCROPe 2019—emerging research priorities towards microbe-assisted crop production. FEMS Microbiol. Ecol. 2020, 96, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, V.A.; Dobereiner, J. A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane. Plant Soil 1988, 108, 23–31. [Google Scholar] [CrossRef]
- Muthukumarasamy, R.; Revathi, G.; Seshadri, S.; Lakshminarasimhan, C. Gluconacetobacter diazotrophicus (syn. Acetobacter diazotrophicus), a promising diazotrophic endophyte in tropics. Curr. Sci. 2002, 83, 137–145. [Google Scholar]
- Stephan, P.M.; Oliveira, M.; Teixeira, K.R.S.; Martinez-Drets, G.; Döbereiner, J. Physiology and dinitrogen fixation ofAcetobacter diazotrophicus. FEMS Microbiol. Lett. 1991, 77, 67–72. [Google Scholar] [CrossRef][Green Version]
- Boddey, R.M.; Polidoro, J.C.; Resende, A.S.; Alves, B.J.R.; Urquiaga, S. Use of the15N natural abundance technique for the quantification of the contribution of N2 fixation to sugar cane and other grasses. Funct. Plant Biol. 2001, 28, 889–895. [Google Scholar] [CrossRef]
- Jimenez-Salgado, T.; Fuentes-Ramirez, L.E.; Tapia-Hernandez, A.; Mascarua-Esparza, M.A.; Martinez-Romero, E.; Caballero-Mellado, J. Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen-fixing acetobacteria. Appl. Environ. Microbiol. 1997, 63, 3676–3683. [Google Scholar] [CrossRef]
- Loganathan, P.; Sunita, R.; Parida, A.K.; Nair, S. Isolation and characterization of two genetically distant groups of Acetobacter diazotrophicus from a new host plant Eleusine coracana L. J. Appl. Microbiol. 1999, 87, 167–172. [Google Scholar] [CrossRef]
- Weber, O.; Baldani, V.L.D.; Teixeira, K.R.S.; Kirchhof, G.; Baldani, J.I.; Dobereiner, J. Isolation and characterization of diazotrophic bacteria from banana and pineapple plants. Plant Soil 1999, 210, 103–113. [Google Scholar] [CrossRef]
- Tapia-Hernández, A.; Bustillos-Cristales, M.; Jiménez-Salgado, T.; Caballero-Mellado, J.; Fuentes-Ramírez, L. Natural Endophytic Occurrence of Acetobacter diazotrophicus in Pineapple Plants. Microb. Ecol. 2000, 39, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, M.; Saravanan, V.; Jovi, D.S.S.; Lee, H.; Thenmozhi, R.; Hari, K.; Sa, T. Occurrence of Gluconacetobacter diazotrophicus in tropical and subtropical plants of Western Ghats, India. Microbiol. Res. 2004, 159, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Muthukumarasamy, R.; Cleenwerck, I.; Revathi, G.; Vadivelu, M.; Janssens, D.; Hoste, B.; Gum, K.U.; Park, K.D.; Son, C.Y.; Sa, T.; et al. Natural association of Gluconacetobacter diazotrophicus and diazotrophic Acetobacter peroxydans with wetland rice. Syst. Appl. Microbiol. 2005, 28, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, R.O. Recent advances in nitrogen-fixing acetic acid bacteria. Int. J. Food Microbiol. 2008, 125, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Riggs, P.J.; Chelius, M.K.; Iniguez, A.L.; Kaeppler, S.M.; Triplett, E.W. Enhanced maize productivity by inoculation with diazotrophic bacteria. Funct. Plant Biol. 2001, 28, 829–836. [Google Scholar] [CrossRef]
- Tian, G.; Pauls, P.; Dong, Z.; Reid, L.M.; Tian, L. Colonization of the nitrogen-fixing bacterium Gluconacetobacter dia-zotrophicus in a large number of Canadian corn plants. Can. J. Plant Sci. 2009, 89, 1009–1016. [Google Scholar] [CrossRef]
- Luna, M.F.; Apreaa, J.; Crespoa, J.M.; Boiardia, J.L. Colonization and yield promotion of tomato by Gluconacetobacter diazotrophicus. Appl. Soil Ecol. 2012, 61, 225–229. [Google Scholar] [CrossRef]
- Paula, M.A.; Reis, V.M. Interactions of Glomus clarum with Acetobacter diazotrophicus in infection of sweet potato (Ipomoea batatas), sugarcane (Saccharum spp.), and sweet sorghum (Sorghum vulgare). Biol. Fertil. Soils 1991, 11, 111–115. [Google Scholar] [CrossRef]
- Yoon, V.; Tian, G.; Vessey, J.K.; MacFie, S.M.; Dangi, O.P.; Kumer, A.K.; Tian, L. Colonization efficiency of different sorghum genotypes by Gluconacetobacter diazotrophicus. Plant Soil 2015, 398, 243–256. [Google Scholar] [CrossRef]
- Rocafell, Y.R.; Álvarez, B.D.; Badía, M.R.; García, M.O.; Daza, N.A.; Sánchez, J.R. Interaction of the bacteria Gluconacetobacter diazotrophicus and root vegetables. Cultiv. Trop. 2016, 37, 28–32. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pagnani, G.; Rossi, M.; D’Egidio, S.; Gallo, M.; Forni, C. Daucus carota L. Seed Inoculation with a Consortium of Bacteria Improves Plant Growth, Soil Fertility Status and Microbial Community. Appl. Sci. 2021, 11, 3274. [Google Scholar] [CrossRef]
- de Oliveira, T.R.A.; Gravina, G.A.; da Cruz, D.P.; Silva, N.D.; de Oliveira, G.H.F.; de Sant’Anna, C.Q.; Magalhães, M.M.; Berbert-Molina, M.A.; Neto, F.A. The performance of bean pod lineage inoculated with Gluconacetobacter diazotrophicus PAL5. Sci. Hortic. 2019, 249, 65–70. [Google Scholar] [CrossRef]
- Lopes, E.A.P.; Da Silva, A.D.A.; Mergulhão, A.C.D.E.S.; Da Silva, E.V.N.; Santiago, A.D.; Figueiredo, M.D.V.B. Co-Inoculation of Growth Promoting Bacteria and Glomus Clarum in Micropropagated Cassava Plants. Rev. Caatinga 2019, 32, 152–166. [Google Scholar] [CrossRef]
- Fisher, K.; Newton, W.E. Nitrogenase proteins from Gluconacetobacter diazotrophicus, a sugarcane-colonizing bacterium. Biochim. et Biophys. Acta (BBA) Proteins Proteom. 2005, 1750, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Andrade, O.; Fuentes-Ramírez, L.E.; Morales-García, Y.E.; Molina-Romero, D.; Bustillos-Cristales, M.R.; Martínez-Contreras, R.D.; Muñoz-Rojas, J. Colonization of sugarcane by Acetobacter diazotrophicus is inhibited by high N-fertilization. FEMS Microbiol. Ecol. 1999, 29, 117–128. [Google Scholar] [CrossRef]
- Rodríguez-Andrade, O.; Fuentes-Ramírez, L.E.; Morales-García, Y.E.; Molina-Romero, D.; Bustillos-Cristales, M.R.; Martínez-Contreras, R.D.; Muñoz-Rojas, J. The decrease in the population of Gluconacetobacter diazotrophicus in sugarcane after nitrogen fertilization is related to plant physiology in split root experiments. Rev. Argent Microbiol. 2015, 47, 335–343. [Google Scholar] [CrossRef]
- Fuentes-Ramírez, L.E.; Jiménez-Salgado, T.; Abarca-Ocampo, I.R.; Caballero-Mellado, J. Acetobacter diazotrophicus, an indoleacetic acid producing bacterium isolated from sugarcane cultivars of Mexico. Plant Soil 1993, 154, 145–150. [Google Scholar] [CrossRef]
- Bastián, F.; Cohen, A.; Piccoli, P.; Luna, V.; Bottini, R.; Baraldi, R. Production of indole-3-acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically-defined culture media. Plant Growth Regul. 1998, 24, 7–11. [Google Scholar] [CrossRef]
- Piñón, D.; Casas, M.; Blanch, M.; Fontaniella, B.; Blanco, Y.; Vicente, C.; Solas, M.-T.; Legaz, M.-E. Gluconacetobacter diazotrophicus, a sugar cane endosymbiont, produces a bacteriocin against Xanthomonas albilineans, a sugar cane pathogen. Res. Microbiol. 2002, 153, 345–351. [Google Scholar] [CrossRef]
- Arencibia, A.D.; Vinagre, F.; Estevez, Y.; Bernal, A.; Perez, J.; Cavalcanti, J.; Santana, I.; Hemerly, A.S. Gluconacetobacter diazotrophicus elicits a sugarcane defense response against a pathogenic bacteria Xanthomonas albilineans. Plant Signal. Behav. 2006, 1, 265–273. [Google Scholar] [CrossRef]
- Vargas, L.; Brígida, A.B.S.; Filho, J.P.M.; De Carvalho, T.G.; Rojas, C.; Vaneechoutte, D.; Van Bel, M.; Farrinelli, L.; Ferreira, P.C.G.; Vandepoele, K.; et al. Drought Tolerance Conferred to Sugarcane by Association with Gluconacetobacter diazotrophicus: A Transcriptomic View of Hormone Pathways. PLoS ONE 2014, 9, e114744. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, L.; Silva, R.; Almeida, I.; Vidal, M.; Baldani, J.I.; Meneses, C.H.S.G. Gluconacetobacter diazotrophicus mitigates drought stress in Oryza sativa L. Plant Soil 2019, 451, 57–73. [Google Scholar] [CrossRef]
- Saravanan, V.S.; Osborne, J.; Madhaiyan, M.; Mathew, L.; Chung, K.; Ahn, K.; Sa, T. Zinc Metal Solubilization by Gluconacetobacter diazotrophicus and Induction of Pleomorphic Cells. J. Microbiol. Biotechnol. 2007, 17, 1477–1482. [Google Scholar] [PubMed]
- Delaporte-Quintana, P.; Lovaisa, N.C.; Rapisarda, V.A.; Pedraza, R.O. The plant growth promoting bacteria Gluconacetobacter diazotrophicus and Azospirillum brasilense contribute to the iron nutrition of strawberry plants through siderophores production. Plant Growth Regul. 2020, 91, 185–199. [Google Scholar] [CrossRef]
- Kratky, B.A. A suspended pot, non-circulating hydroponic method. Proceedings of the South Pacific Soilless Culture Conference. Acta Hort. 2004, 648, 83–89. [Google Scholar] [CrossRef]
- Cocking, E.C.; Stone, P.J.; Davey, M.R. Intracellular colonization of roots of Arabidopsis and crop plants by Gluconacetobacter diazotrophicus. In Vitro Cell. Dev. Biol. Plant 2006, 42, 74–82. [Google Scholar] [CrossRef]
- Mattson, N.S.; Peters, C. A Recipe for Hydroponic Success. Inside Grower 16–19 January 2014. Available online: http://www.greenhouse.cornell.edu/crops/factsheets/hydroponic-recipes.pdf (accessed on 24 September 2021).
- Vysotskaya, L.B.; Veselov, S.Y.; Kudoyarova, G.R. Effect of Competition and Treatment with Inhibitor of Ethylene Perception on Growth and Hormone Content of Lettuce Plants. J. Plant Growth Regul. 2017, 36, 450–459. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef]
- Di Gioia, F.; Gonnella, M.; Buono, V.; Santamaria, P. Nitrogen use efficiency, yield and quality response of lettuce crop to nitrogen input. In Proceedings of the Conference on Nitrogen, Environment and Vegetables (NEV2013), Torino, Italy, 15—17 April 2013. [Google Scholar]
- Amirouche, M.; Smadhi, D.; Zella, L. Modeling of Nitrogen Use Efficiency in Lettuce Culture (Lactuca sativa): Isotopic Nitrogen (15 N) and AquaCrop. In Nitrogen in Agriculture—Physiological, Agricultural and Ecological Aspec; IntechOpen: London, UK, 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Bacterial Indole-3-Acetic Acid Influences Soil Nitrogen Acquisition in Barley and Chickpea. Plants 2021, 10, 780. [Google Scholar] [CrossRef]
- Eskin, N.; Vessey, K.; Tian, L. Research Progress and Perspectives of Nitrogen Fixing Bacterium, Gluconacetobacter diazotrophicus, in Monocot Plants. Int. J. Agron. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- de Souza, A.L.S.R.; De Souza, S.A.; De Oliveira, M.V.V.; Ferraz, T.M.; Figueiredo, F.A.M.M.A.; Da Silva, N.D.; Rangel, P.L.; Panisset, C.R.S.; Olivares, F.L.; Campostrini, E.; et al. Endophytic colonization of Arabidopsis thaliana by Gluconacetobacter diazotrophicus and its effect on plant growth promotion, plant physiology, and activation of plant defense. Plant Soil 2015, 399, 257–270. [Google Scholar] [CrossRef]
- Di Gioia, F.; Gonnella, M.; Buono, V.; Ayala, O.; Santamaria, P. Agronomic, physiological and quality response of romaine and red oak-leaf lettuce to nitrogen input. Ital. J. Agron. 2017, 12, 47–58. [Google Scholar] [CrossRef]
- Sebring, R.L.; Schlossberg, M.; Regan, J.; Bryant, R.B. Establishment of Kentucky Bluegrass Inoculated with the Novel Nitrogen Fixing Bacterial Endophyte, Gluconacetobacter Diazotrophicus. Ph.D. Thesis, The Pennsylvania State Univiversity, University Park, State College, PA, USA, 2017. [Google Scholar]
- Day, J.A.; Diener, C.; Otwell, A.E.; Tams, K.E.; Bebout, B.; Detweiler, A.M.; Lee, M.D.; Scott, M.T.; Ta, W.; Ha, M.; et al. Lettuce (Lactuca sativa) productivity influenced by microbial inocula under nitrogen-limited conditions in aquaponics. PLoS ONE 2021, 16, e0247534. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).