Quantitative Proteome Analysis in Response to Glucose Concentration in C2C12 Myotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Proteomics
2.3. ATP Measurement
2.4. Quantitative Real-Time PCR Analysis (qRT-PCR)
2.5. Statistical Analysis
3. Results
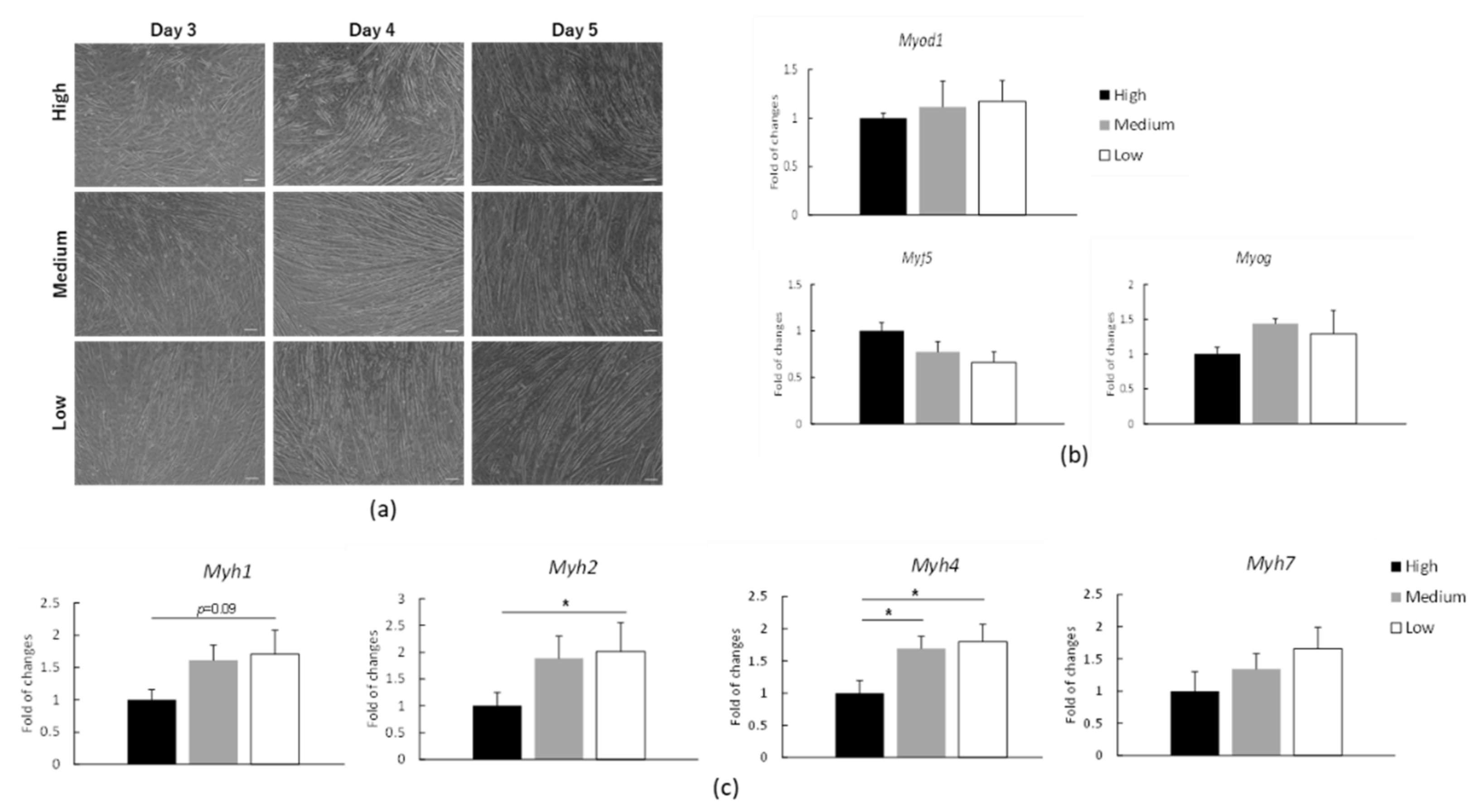
3.1. Proteome Analysis in C2C12 Myotubes under Low, Medium or High Glucose Conditions
3.2. ATP Levels in C2C12 Myotubes under Low, Medium or High Glucose Conditions
3.3. mRNA Expression of Myosin Heavy Chain (Myh) in C2C12 Myotubes under Low, Medium or High Glucose Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Ghani, M.A.; Defronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.R.; Kim, H.K.; Song, I.S.; Youm, J.; Dizon, L.A.; Jeong, S.H.; Ko, T.H.; Heo, H.J.; Ko, K.S.; Rhee, B.D.; et al. Glucocorticoids and their receptors: Insights into specific roles in mitochondria. Prog. Biophys. Mol. Biol. 2013, 112, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1057–1072. [Google Scholar] [CrossRef] [Green Version]
- Seok, W.P.; Goodpaster, B.H.; Jung, S.L.; Kuller, L.H.; Boudreau, R.; De Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.X.; Gong, J.; Chen, Z.; Sun, J.; Xiao, Y.; Wang, L.; Li, Y.; Liu, J.; Xu, X.Z.S.; Lin, J.D. Glucose Sensing by Skeletal Myocytes Couples Nutrient Signaling to Systemic Homeostasis. Mol. Cell 2017, 66, 332–344.e4. [Google Scholar] [CrossRef] [Green Version]
- Elkalaf, M.; Anděl, M.; Trnka, J. Low Glucose but Not Galactose Enhances Oxidative Mitochondrial Metabolism in C2C12 Myoblasts and Myotubes. PLoS ONE 2013, 8, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Nakai, N.; Kitai, S.; Iida, N.; Inoue, S.; Nakata, K.; Murakami, T.; Higashida, K. Induction of Autophagy and Changes in Cellular Metabolism in Glucose Starved C2C12 Myotubes. J. Nutr. Sci. Vitaminol. 2020, 66, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Furuichi, Y.; Kawabata, Y.; Aoki, M.; Mita, Y.; Fujii, N.L.; Manabe, Y. Excess Glucose Impedes the Proliferation of Skeletal Muscle Satellite Cells Under Adherent Culture Conditions. Front. Cell Dev. Biol. 2021, 9, 640399. [Google Scholar] [CrossRef]
- Bekebrede, A.F.; van Deuren, T.; Gerrits, W.J.J.; Keijer, J.; de Boer, V.C.J. Butyrate alters pyruvate flux and induces lipid accumulation in cultured colonocytes. Int. J. Mol. Sci. 2021, 22, 10937. [Google Scholar] [CrossRef]
- Ohigashi, I.; Tanaka, Y.; Kondo, K.; Fujimori, S.; Kondo, H.; Palin, A.C.; Hoffmann, V.; Kozai, M.; Matsushita, Y.; Uda, S.; et al. Trans-omics Impact of Thymoproteasome in Cortical Thymic Epithelial Cells. Cell Rep. 2019, 29, 2901–2916.e6. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, C.; Brandt, U.; Hunte, C.; Zickermann, V. Structure and function of mitochondrial complex I. Biochim. Biophys. Acta 2016, 1857, 902–914. [Google Scholar] [CrossRef]
- Storch, J.; Thumser, A.E. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta 2000, 1486, 28–44. [Google Scholar] [CrossRef] [Green Version]
- Lis, P.; Dylag, M.; Niedźwiecka, K.; Ko, Y.H.; Pedersen, P.L.; Goffeau, A.; Ułaszewski, S. The HK2 dependent “Warburg effect” and mitochondrial oxidative phosphorylation in cancer: Targets for effective therapy with 3-bromopyruvate. Molecules 2016, 21, 1730. [Google Scholar] [CrossRef] [Green Version]
- Baar, M.P.; Perdiguero, E.; Muñoz-Cánoves, P.; de Keizer, P.L. Musculoskeletal senescence: A moving target ready to be eliminated. Curr. Opin. Pharmacol. 2018, 40, 147–155. [Google Scholar] [CrossRef]
- Tang, J.E.; Hartman, J.W.; Phillips, S.M. Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men. Appl. Physiol. Nutr. Metab. 2006, 31, 495–501. [Google Scholar] [CrossRef]
- Kusudo, T.; Kontani, Y.; Kataoka, N.; Ando, F.; Shimokata, H.; Yamashita, H. Fatty acid-binding protein 3 stimulates glucose uptake by facilitating AS160 phosphorylation in mouse muscle cells. Genes Cells 2011, 16, 681–691. [Google Scholar] [CrossRef]
- Sharma, L.K.; Lu, J.; Bai, Y. Mitochondrial respiratory complex I: Structure, function and implication in human diseases. Curr. Med. Chem. 2009, 16, 1266–1277. [Google Scholar] [CrossRef] [Green Version]
- Lazarou, M.; Thorburn, D.R.; Ryan, M.T.; McKenzie, M. Assembly of mitochondrial complex I and defects in disease. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 78–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, C.V.; Peralta, S.; Arguello, T.; Bacman, S.R.; Diaz, F.; Moraes, C.T. Myopathy reversion in mice after restauration of mitochondrial complex I. EMBO Mol. Med. 2020, 12, e10674. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venditti, P.; Di Meo, S. The role of reactive oxygen species in the life cycle of the mitochondrion. Int. J. Mol. Sci. 2020, 21, 2173. [Google Scholar] [CrossRef] [Green Version]
- Cannavino, J.; Brocca, L.; Sandri, M.; Grassi, B.; Bottinelli, R.; Pellegrino, M.A. The role of alterations in mitochondrial dynamics and PGC-1α over-expression in fast muscle atrophy following hindlimb unloading. J. Physiol. 2015, 593, 1981–1995. [Google Scholar] [CrossRef] [Green Version]
- Geng, T.; Li, P.; Yin, X.; Yan, Z. PGC-1α promotes nitric oxide antioxidant defenses and inhibits FOXO signaling against cardiac cachexia in mice. Am. J. Pathol. 2011, 178, 1738–1748. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Olson, L.C.; Redden, J.T.; Schwartz, Z.; Cohen, D.J.; Mcclure, M.J. Advanced Glycation End-Products in Skeletal Muscle Aging. Bioengineering 2021, 8, 168. [Google Scholar] [CrossRef]
- Zheng, Q.; Osunsade, A.; David, Y. Protein arginine deiminase 4 antagonizes methylglyoxal-induced histone glycation. Nat. Commun. 2020, 11, 3241. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Omans, N.D.; Leicher, R.; Osunsade, A.; Agustinus, A.S.; Finkin-Groner, E.; D’Ambrosio, H.; Liu, B.; Chandarlapaty, S.; Liu, S.; et al. Reversible histone glycation is associated with disease-related changes in chromatin architecture. Nat. Commun. 2019, 10, 1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Myod1 | AGGAGCACGCACACTTCTCT | TCTCGAAGGCCTCATTCACT |
| Myf5 | AGACGCCTGAAGAAGGTCAA | GTTCTCCACCTGTTCCCTCA |
| Myog | CTACAGGCCTTGCTCAGCTC | ACGATGGACGTAAGGGAGTG |
| Myh1 | AATCAAAGGTCAAGGCCTACAA | GAATTTGGCCAGGTTGACAT |
| Myh2 | CAGCACGAGCTGGAGGAAG | CTCGCTTCGGTCATTCCAC |
| Myh4 | GCTGAGGAGGCTGAGGAAC | GTGTGAACCTCTCGGCTCTT |
| Myh7 | AGTCCCAGGTCAACAAGCTG | TTCCACCTAAAGGGCTGTTG |
| 18S rRNA | CATTCGAACGTCTGCCCTA | CCTGCTGCCTTCCTTGGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, A.; Sakakibara, I.; Fukushima, T.; Sugiura, K.; Fukawa, T.; Nikawa, T.; Uchida, T. Quantitative Proteome Analysis in Response to Glucose Concentration in C2C12 Myotubes. Appl. Sci. 2022, 12, 1553. https://doi.org/10.3390/app12031553
Kato A, Sakakibara I, Fukushima T, Sugiura K, Fukawa T, Nikawa T, Uchida T. Quantitative Proteome Analysis in Response to Glucose Concentration in C2C12 Myotubes. Applied Sciences. 2022; 12(3):1553. https://doi.org/10.3390/app12031553
Chicago/Turabian StyleKato, Ayano, Iori Sakakibara, Taku Fukushima, Kosuke Sugiura, Tomoya Fukawa, Takeshi Nikawa, and Takayuki Uchida. 2022. "Quantitative Proteome Analysis in Response to Glucose Concentration in C2C12 Myotubes" Applied Sciences 12, no. 3: 1553. https://doi.org/10.3390/app12031553
APA StyleKato, A., Sakakibara, I., Fukushima, T., Sugiura, K., Fukawa, T., Nikawa, T., & Uchida, T. (2022). Quantitative Proteome Analysis in Response to Glucose Concentration in C2C12 Myotubes. Applied Sciences, 12(3), 1553. https://doi.org/10.3390/app12031553







