Abstract
Regarding the use of SF6 in medium voltage switchgear (MVS), a review of alternatives was encouraged by the European Parliament in Regulation No 517/2014. This is aimed at a new regulatory change, that is expected soon, which will include its prohibition, similar to what has happened with other fluorinated greenhouse gases in other fields, like refrigeration. Therefore, there is an urgent need to study the physical and chemical properties of alternative gas mixtures to determine if they are suitable to replace SF6. In this context, this work addresses the difusional analysis of new gases. Binary and ternary mixtures made of 1,3,3,3-tetrafluoropropene (C3F4H2) and heptafluoroisopropyl trifluoromethyl ketone (C5F10O), using dry air as a carrier gas, were studied. The mixtures were analyzed using original equipment, composed of UV-Vis spectroscopy technology in a sealed gas chamber, which is similar to MVS. Consequently, an experimental equipment that monitors the concentration of a gas mixture online and a model that predicts the mixing process were designed and tested. The concentration profiles were obtained concerning both the time and position in the gas chamber, and the diffusional and convectional parameters were numerically calculated and optimized in an algorithm created in Scilab.
1. Introduction
Sulfur hexafluoride, SF6, is the most widely applied gas in the electric market for insulation and electric arc quenching due to its high stability, high dielectric strength, and non-toxicity [1]. It is commonly used in gas-insulated switchgear (GIS), gas-insulated transformers (GIT), gas-insulated lines (GIL), and gas-insulated circuit breakers (GICB) [2]. However, it is also considered a very strong greenhouse gas, with a global warming potential (GWP) of about 23,500 on a 100-year horizon, making SF6 the compound with the highest value according to the Fifth Assessment Report (AR5) of the Intergovernmental Panel on Climate Change (IPCC) [3]. In 1997, in the Kyoto Protocol [4], the reduction of greenhouse gas emissions was established, which included SF6, so alternative gases for applications that use SF6 have been investigated ever since. Although the equipment in which sulfur hexafluoride is used is sealed, leaks between the sealing surfaces, permeation of the gas through the thermoplastic materials, or accidents can happen [5], resulting in gas emissions. SF6 and other common fluorinated greenhouse gases, their lifetime (LT) in the atmosphere, GWP values, and applications are shown in Table 1.

Table 1.
The lifetime (LT) in the atmosphere, global warming potential values in a 100-year horizon (GWP100), and applications of SF6 and other fluorinated greenhouse gases.
In 2014, the European Parliament developed Regulation (EU) No 517/2014 [11], on fluorinated greenhouse gases, where rules for the use, containment, recovery, and destruction of some fluorinated greenhouse gases are collected. These fluorinated gases include not only SF6, but also hydrofluorocarbons (HFC) and perfluorocarbons (PFC). The European Commission is currently reviewing the impact that the actual regulation has had, and is expected to propose a new regulation soon [12]. For that reason, it is urgent to find an alternative that replaces SF6 in medium voltage switchgear (MVS) before a possible ban on its use comes into force.
Initially, SF6 alternatives were focused on the use of existing technologies such as air-insulated switchgear (AIS) [13] or solid-insulated switchgear (SIS) [14]. Even though both have been commonly used in low-voltage equipment, their use in higher voltage systems would require higher dimensions and, therefore, higher costs.
The mixture between SF6 with a carrier gas, like air, N2, or CO2, was then considered for insulation [15]. Although the use of SF6 is reduced, the environmental impact of the mixture, even with low SF6 concentrations, is still significantly high. Consequently, researchers are more focused on finding other gas or gas mixtures that replace SF6 completely.
There are some important requirements that this gas or gas mixture must meet, according to Kieffel et al. [16], to be considered a suitable option: high dielectric strength, good arc quenching capability, low boiling point, high vapor pressure at low temperature, high heat dissipation, and compatibility with the materials used in electrical switchgear, among others. Moreover, the gases must have low toxicity, no flammability, no ozone depletion potential (ODP), minimal environmental impact, and low GWP, to meet the environmental, health, and safety requirements.
The most important of these characteristics is the dielectric strength, or electric field strength (Ecr), which is defined as the maximum voltage that an insulating component can withstand before suffering electrical breakdown [17]. Currently, the different gases that are being researched are hydrofluorocarbons (HFC), perfluorocarbons (PFC), fluoroketones (FK), fluoronitriles (FN), and hydrofluoroolefins (HFO) [15,16]. HFCs and PFCs have excellent dielectric properties [2], but their GWP is high, between 5000 and 12,000 in a 100-year horizon [16], making them not suitable environmentally. FKs and FNs have better dielectric properties than SF6 [15]. One of the most researched FK, C5F10O, has almost the same GWP as CO2, but its boiling point reaches 300 K. Although FNs have higher GWP, they present a lower boiling point, high stability, and material compatibility [18]. Finally, a certain researched HFO, C3F4H2, has slightly lower dielectric strength than SF6, a low boiling point, and low GWP [16].
Table 2 presents some of the gases and gas mixtures that have been researched for the replacement of SF6 in medium and high voltage electrical switchgear, and their relative breakdown strength (Erel), GWP, boiling temperature (TB), lifetime (LT) in the atmosphere, toxicity threshold limit value–time weighted average (TLV-TWA), and decomposition products. The boiling temperature of all these gases is higher than the minimum operating temperature of outdoor medium voltage switchgear of 248 K, according to IEC 62271-200, except C3F8, so the gases must be diluted with a carrier gas to ensure that they do not liquefy in any circumstance. The most common carrier gases are the natural gases, CO2, N2, and dry air. The dielectric strength of the components is similar to that of SF6, even after mixing them with the carrier gas, and the highest GWP is 70% lower. Besides, the lifetime in the atmosphere is lower than 20 days for the components CF3I, C3F4H2, C5F10O, and C6F12O. Furthermore, the TLV-TWA values of all the gas mixtures are lower than the one of SF6; however, as they need to be mixed with a carrier gas, the toxicity decreases.

Table 2.
Relative breakdown strength (Erel), global warming potential values in a 100-year horizon (GWP100), boiling point (TB), toxicity threshold limit values–time weighted average (TLV-TWA), and decomposition products of the main researched alternative gas or gas mixtures to replace SF6 in medium and high voltage switchgear.
The key to identifying what could be a suitable alternative is to find a component with low GWP that preserves a high dielectric strength when it is diluted with a vector gas to reduce the boiling temperature. Various researchers have already presented patents for some of these gas mixtures for their use in medium and high voltage switchgear [19,20,21,22].
Binary and ternary mixtures made with the hydrofluoroolefin C3F4H2, also known as HFO-1234ze(E), and the perfluoroketone C5F10O, have been studied. They will be referred to as HFO3E and PFK5, respectively, and their molecular structures are shown in Figure 1. These gases have been selected by Ormazabal Corporate Technology. Due to their boiling point being higher than that of SF6, as shown in Table 2, dry air has been used as a carrier gas. Because of that, the dielectric strength of the gas mixture is also reduced, so a balance must be found between getting the boiling point low enough, while maintaining the dielectric properties. The boiling point of PFK5 is higher than the one of HFO3E, which means that it should be more diluted. Moreover, this favors the fact that HFO3E has lower dielectric strength.
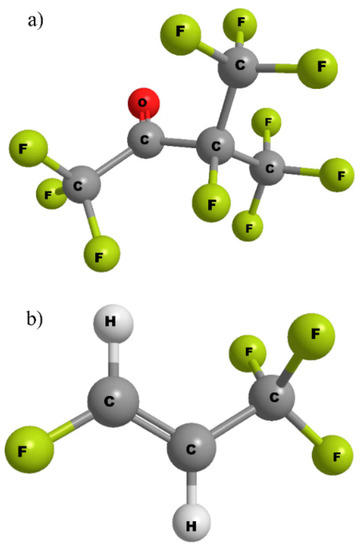
Figure 1.
Molecular structures of (a) C5F10O (PFK5) and (b) C3F4H2 (HFO3E).
The understanding of the mixing process of these new gas mixtures is critical to define the physical and chemical properties that affect the electrical insulation and arc quenching properties. Therefore, the goal of this paper is the study of the diffusional behavior of the binary and ternary mixtures made with PFK5, HFO3E, and dry air, as possible alternatives for SF6 for medium voltage switchgear, to anticipate the oncoming change of regulations. A chamber has been designed and built to study the diffusion of these binary and ternary mixtures, using UV-Vis spectroscopy to measure the concentration in the chamber during the mixing process. This technique was already used by some authors to measure these kinds of gases [46,47].
A diffusion process model is proposed to describe the mixture process; the model is fitted to the experimental concentration values in an algorithm developed in Scilab, which numerically calculates the diffusion parameters of the mixtures.
In this work, a novel experimental equipment capable of measuring and collecting the online concentration of a gas mixture has been designed and tested with fluorinated gas mixtures, candidates to replace SF6 in medium voltage switchgear. Although this study has been focused on the analysis of a specific case of new fluorinated gas mixtures, this non-destructive technology would be of great interest for any application in which it is necessary to determine the physicochemical properties of a gas mixture through its composition. Some examples include the design of gas mixtures that require specific characteristics, the monitoring of reactive mixtures, or the online malfunction detection and maintenance without the need to take samples that could compromise the integrity of the gas mixtures.
Moreover, a mathematical model that simulates the diffusion process of a component in a binary or ternary gas mixture has been proposed and implemented. The mixing process of the new insulating gas mixtures and the time it takes for them to reach stability have been determined.
This article intends to lay the groundwork for future experiments to study the effect of different perturbations, such as temperature, humidity, and concentration changes, among others, during the filling, mixing, and post-mixing process of the new insulating gas mixtures.
2. Materials and Methods
2.1. Experimental Equipment
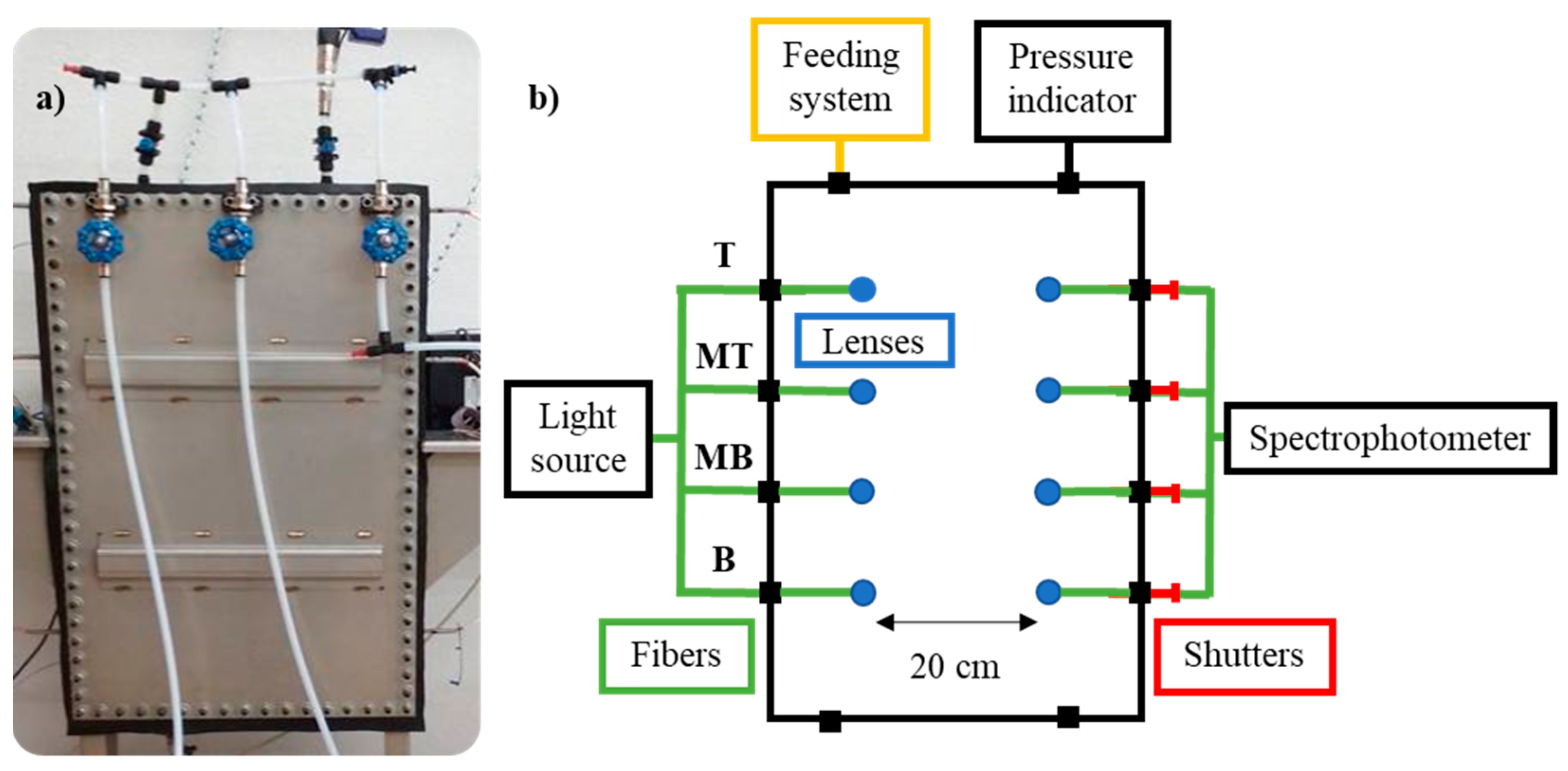
The gas diffusion was determined using an experimental system that was specifically designed for this project to contain the gas mixtures and measure their concentration over time, using UV-Vis spectroscopy technology. The experimental equipment is shown in Figure 2.
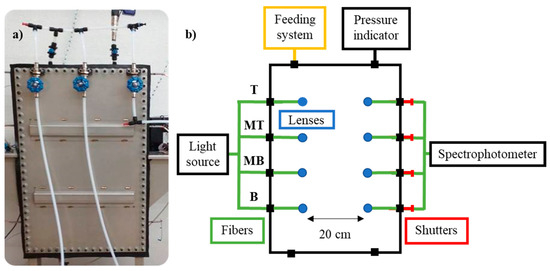
Figure 2.
(a) Gas chamber and (b) scheme of the experimental equipment used for the gas diffusion study and its components. T (Top), MT (Medium Top), MB (Medium Bottom), and B (Bottom) correspond to the different heights of the measurement lines.
The designed system consisted of a 60 L tank, a DH-2000-S-DUV-TTL light source (Ocean Insight, Orlando, FL, USA), a Maya2000Pro spectrophotometer (Ocean Insight), a pressure indicator (WIKA, Klingenberg, Bayern, Deutschland), and a feeding system composed of polytetrafluoroethylene (PTFE) tubes to introduce the gas mixtures in the tank. The tank had similar dimensions to an actual medium voltage switchgear, but was slightly narrower, 0.8 × 0.5 × 0.15 m, which favors vertical gas diffusion.
The light source emits a continuous UV-Vis spectrum, between 190–750 nm, which is split into the four fibers that end in four lenses that are located at four different heights of the tank, as the interest is focused on the diffusion that happens vertically. The measurement lines are located at 0.13, 0.35, 0.57, and 0.78 m; for simplicity reasons, the lines are referred to as Bottom (B), Medium Bottom (MB), Medium Top (MT), and Top (T), respectively.
The emitted light passes through the tank and is collected in four other lenses and fibers that merge and terminate in the spectrophotometer, which provides the corresponding ultraviolet absorbance spectra. A shutter is installed in each line, so the signal of each line is measured individually.
2.2. Mixing Process Tracking
The concentration (C) of the components in the gas mixtures that were studied are shown in Table 3. Each gas mixture was replicated three times. The components of the mixture were introduced in the tank one at a time through the feeding system, according to their molecular weight. An initial state of stratification by density was created by inserting the gases from heaviest to lightest, favoring the monitoring of the mixing process.

Table 3.
Nomenclature and concentration (C) of the components of the studied gas mixtures.
The dielectric strength, or critical field strength (Ecr), of the gas mixtures mentioned above can be estimated by a linear scaling according to Saxegaard et al. [48]:
where xi and Ecr,i are the molar fraction and the electrical field strength of each component of the mixture, taking into consideration the dielectric strength of SF6 and dry air (89 and 27 V m−1 Pa−1) [49], and the Erel values of PFK5 and HFO3E that are shown on Table 2. The Ecr of components PFK5 and HFO3E are 178 and 71 V m−1 Pa−1, and the estimated values are 42, 57, 37, and 53 V m−1 Pa−1 for the gas mixtures B-10P, B-20P, B-40H, and T-10P/40H, respectively.
The measurement process of absorbance was automatized using LabVIEW, identifying the values of the absorbance for the 196 and 300 nm wavelengths, which correspond to the maximum absorption of HFO3E and PFK5, respectively. The system was programmed to open and close the shutters of the light source and the fibers, and to control the spectrophotometer to measure the reference intensities and calculate the absorbance of the four lines sequentially. The absorbance was calculated every 15 min for the first hour, every 20 min for the second hour, and every 30 min until the end of the experiment, which ends after 20 h.
Absorbance (A) was calculated by the Beer–Lambert Law [50]:
where I0 is the reference intensity, I is the intensity that is read after the absorption, and D0 is the dark intensity, the one that the spectrophotometer reads when no light is emitted. A can also be expressed by the molar absorptivity (εM, m3 mol−1 m−1) multiplied by the molar concentration of the species (C) and the path length (l).
The mean value of the absorbance that is obtained in the three replicas of each experiment was the one used for the numerical calculation of the diffusion parameters.
2.2.1. Determination of the Concentration
The concentration was obtained through a calibration that relates it with the absorbance of each component. Four gas mixtures of known concentration were prepared for each component: 5%, 10%, 15% and 20% mixtures for PFK5, and 10%, 20%, 30% and 40% for HFO3E.
Each mixture was introduced in the gas chamber and the absorbance was monitored until the mixing process was completed, which means when all measurement lines displayed a similar value. The absorbance value that was obtained is the one related to the specific concentration that was introduced.
The A-C data were plotted and adjusted to linear regression to calculate the equations of the calibration curves. These equations allowed the calculation of the concentration of the components from the measurements of absorbance.
2.2.2. Diffusional Model
Two effects needed to be studied to determine the variation of concentration over time: (a) molecular diffusion, the transfer of individual molecules due to a concentration, pressure, or temperature gradient, among others; and (b) convection transport, caused by the overall movement of the fluid [51]. As the gases used in these experiments have high molecular weight, it was considered that the gas mixing process happened not only due to the concentration gradient, but also to the differences in the density of the components or natural convection.
Considering the described two effects, a modified Fick’s generalized expression was obtained:
where Ci is the concentration, D (m2 s−1) is the diffusion coefficient, t represents the time, and z the height. Vi is the velocity caused by the differences between densities:
where K (m4 kg−1 s−1) is the convection constant. The density of the heavy component in the binary mixture (ρm) and the density of the mixture at each point in time (ρi) were calculated by:
T and P are the temperature and pressure at which the experiments were carried out, 298 K and 1 atm (101,325 Pa), and R is the ideal gas constant. MWm is the molecular weight of the heavy component of the binary mixture, and MWi indicates the molecular weight of the mixture at each point in time, which was calculated by the weighted average of the MWm of each component of the mixture:
The equations were based on the fact that for a binary mixture consisting of components A and B, the diffusivity of A in B (DAB), and vice versa (DBA), are the same [51]. Both molecular diffusion and natural convection were considered to determine the molar flux (J):
Theoretical studies of multicomponent diffusion are based on the Stefan–Maxwell equation, which, in turn, was derived from the solution of the Boltzmann equation [52]. For isothermal and isobaric conditions, this equation is a strong approximation that satisfies the practical requirement. The variation of concentration over time for multicomponent mixtures is represented in the following equation:
The element [D] is a size n − 1 squared matrix that represents the diffusion coefficients, n being the number of components in the mixture. The elements of the square matrix [D] are called practical diffusion coefficients [52]. In this study, a gas mixture composed of three elements was made; therefore, the matrix was composed of 2 × 2 elements. As well as in binary gas mixtures, the convection effect was also considered in multicomponent mixing:
Hence, the concentration differential of each component over time depends on the practical diffusion coefficients of both components and their concentration differential over the position in the gas chamber [52]:
Furthermore, the effective diffusivity coefficients (Deff) were calculated using the practical diffusion coefficients [52]. Bird et al. [53] stated that the molar fraction gradients could be replaced by molar fraction differences to simplify the calculations:
Furthermore, according to Taylor and Krisna [52], the diffusivity eigenvalues of the components ( and ) must be positive:
where tr[D] is the trace and disc[D] is the discriminant of the matrix. Because of Equations (14) and (15), the conditions presented in the following equations must be met:
The proposed mathematical model was reproduced in an algorithm created in Scilab. The purpose of the program is the numerical calculation of the differential Equations (3), (11) and (12) to obtain the optimized diffusion and convection coefficients by minimizing an error objective function (OF):
Such OF was defined as the sum of the squared differences between the experimental values of the concentration (Cexp) and the ones obtained in the model (Cmodel) divided by the whole amount of measured concentrations in the lines (N). The relative standard error (RSE) [54] between the experimental concentration values and the model was also calculated by referencing them to the final concentration value (Cfinal):
The optimization algorithm looked for the values of D and K in the differential equations so that the theoretical model fitted the experimental values and also calculated the relative standard error.
Additionally, the binary diffusion coefficients (DE) were also calculated using the correlation proposed by Fuller, Schettler, and Giddings [52,55]:
The results were compared with the numerically obtained optimized diffusion constants of all binary mixtures. The binary diffusion coefficient between components A and B (DAB) depends on the temperature (T, K), pressure (P, Pa), molecular weight (M, g mol−1), and atomic diffusion volumes (v). The values of v for the atoms C, H, O, and F are 15.9, 2.31, 6.11, and 14.7, respectively [56].
The values of DE of the ternary mixture were calculated by dividing the mixture into two pseudo-binary mixtures between one heavy component against the other two components as a whole. The estimated value for each heavy component was compared with the numerically calculated Deff values.
3. Results and Discussion
3.1. Mixing Process Tracking
3.1.1. Determination of the Concentration
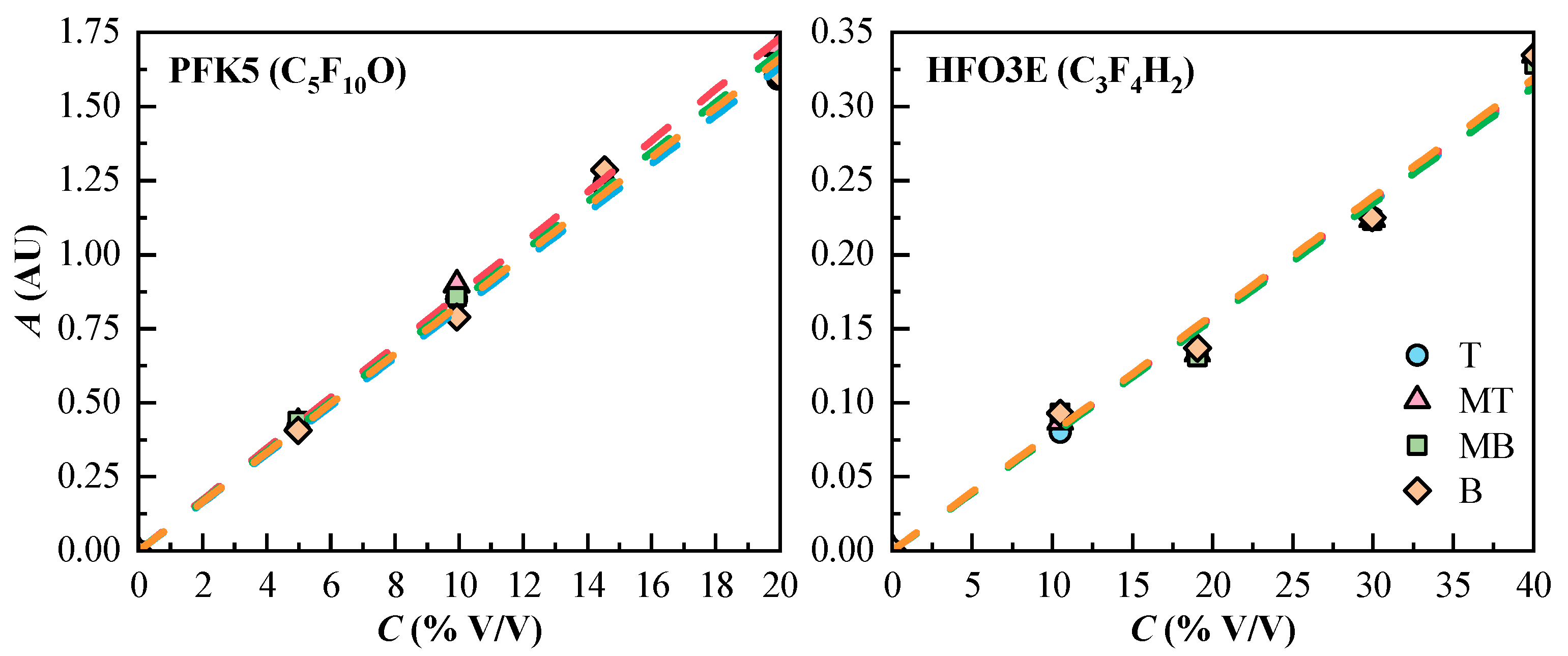
The calibration curve of each component and measurement line is shown in Figure 3. The curves present a linear tendency. The linear regression equations, correlation coefficients (r2), and the molar absorptivity of PFK5 and HFO3E were calculated, and they are collected in Table 4. The results show that the sensitivity of the measuring system for HFO3E is considerably lower than the one of PFK5, due to the great difference in the molar absorptivity of both gases.
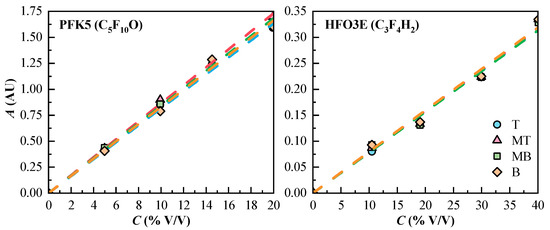
Figure 3.
Experimental concentration values (Cexp, dots) and calibration curves (dashed lines) of the components of the gas mixtures. T (Top, ◯), MT (Medium Top, △), MB (Medium Bottom, ☐), and B (Bottom, ◇) correspond to the different heights of the measurement lines.

Table 4.
Linear regression equations, correlation coefficients (r2), and molar absorptivities (εM), of PFK5 and HFO3E.
The maximum absorption of the component HFO3E is located at 196 nm in the UV-Vis spectrum, where it is common to find noise when measuring the absorbance since this wavelength shows very poor selectivity. Because of this, the concentration, velocity and molar flux profiles of HFO3E have more deviations and are less accurate.
3.1.2. Diffusional Model
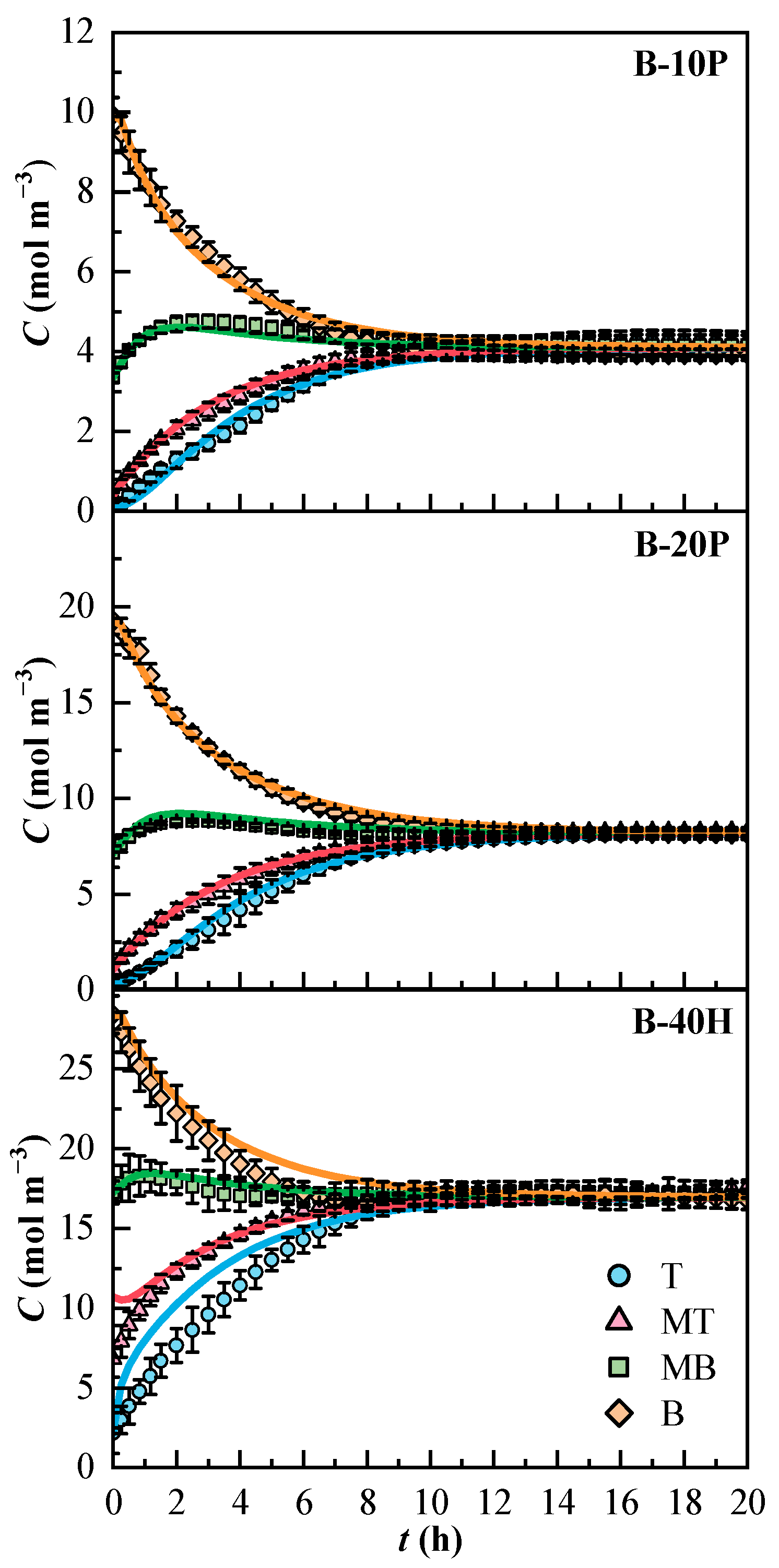
The concentration profiles of the model and the diffusional coefficients were obtained thanks to the optimization process in Scilab. The concentration profiles of the binary mixtures are plotted in Figure 4. The 95% confidence interval of the experimental values is indicated. The B-40H mixture showed broader confidence intervals than the binary mixtures of PFK5. The concentration of all binary mixtures reached stabilization after 10 h. The molar concentrations at the end of the experiments were 4.1 and 8.2 mol m−3 for the PFK5 mixtures and 17.1 mol m−3 for the HFO3E mixture.
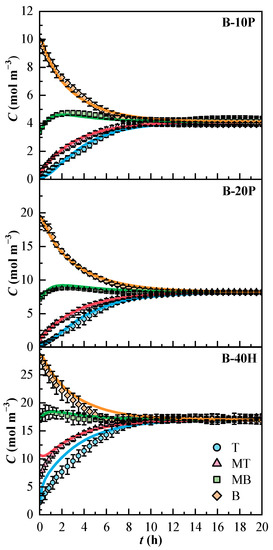
Figure 4.
Experimental (Cexp, dots) and estimated (Cmodel, solid lines) concentration profiles of the binary mixtures over time. T (Top, ◯), MT (Medium Top, △), MB (Medium Bottom, ☐), and B (Bottom, ◇) correspond to the different heights of the measurement lines.
The model fitted the experimental data of B-10P and B-20P mixtures correctly. The concentration profiles from the mixture B-40H show that the model deviated in lines T and B at the beginning of the experiment. The molar absorptivity of HFO3E is ten times lower than that of PFK5 in the UV spectrum. T is the line that shows the lowest concentration and B is the line that shows the highest molar flux, as will be discussed later.
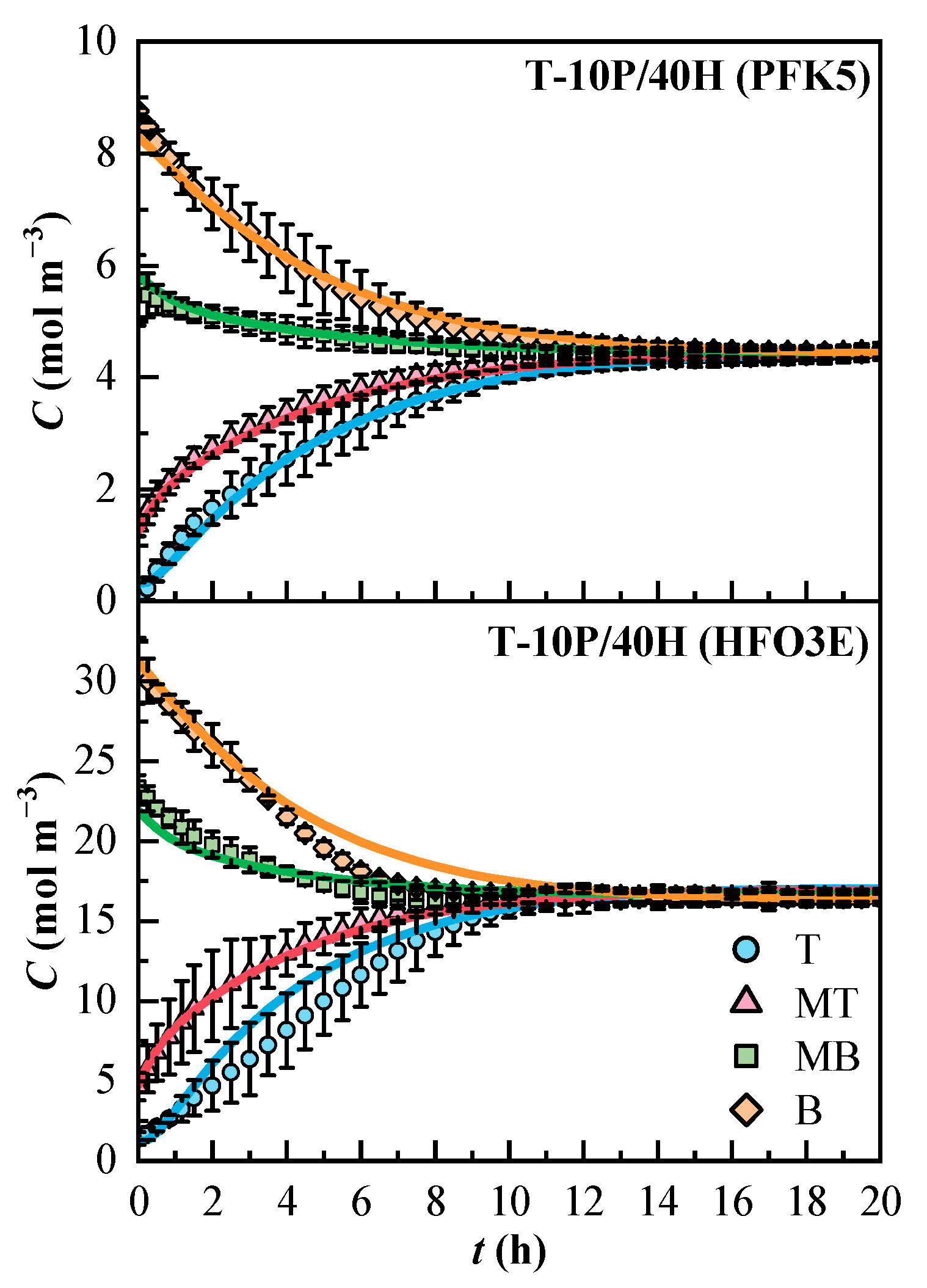
The concentration profiles obtained for the T-10P/40H mixture are shown in Figure 5. In general, the ternary mixture showed greater confidence intervals than the binary mixtures. Line MB exhibited less variation in both components than the others. Even though the whole mixture stabilized after 12 h, the concentration of HFO3E was stable after 10 h. The compound PFK5 is the one that determines the duration of the mixing process, as it is the one with the lower effective diffusion coefficient. The molar concentrations at the end of the experiments were 4.4 mol m−3 for PFK5 and 16.6 mol m−3 for HFO3E. The model fitted the experimental data correctly, although the concentration profile of HFO3E also deviated from the confidence interval in lines T and B, like in mixture B-40H.
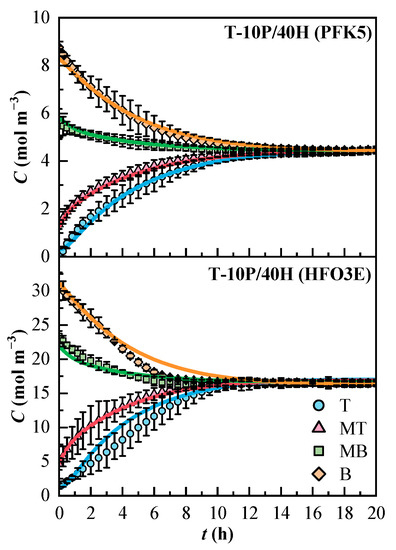
Figure 5.
Experimental (Cexp, dots) and estimated (Cmodel, solid lines) concentration profiles of the components of the ternary mixture over time. T (Top, ◯), MT (Medium Top, △), MB (Medium Bottom, ☐), and B (Bottom, ◇) correspond to the different heights of the measurement lines.
The optimized diffusion and convective coefficients, the theoretically estimated coefficients, and relative standard error for the binary mixtures are shown in Table 5. As diffusion does not depend on concentration, the D of mixtures B-10P and B-20P should be equal, and they are similar. Additionally, the K values are very low. It can be assumed that the convection mechanism had little effect on the mixing process. The RSE for all binary mixtures is lower than 6%, and the mixture whose model best fitted the experimental data was B-20P, which also has the lowest diffusion coefficient. The optimized coefficients of the PFK5 mixtures are more similar to the estimated one than the HFO3E mixture, and the error increases with the concentration.

Table 5.
Diffusion (DE and D) and convective (K) coefficients and relative standard error (RSE) of the binary mixtures after the optimization process using Scilab.
For the ternary mixture, the values of the practical diffusion coefficients were the following:
The conditions that Taylor and Krisna described [52], which are shown in Equations (16)–(18), were met.
The diffusion and convection coefficients and RSE values are shown in Table 6. Both values of K are also very low in the ternary mixture. The Deff for PFK5 and HFO3E are calculated from Equation (13). They are lower than the D obtained for the binary mixtures, which states that the ternary mixture diffuses slower than the binary mixtures. The Deff for HFO3E is higher than the Deff for PFK5. That of HFO3E is closer to its estimated value than the one of PFK5. HFO3E diffuses slower and PFK5 diffuses faster than what was theoretically estimated. Overall, the model fitted better the experimental data of PFK5 than the data of HFO3E. The results are consistent with what is shown in Figure 5.

Table 6.
Diffusion (Deff,E and Deff) and convective (K) coefficients and relative standard error (RSE) of the components of the ternary mixture after the optimization process in Scilab.
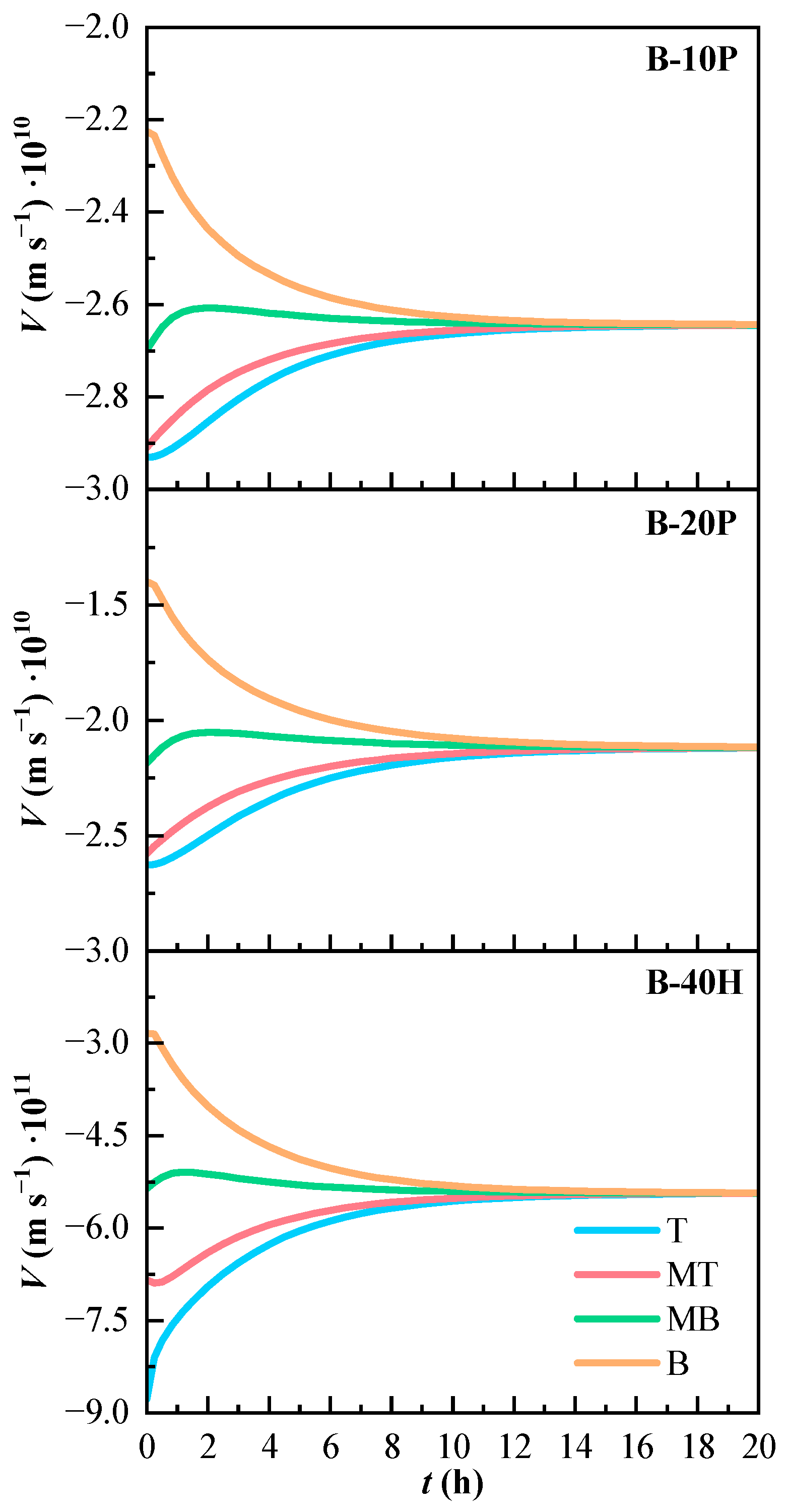
The convection velocity of the binary mixtures is shown in Figure 6. The velocity is negative, as K is positive and the mixture with air has lower density than PFK5 or HFO3E. The convection phenomenon pushes down the heavy components of the mixtures. The most negative velocity values are found in line T, which is the highest line in the gas chamber and therefore the most affected by the high density of the components. The velocities are less negative as the height in which the measurement line is located decreases. Nonetheless, the convection velocity is so small that it is almost negligible.
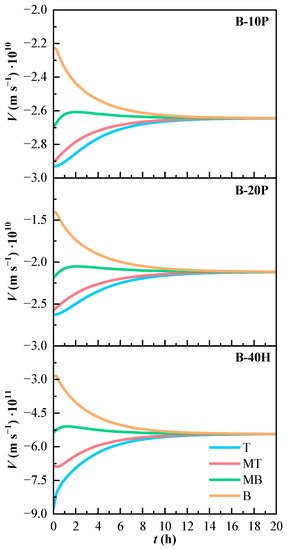
Figure 6.
Variation of the estimated convection velocity (V) of the binary mixtures over time. T (Top), MT (Medium Top), MB (Medium Bottom), and B (Bottom) correspond to the different heights of the measurement lines.
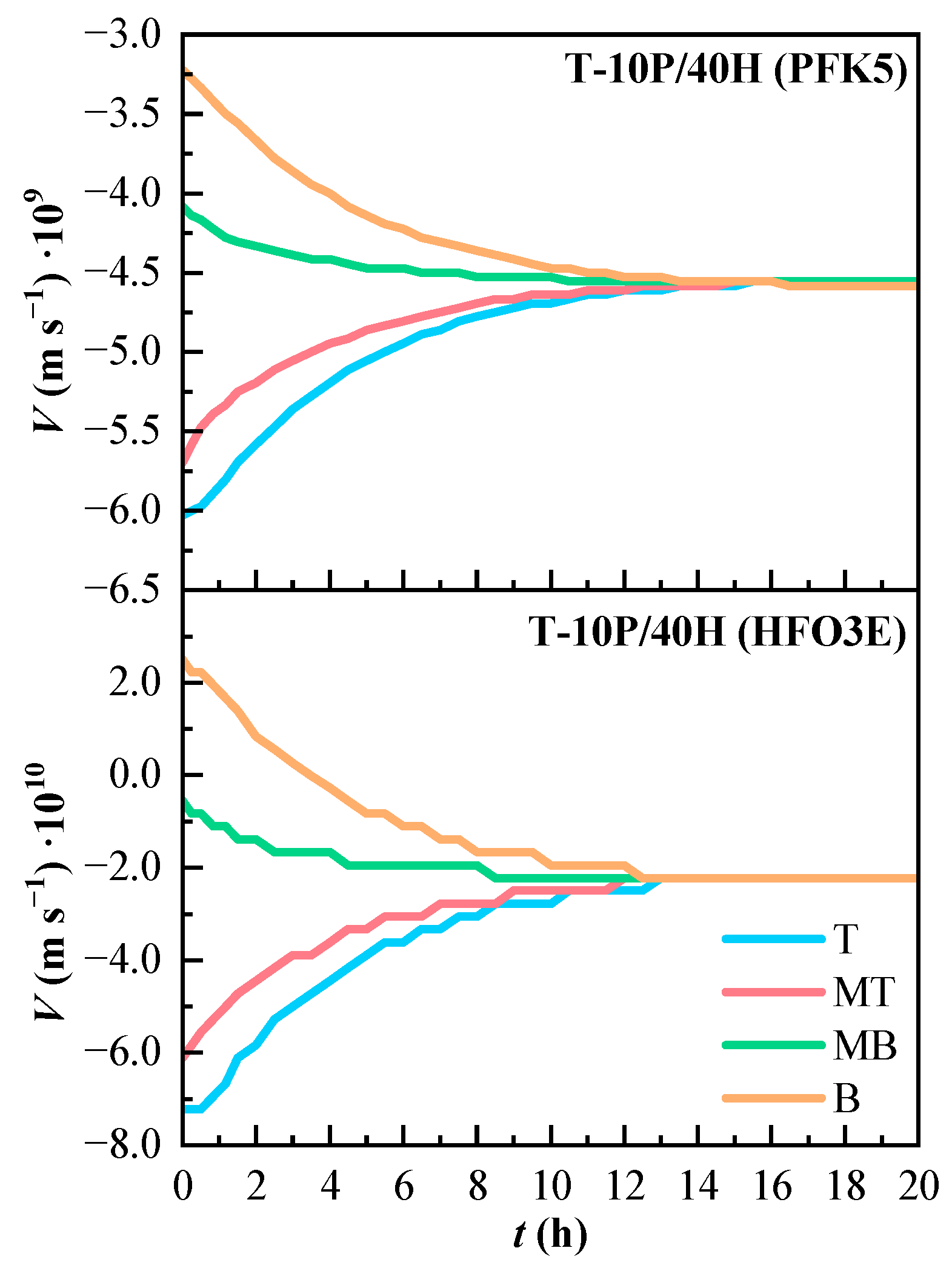
The convection velocity of T-10P/40H is shown in Figure 7. As the component PFK5 is the heaviest, its convection velocity is always negative, because any mixture between HFO3E and air will always have lower density than PFK5. The convection velocity of component HFO3E is positive in line B at the beginning of the experiment, which happens because the higher concentration of PFK5 pushes up HFO3E and the carrier gas.
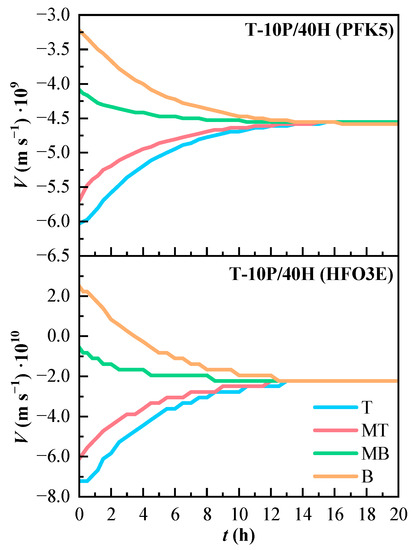
Figure 7.
Variation of the estimated convection velocity (V) of the components of the ternary mixture over time. T (Top), MT (Medium Top), MB (Medium Bottom), and B (Bottom) correspond to the different heights of the measurement lines.
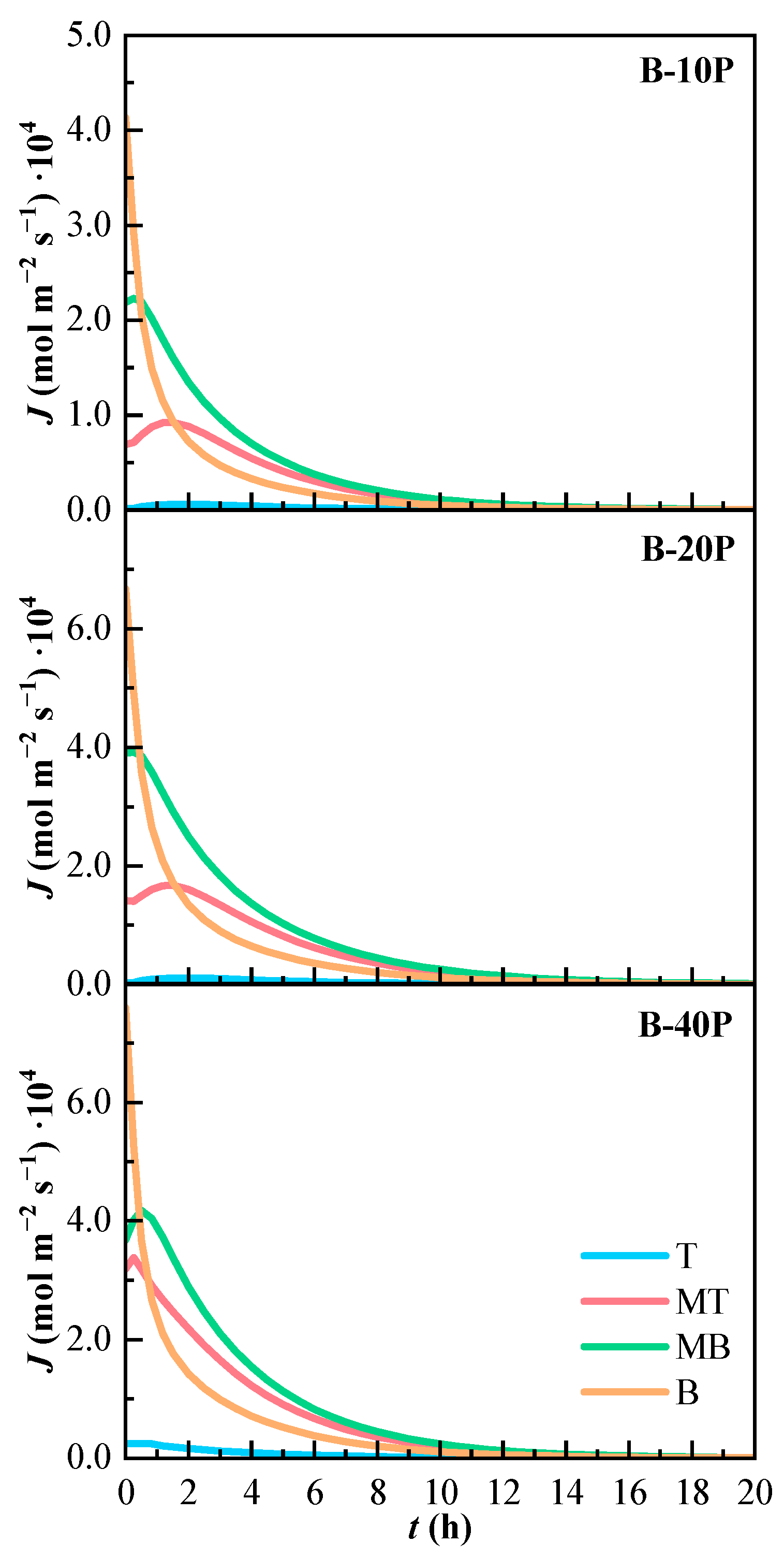
The molar flux of the binary mixtures is shown in Figure 8. All binary mixtures have a similar tendency. The molar flow is higher in line B, the one that presents the highest concentration values. The molar flow is almost zero in line T. The molar flow of lines MT and MB slightly increase at the beginning of the experiments.
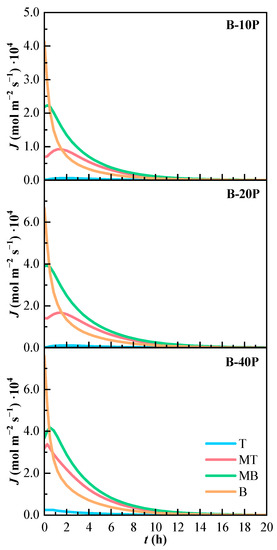
Figure 8.
Variation of the estimated molar flux (J) of the binary mixtures over time. T (Top), MT (Medium Top), MB (Medium Bottom), and B (Bottom) correspond to the different heights of the measurement lines.
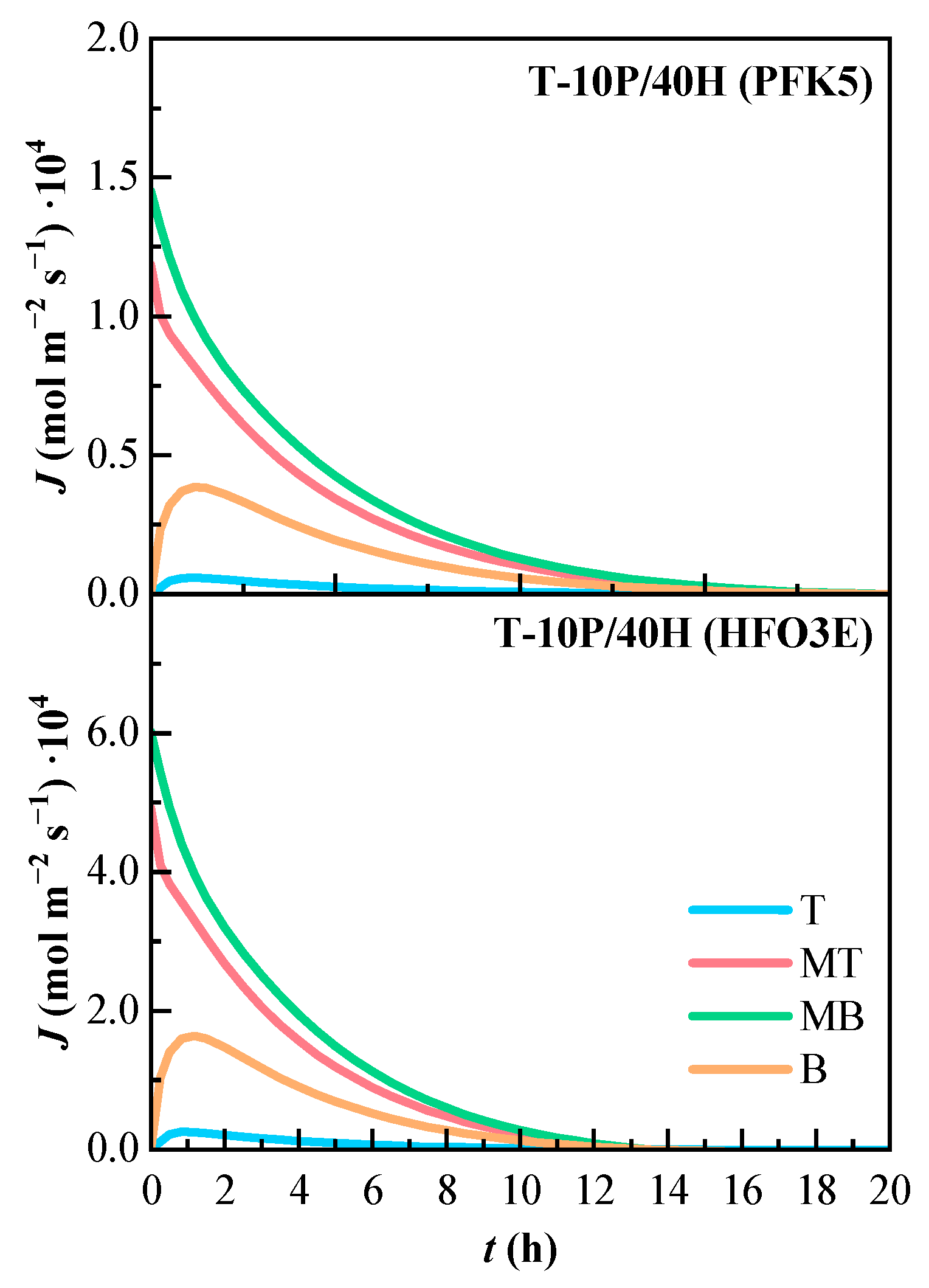
The molar flux of the ternary mixture can be observed in Figure 9. Both molar flux profiles share the same tendency, are very similar, and reach zero approximately at the same time. The lines that present higher molar flux are MT and MB, as they show the highest concentration gradients between all components. The molar flux of line B is the one that increased at the beginning of the mixing process, and line T is also almost zero.
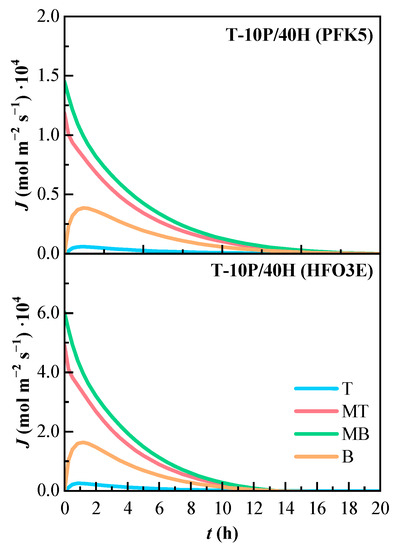
Figure 9.
Variation of the estimated molar flux (J) of the components of the ternary mixture over time. T (Top), MT (Medium Top), MB (Medium Bottom), and B (Bottom) correspond to the different heights of the measurement lines.
The molar flux profiles, together with the obtained concentration and velocity profiles, represent that all the mixtures reached homogeneity and stability at the end of the experiments.
3.1.3. Model Validation
The validation of the model was carried out to determine its accuracy not only for the gas mixtures used to build it, but also for mixtures made with other concentration values too [53]. Five gas mixtures were proposed, different from the ones used to prepare the model. The composition of the gas mixtures is shown in Table 7.

Table 7.
Nomenclature and concentration (C) of the components of the gas mixtures used for validation.
The concentration profiles of these mixtures were simulated. The D and K coefficients that were used for the binary mixtures are the ones shown in Table 5, and the Deff and K coefficients that were used for the ternary mixture are obtained from Table 6. The residuals of each mixture, throughout the whole experiment, were calculated:
The mean residual value of all four measurement lines at a specific time () and the mean residual value of a specific measurement line () until the end of the experiment (tf) were also calculated:
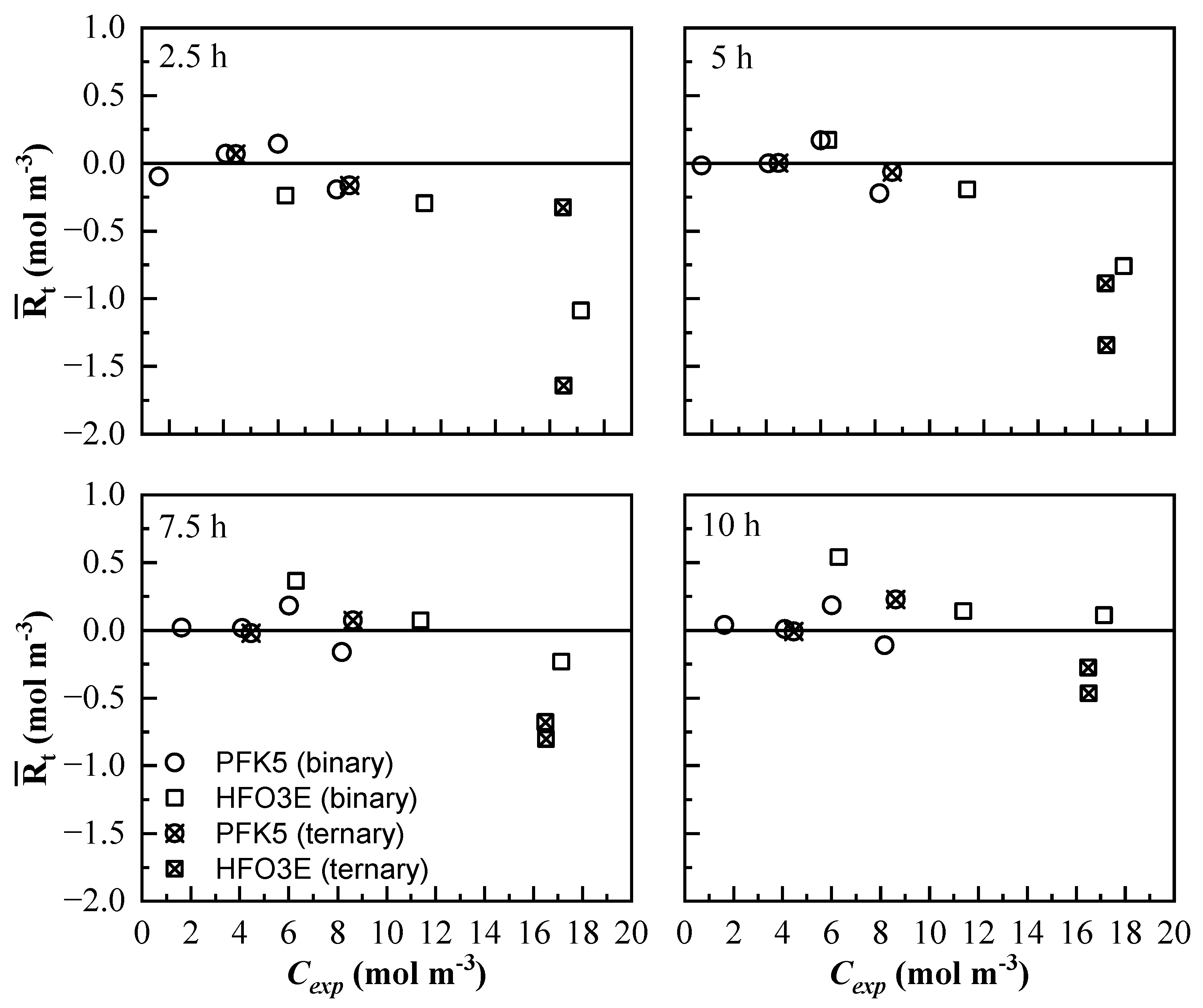
The end of the experiment was considered when the concentration of the four lines reached stabilization. All the mean residual values were represented against Cexp, including the mixtures that were used for building the model [53]. is represented against Cexp in Figure 10. The residuals of PFK5 do not show a distinct tendency; they are distributed almost evenly in the positive and negative sides of the y-axis and are near zero. This shows that the error of PFK5 is not carried over with the model. There is no significant difference between the of PFK5 of binary and ternary mixtures.
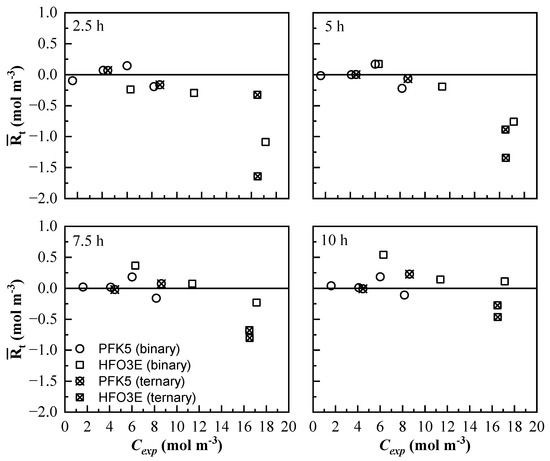
Figure 10.
Mean residual value of all measurement lines at different times () of the mixing process. The results of all the binary (◯ and ☐) and ternary (⊗ and ⊠) mixtures are represented for PFK5 and HFO3E, respectively.
The results for HFO3E show higher residual values and they are not evenly distributed. Most of them are negative values, which shows that the model is more likely to present higher values than the experimental ones. The residuals also decrease with time, so the model showed better results near stabilization. The low absorptivity of this component, together with the higher molar flow at the beginning of the experiments, makes it more difficult to predict the values of concentration. Besides, the concentration of HFO3E of the binary mixtures is easier to calculate as the residuals are lower.
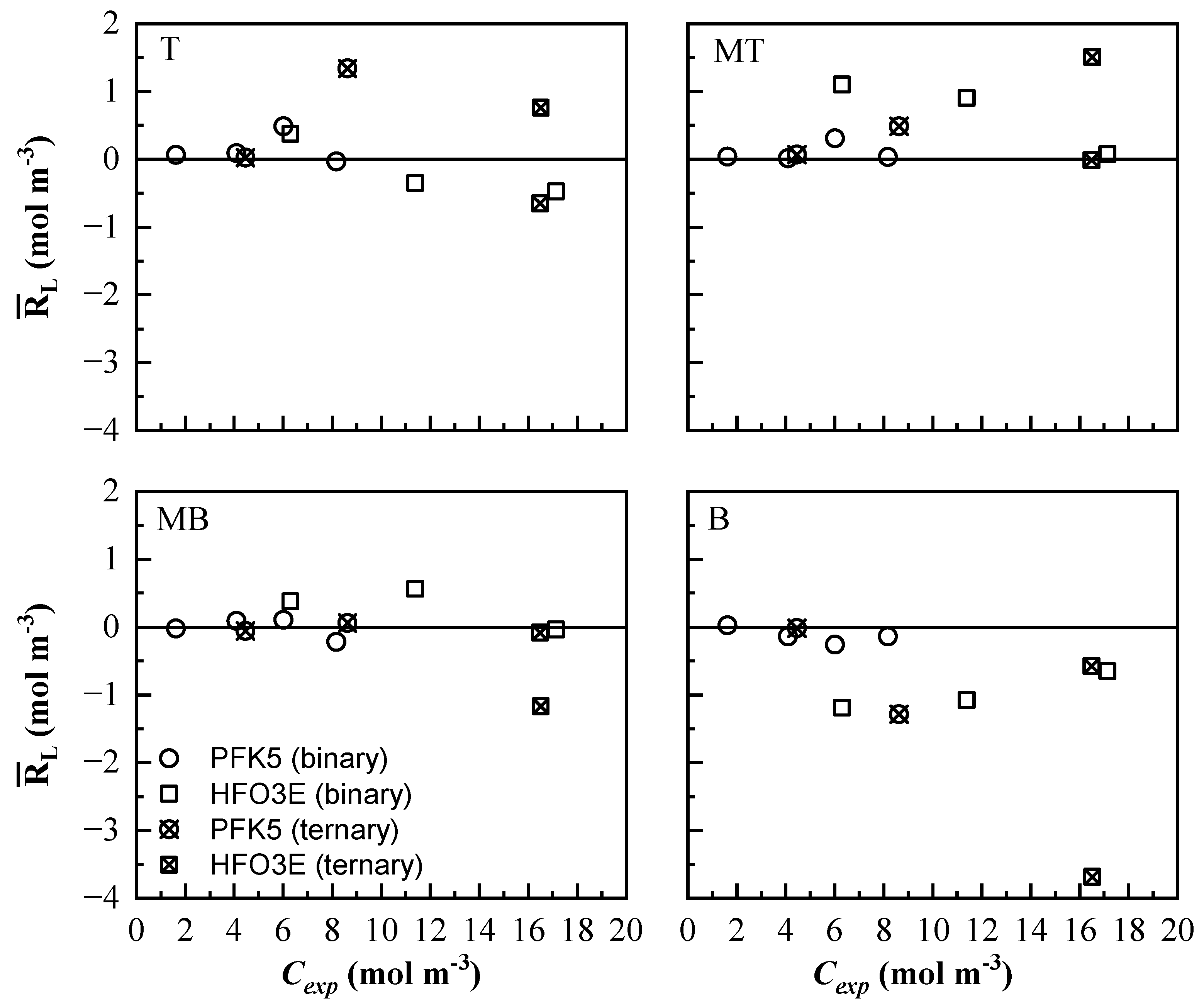
is represented against Cexp in Figure 11. Lines T and MB show lower residual values for both components. PFK5 clearly shows lower residual values in both binary and ternary mixtures. The values of HFO3E of the ternary mixtures are higher than the ones of the binary mixtures. The model will predict lower values than the experimental ones in line MT and higher ones in line B. Line B shows the highest absolute residual values, especially for HFO3E. The higher molar concentration and molar flux that was found in this line complicated the ability of the model to predict the concentration values.
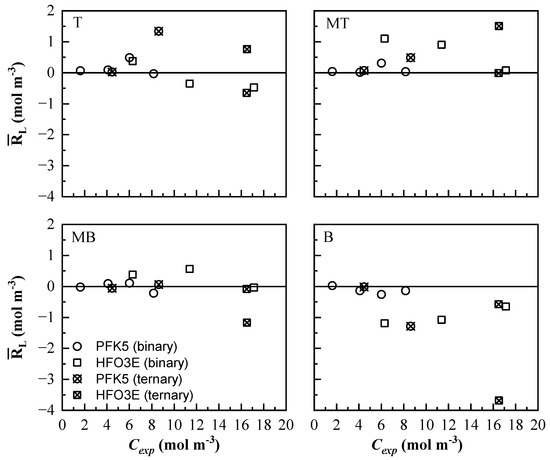
Figure 11.
Mean residual value of the measurement lines at all times () of the mixing process. The results of all the binary (◯ and ☐) and ternary (⊗ and ⊠) mixtures are represented for PFK5 and HFO3E, respectively.
The minimum and maximum absolute residual values for all mixtures are shown in Table 8. As all gas mixtures reach stability after 10 h, only results before that were considered. The maximum residual values of PFK5 were found in line B at the beginning of the experiments. The minimum ones were distributed into all lines.

Table 8.
Minimum and maximum absolute residual values of the mixtures.
HFO3E showed its maximum residuals distributed between lines T and B at the beginning of the experiments. The minimum residual values of HFO3E increase when the concentration increases. The T-20P/40H mixture was the one that showed the highest maximum absolute residuals, which were presented in line B.
4. Conclusions
A novel piece of experimental equipment has been designed, and an automatized measurement system, which has been programmed in LabVIEW, has been implemented for the online monitoring of the mixing process of the gas mixtures.
Consequently, it has been possible to determine the duration of the mixing process of new insulating gas mixtures made of PFK5 and HFO3E, candidates to replace SF6 in MVS. Starting from an initial stratification state caused by the feeding of the gases depending on their molecular weight, stability in the mixtures has been reached between 10 and 12 h, depending on the composition of the mixture. Besides, binary mixtures have reached stability before the ternary mixture. In addition, it has been seen that the molar absorptivity of HFO3E is lower than of PFK5, and that it absorbs at 196 nm in the UV-Vis spectrum, which has hindered measurements.
A mathematical model that describes the mixing process has been proposed, which takes both natural convection and molecular diffusion into consideration. The model has made it possible to obtain the diffusion and convection coefficients of the mixtures with a relative standard error lower than 6%. In comparison with the calculation of the diffusion coefficient of HFO3E that Hu et al. [57] performed, the estimation shares the same order of magnitude, although it is slightly higher in this work. The convection coefficients of all the mixtures have been significantly low, which could prove that the effect of natural convection is negligible in comparison with molecular diffusion of the order of 105 (in the ternary mixture) to 108 (in the binary mixtures) times smaller.
The residuals of PFK5, which have shown no significant tendency, neither in sign (positive or negative) nor in value, are lower than 1.9 mol m−3 for the binary mixtures and 4.1 for the ternary mixtures. Those of HFO3E have been higher at the beginning of the experiment, between 2.3 and 7.4 mol m−3, and have decreased with time, reaching values as low as 2.4 × 10−3 mol m−3. The model has predicted the concentration values of line MB, for example, better than that of the bottom line, probably due to high variability in the concentration.
It has been possible to predict the concentration profiles of a mixing process of gas mixtures made of PFK5 to be better than the ones made of HFO3E, using air as the carrier gas. The low absorptivity of HFO3E in the UV spectrum has led to higher residual values in the lines that have the highest and lowest concentration of both binary and ternary mixtures. This indicates that UV-Vis could not be the best technique to monitor the concentration changes of this component.
Through this work, the physical characterization of the diffusion process and mixing stability of binary and ternary gas mixtures made of PFK5 and HFO3E was started. All this information is postulated as a methodological and diffusional modeling basis for future studies that take into account other disturbances, such as temperature, humidity, and electrical discharge, among others. Nevertheless, attention also needs to be paid to the chemical stability of the mixtures using analytical methods in future studies.
Author Contributions
Conceptualization, J.I.L.; methodology, A.E. and L.C.; software, E.A., A.E. and B.P.-A.; validation, A.E., M.L.A. and R.M.A.; formal analysis, A.E.; investigation, A.E. and L.C.; resources, J.I. (Jesús Izcara) and J.I. (Josu Izagirre); data curation, A.E.; writing—original draft preparation, A.E. and L.C.; writing—review and editing, J.I.L., M.L.A. and R.M.A.; visualization, J.I.L.; supervision, J.I.L.; project administration, J.I.L., R.M.A. and J.I. (Josu Izagirre); funding acquisition, J.I.L., R.M.A. and J.I. (Josu Izagirre). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basque Government, grant numbers KK-2017/00090 and KK-2019/00017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsai, W.-T. The decomposition products of sulfur hexafluoride (SF6): Reviews of environmental and health risk analysis. J. Fluor. Chem. 2007, 128, 1345–1352. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, X.; Tang, J.; Liu, S. A review on SF6 substitute gases and research status on CF3I gases. Energy Rep. 2018, 4, 486–496. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.M.B.; Allen, S.K.; Boschung, J. Climate Change 2013: The Physical Science Basis. Intergovernmental Panel on Climate Change. 2013. Available online: https://www.ipcc.ch/report/ar5/wg1/ (accessed on 13 December 2021).
- United Nations. Kyoto Protocol to the United Nations Framework Convention on Climate Change. 1998. Available online: https://unfccc.int/kyoto_protocol (accessed on 14 December 2021).
- Hyrenbach, M.; Paul, T.A.; Owens, J. Environmental and safety aspects of AirPlus insulated GIS. In Proceedings of the 24th International Conference & Exhibition on Electricity Distribution, Glasgow, UK, 12–15 June 2017. [Google Scholar]
- Weiss, R.F.; Mühle, J.; Salameh, P.K.; Harth, C.M. Nitrogen trifluoride in the global atmosphere. Geophys. Res. Lett. 2008, 35, L20821. [Google Scholar] [CrossRef] [Green Version]
- Morais, A.R.C.; Harders, A.N.; Baca, K.R.; Olsen, G.M.; Befort, B.J.; Dowling, A.W.; Maginn, E.J.; Shiflett, M.B. Phase Equilibria, Diffusivities, and Equation of State Modeling of HFC-32 and HFC-125 in Imidazolium-Based Ionic Liquids for the Separation of R-410A. Ind. Eng. Chem. Res. 2020, 59, 18222–18235. [Google Scholar] [CrossRef]
- Mühle, J.; Ganesan, A.L.; Miller, B.R.; Salameh, P.K.; Harth, C.M.; Greally, B.R.; Rigby, M.; Porter, L.W.; Steele, L.P.; Trudinger, C.M.; et al. Perfluorocarbons in the global atmosphere: Tetrafluoromethane, hexafluoroethane, and octafluoropropane. Atmos. Chem. Phys. 2010, 10, 5145–5164. [Google Scholar] [CrossRef] [Green Version]
- Corr, S. 1,1,1,2-Tetrafluoroethane; from refrigerant and propellant to solvent. J. Fluor. Chem. 2002, 118, 55–67. [Google Scholar] [CrossRef]
- Akasaka, R.; Zhou, Y.; Lemmon, E.W. A Fundamental Equation of State for 1,1,1,3,3-Pentafluoropropane (R-245fa). J. Phys. Chem. Ref. Data 2015, 44, 013104. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 on Fluorinated Greenhouse Gases and Repealing Regulation (EC) No 842/2006. 2014, 150/195-230. Available online: https://eur-lex.europa.eu/eli/reg/2014/517/oj (accessed on 14 December 2021).
- European Commission. Climate Action: EU Legislation to Control F-Gases. Available online: https://ec.europa.eu/clima/policies/f-gas/legislation_en (accessed on 14 December 2021).
- Nagarsheth, R.; Singh, S. Study of gas insulated substation and its comparison with air insulated substation. Int. J. Electr. Power Energy Syst. 2014, 55, 481–485. [Google Scholar] [CrossRef]
- Sato, J.; Sakaguchi, O.; Kubota, N.; Makishima, S.; Kinoshita, S.; Shioiri, T.; Yoshida, T.; Miyagawa, M.; Homma, M.; Kaneko, E. New technology for medium voltage solid insulated switchgear. In Proceedings of the IEEE Transmission and Distribution Conference and Exhibition, Yokohama, Japan, 6–10 October 2002. [Google Scholar]
- Hyrenbach, M.; Zache, S. Alternative insulation gas for medium-voltage switchgear. In Proceedings of the 2016 Petroleum and Chemical Industry Conference Europe, Berlin, Germany, 14–16 June 2016. [Google Scholar]
- Kieffel, Y.; Irwin, T.; Ponchon, P.; Owens, J. Green Gas to Replace SF6 in Electrical Grids. IEEE Power Energy Mag. 2016, 14, 32–39. [Google Scholar] [CrossRef]
- Pionteck, J.; Wypych, G. Handbook of Antistatics, 2nd ed.; ChemTech Publishing: Toronto, ON, Canada, 2016. [Google Scholar]
- Kieffel, Y.; Biquez, F. SF6 alternative development for high voltage switchgears. In Proceedings of the 2015 IEEE Electrical Insulation Conference, Seattle, WA, USA, 7–10 June 2015. [Google Scholar]
- Izcara, J.; Larrieta, J. Electrical Insulation System for Medium- and High-voltage Electrical Switchgear. U.S. Patent 10,607,748 B2, 31 March 2020. [Google Scholar]
- Glasmacher, P. Fluorinated Ketones as a High-Voltage Insulating Medium. WO Patent 2010/146022A1, 23 December 2010. [Google Scholar]
- Mantilla, J.; Claessens, M.-S.; Gariboldi, N.; Grob, S.; Skarby, P.; Paul, T.A.; Mahdizabed, N. Dielectric Insulation Medium. U.S. Patent 8,822,870 B2, 2 September 2014. [Google Scholar]
- Kieffel, Y.; Girodet, A.; Piccoz, D.; Maladen, R. Use of a Mixture Comprising a Hydrofluoolefin as a Medium-voltage Arc-extiguishing and/or Insulating Gas and Medium-Voltage Electrical Device Comprising Same. U.S. Patent 9,491,877, 8 November 2016. [Google Scholar]
- Preve, C.; Piccoz, D.; Maladen, R. Validation methods of SF6 alternatives gas. In Proceedings of the 23rd International Conference on Electricity Distribution, Lyon, France, 15–18 June 2015. [Google Scholar]
- Pang, X.; Wu, H.; Pan, J.; Qi, Y.; Li, X.; Zhang, J.; Xie, Q. Analysis of correlation between Internal discharge in GIS and SF6 decomposition products. In Proceedings of the IEEE International Conference on High Voltage Engineering and Application, Athens, Greece, 10–13 September 2018. [Google Scholar]
- Katagiri, H.; Kasuya, H.; Mizoguchi, H.; Yanabu, S. Investigation of the performance of CF3I Gas as a Possible Substitute for SF6. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1424–1429. [Google Scholar] [CrossRef]
- Skaggs, S.R.; Rubenstein, R. Setting the occupational exposure limit for CF3I. In Proceedings of the Halon Options Technical Working Conference, Albuquerque, NM, USA, 27–29 April 1999. [Google Scholar]
- Widger, P.; Haddad, A. Analysis of gaseous by-products of CF3I and CF3I-CO2 after high voltage arcing using a GCMS. Molecules 2019, 24, 1599. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gao, Z.; Wang, B.; Zhou, W.; Yu, P.; Luo, Y. Synthesis and dielectric properties of trifluoromethanesulfonyl fluoride: An alternative gas to SF6. Ind. Eng. Chem. Res. 2019, 58, 21913–21920. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Zhou, W.; Qiu, R.; Luo, Y.; Wang, B. Dielectric Properties of CF3SO2F/N2 and CF3SO2F/CO2 Mixtures as a Substitute to SF6. Ind. Eng. Chem. Res. 2020, 59, 15796–15804. [Google Scholar] [CrossRef]
- Long, Y.; Guo, L.; Wang, Y.; Chen, C.; Chen, Y.; Li, F.; Zhou, W. Electron Swarms Parameters in CF3SO2F as an Alternative Gas to SF6. Ind. Eng. Chem. Res. 2020, 59, 11355–11358. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, L.; Yan, J.; Yang, A.; Han, G.; Wu, Y.; Rong, M. Investigation of dielectric properties of cold C3F8 mixtures and hot C3F8 gas as Substitutes for SF6. Eur. Phys. J. D 2015, 69, 240. [Google Scholar] [CrossRef]
- Deng, Y.; Li, B.; Xiao, D. Analysis of the insulation characteristics of C3F8 gas mixtures with N2 and CO2 using boltzmann equation method. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3253–3259. [Google Scholar] [CrossRef]
- Trudinger, C.M.; Fraser, P.J.; Etheridge, D.M.; Sturges, W.T.; Vollmer, M.K.; Rigby, M.; Martinerie, P.; Mühle, J.; Worton, D.R.; Krummel, P.B.; et al. Atmospheric abundance and global emissions of perfluorocarbons CF4, C2F6 and C3F8 since 1800 inferred from ice core, firn, air archive and in situ measurements. Atmos. Chem. Phys. 2016, 16, 11733–11754. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, Q.; Tang, J.; Chen, D.; Wang, D. Decomposition Properties of C4F7N/N2 Gas Mixture: An Environmentally Friendly Gas to Replace SF6. Ind. Eng. Chem. Res. 2018, 57, 5173–5182. [Google Scholar] [CrossRef]
- Chachereau, A.; Hösl, A.; Franck, C.M. Electrical insulation properties of the perfluoronitrile C4F7N. J. Phys. D. Appl. Phys. 2018, 51, 495201. [Google Scholar] [CrossRef] [Green Version]
- Andersen, M.P.S.; Kyte, M.; Andersen, S.T.; Nielsen, C.J.; Nielsen, O.J. Atmospheric Chemistry of (CF3)2CF–C≡N: A Replacement Compound for the Most Potent Industrial Greenhouse Gas, SF6. Environ. Sci. Technol. 2016, 51, 1321–1329. [Google Scholar] [CrossRef]
- Zhao, M.; Han, D.; Zhou, Z.; Zhang, G. Experimental and theoretical analysis on decomposition and by-product formation process of (CF3)2CFCN mixture. AIP Adv. 2019, 9, 105204. [Google Scholar] [CrossRef]
- Koch, M.; Franck, C.M. High voltage insulation properties of HFO1234ze. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3260–3268. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, F.; Sun, Q.; Zhai, Y. Molecular structural and electrical properties of trans-1,3,3,3-tetrafluoropropene and 2,3,3,3-tetrafluoropropene under external electric fields. Comput. Theor. Chem. 2017, 1120, 79–83. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Liu, H.; Huang, X.; Wang, J. Theoretical and experimental investigation on decomposition mechanism of eco-friendly insulation gas HFO1234zeE. J. Mol. Graph. Model. 2020, 100, 107671. [Google Scholar] [CrossRef] [PubMed]
- Mantilla, J.D.; Gariboldi, N.; Grob, S.; Claessens, M. Investigation of the insulation performance of a new gas mixture with extremely low GWP. In Proceedings of the IEEE Electrical Insulation Conference, Philadelphia, PA, USA, 8–11 June 2014. [Google Scholar]
- Simka, P.; Ranjan, N. Dielectric strength of C5 perfluoroketone. In Proceedings of the 19th International Symposium on High Voltage Engineering, Pilsen, Czech Republic, 23–28 August 2015. [Google Scholar]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, Q.; Wang, D. Decomposition characteristics of C5F10O/air mixture as substitutes for SF6 to reduce global warming. J. Fluor. Chem. 2018, 208, 65–72. [Google Scholar] [CrossRef]
- Preve, C.; Piccoz, D.; France, P.S.; Maladen, R. Comparison of Alternatives to SF6 Regarding EHS and End of Life. In Proceedings of the 25th International Conference on Electricity Distribution, Madrid, Spain, 3–6 June 2019. [Google Scholar]
- Zhuo, R.; Chen, Q.; Wang, D.; Fu, M.; Tang, J.; Hu, J.; Jiang, Y. Compatibility between C6F12O–N2 Gas Mixture and Metal Used in Medium-Voltage Switchgears. Energies 2019, 12, 4639. [Google Scholar] [CrossRef] [Green Version]
- Blázquez, S.; Antiñolo, M.; Nielsen, O.J.; Albaladejo, J.; Jiménez, E. Reaction kinetics of (CF3)2CFCN with OH radicals as a function of temperature (278–358 K): A good replacement for greenhouse SF6? Chem. Phys. Lett. 2017, 687, 297–302. [Google Scholar] [CrossRef]
- Kramer, A.; Over, D.; Stoller, P.; Paul, T.A. Fiber-coupled LED gas sensor and its application to online monitoring of ecoefficient dielectric insulation gases in high-voltage circuit breakers. Appl. Opt. 2017, 56, 4505. [Google Scholar] [CrossRef]
- Saxegaard, M.; Seeger, M.; Kristoffersen, M.; Germany, A.; Stoller, P.; Landsverk, H. Dielectric properties of gases suitable for secondary medium voltage switchgear. In Proceedings of the 23rd International Conference om Electricity Distribution, Lyon, France, 15–18 June 2015. [Google Scholar]
- Kuffel, E.; Zaengl, W.S.; Kuffel, J. High Voltage Engigeering Fundamentals, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Maikala, R.V. Modified Beer’s Law—Historical perspectives and relevance in near-infrared monitoring of optical properties of human tissue. Int. J. Ind. Ergon. 2010, 40, 125–134. [Google Scholar] [CrossRef]
- Seader, J.D.; Henley, E.J.; Roper, D.K. Separation Process Principles; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Taylor, R.; Krishna, R. Multicomponent Mass Transfer; Wiley: Hoboken, NJ, USA, 1993. [Google Scholar]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Johnson, R.A. Miller and Freud’s Probability and Statistics for Engineers, 9th ed.; Pearson: London, UK, 2018. [Google Scholar]
- Fuller, E.N.; Schettler, P.D.; Giddings, J.C. A new method for prediction of binary gas-phase diffusion coefficients. Ind. Eng. Chem. 1966, 58, 18–27. [Google Scholar] [CrossRef]
- Fuller, E.N.; Ensley, K.; Giddings, J.C. Diffusion of halogenated hydrocarbons in helium. The effect of structure on collision cross sections. J. Phys. Chem. 1969, 73, 3679–3685. [Google Scholar] [CrossRef]
- Hu, X.; Yu, X.; Hou, H.; Wang, B. Stereo-dependent dimerization, boiling points, diffusion coefficients, and dielectric constants of E/Z-HFO-1234ze. Int. J. Quantum Chem. 2022, 122, e26848. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).