Body’s Center of Mass Motion Relative to the Center of Pressure during Gait, and Its Correlation with Standing Balance in Patients with Lumbar Spondylosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Protocol
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prescher, A. Anatomy and pathology of the aging spine. Eur. J. Radiol. 1998, 27, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Shedid, D.; Benzel, E.C. Cervical spondylosis anatomy: Pathophysiology and biomechanics. Neurosurgery 2007, 60, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.H.; Foster, J.B.; Newell, D.J.; Klukvin, B.N. Prevalence of Cervical Spondylosis in a General Practice. Lancet 1965, 1, 1089–1092. [Google Scholar] [CrossRef]
- Rao, R.D.; Currier, B.L.; Albert, T.J.; Bono, C.M.; Marawar, S.V.; Poelstra, K.A.; Eck, J.C. Degenerative cervical spondylosis: Clinical syndromes, pathogenesis, and management. J. Bone Jt. Surg.-Am. 2007, 89A, 1360–1378. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.D.; Currier, B.L.; Albert, T.J.; Bono, C.M.; Marawar, S.V.; Poelstra, K.A.; Eck, J.C. Analysis of gait in cervical myelopathy. Gait Posture 1999, 9, 184–189. [Google Scholar]
- Papadakis, N.; Christakis, D.; Tzagarakis, G.; Chlouverakis, G.; Kampanis, N.; Stergiopoulos, K.; Katonis, P. Gait variability measurements in lumbar spinal stenosis patients: Part B. Preoperative versus postoperative gait variability. Physiol. Meas. 2009, 30, 1187. [Google Scholar] [CrossRef] [PubMed]
- Edwards, W.C.; Larocca, S.H. The Developmental Segmental Sagittal Diameter in Combined Cervical and Lumbar Spondylosis. Spine 1985, 10, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Seidler, A.; Bolm-Audorff, U.; Heiskel, H.; Henkel, N.; Roth-Küver, B.; Kaiser, U.; Bickeböller, R.; Willingstorfer, W.; Beck, W.; Elsner, G. The role of cumulative physical work load in lumbar spine disease: Risk factors for lumbar osteochondrosis and spondylosis associated with chronic complaints. Occup. Environ. Med. 2001, 58, 735–746. [Google Scholar] [CrossRef]
- Epstein, J.A. Diagnosis and Treatment of Painful Neurological Disorders Caused by Spondylosis of the Lumbar Spine. J. Neurosurg. 1960, 17, 991–1001. [Google Scholar] [CrossRef]
- Sato, K.; Kikuchi, S. Clinical analysis of two-level compression of the cauda equina and the nerve roots in lumbar spinal canal stenosis. Spine 1997, 22, 1898–1903. [Google Scholar] [CrossRef]
- Kirkaldy-Willis, W.H.; Wedge, J.H.; Yong-Hing, K.; Reilly, J. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine 1978, 3, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Niu, C.C.; Nikkhoo, M.; Lu, M.L.; Chen, W.C.; Fu, C.J.; Cheng, C.H. Postural stability and trunk muscle responses to the static and perturbed balance tasks in individuals with and without symptomatic degenerative lumbar disease. Gait Posture 2018, 64, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.J.; Lai, D.M.; Wang, S.F.; Wang, J.L.; Hsu, W.L. Changes of balance control in individuals with lumbar degenerative spine disease after lumbar surgery: A longitudinal study. Spine J. 2019, 19, 1210–1220. [Google Scholar] [CrossRef]
- Easton, J.D. Cervical spondylosis-the overlooked cause of impaired gait. Calif. Med. 1971, 115, 51. [Google Scholar] [PubMed]
- Muraki, S.; Akune, T.; Oka, H.; En-yo, Y.; Yoshida, M.; Nakamura, K.; Kawaguchi, H.; Yoshimura, N. Prevalence of falls and the association with knee osteoarthritis and lumbar spondylosis as well as knee and lower back pain in Japanese men and women. Arthritis Care Res. 2011, 63, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sakai, Y.; Nishio, R.; Ito, Y.; Yamazaki, K.; Morita, Y. Relationship between postural stability and fall risk in elderly people with lumbar spondylosis during local vibratory stimulation for proprioception: A retrospective study. Somatosens. Mot. Res. 2020, 37, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Patla, A.; Frank, J.; Winter, D. Assessment of balance control in the elderly: Major issues. Physiother. Can. 1990, 42, 89–97. [Google Scholar] [CrossRef]
- Kuo, A.D. An optimal control model for analyzing human postural balance. IEEE Trans. Biomed. Eng. 1995, 42, 87–101. [Google Scholar] [CrossRef]
- Salavati, M.; Hadian, M.R.; Mazaheri, M.; Negahban, H.; Ebrahimi, I.; Talebian, S.; Jafari, A.H.; Sanjari, M.A.; Sohani, S.M.; Parnianpour, M. Test-retest reliability of center of pressure measures of postural stability during quiet standing in a group with musculoskeletal disorders consisting of low back pain, anterior cruciate ligament injury and functional ankle instability (vol 29, pg 460, 2009). Gait Posture 2009, 30, 126. [Google Scholar]
- Bauer, C.; Groger, I.; Rupprecht, R.; Gassmann, K.G. Intrasession Reliability of Force Platform Parameters in Community-Dwelling Older Adults. Arch. Phys. Med. Rehabil. 2008, 89, 1977–1982. [Google Scholar] [CrossRef]
- Liu, H.; McGee, M.; Prati, V.; Garrison, K.; Fu, Q. Comparison of Standing Balance Parameters Between Rolling Walker Users and Potential Rolling Walker Users: A Pilot Study. Phys. Occup. Ther. Geriatr. 2009, 27, 298–309. [Google Scholar] [CrossRef]
- Garland, S.J.; Ivanova, T.D.; Mochizuki, G. Recovery of standing balance and health-related quality of life after mild or moderately severe stroke. Arch. Phys. Med. Rehabil. 2007, 88, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, P.G.; Griffin, E.D.; Zurakowski, D. Comparison of standing balance between female collegiate dancers and soccer players. Gait Posture 2007, 26, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Schieppati, M.; Hugon, M.; Grasso, M.; Nardone, A.; Galante, M. The Limits of Equilibrium in Young and Elderly Normal Subjects and in Parkinsonians. Electroencephalogr. Clin. Neurophysiol. 1994, 93, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Hufschmidt, A.; Dichgans, J.; Mauritz, K.H.; Hufschmidt, M. Some Methods and Parameters of Body Sway Quantification and Their Neurological Applications. Arch. Psychiatry Nerve Dis. 1980, 228, 135–150. [Google Scholar] [CrossRef]
- Palmieri, R.M.; Ingersoll, C.D.; Stone, M.B.; Krause, B.A. Center-of-pressure parameters used in the assessment of postural control. J. Sport Rehabil. 2002, 11, 51–66. [Google Scholar] [CrossRef]
- Paul, J.C.; Patel, A.; Bianco, K.; Godwin, E.; Naziri, Q.; Maier, S.; Lafage, V.; Paulino, C.; Errico, T.J. Gait stability improvement after fusion surgery for adolescent idiopathic scoliosis is influenced by corrective measures in coronal and sagittal planes. Gait Posture 2014, 40, 510–515. [Google Scholar] [CrossRef]
- Hong, S.-W.; Leu, T.-H.; Wang, T.-M.; Li, J.-D.; Ho, W.-P.; Lu, T.-W. Control of body’s center of mass motion relative to center of pressure during uphill walking in the elderly. Gait Posture 2015, 42, 523–528. [Google Scholar] [CrossRef]
- Huang, S.-C.; Lu, T.-W.; Chen, H.-L.; Wang, T.-M.; Chou, L.-S. Age and height effects on the center of mass and center of pressure inclination angles during obstacle-crossing. Med. Eng. Phys. 2008, 30, 968–975. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chou, L.-S. Detection of gait instability using the center of mass and center of pressure inclination angles. Arch. Phys. Med. Rehabil. 2006, 87, 569–575. [Google Scholar] [CrossRef]
- Lu, H.-L.; Kuo, M.-Y.; Chang, C.-F.; Lu, T.-W.; Hong, S.-W. Effects of Gait Speed on the Body’s Center of Mass Motion Relative to the Center of Pressure During Over-Ground Walking; Human Movement Science: Amsterdam, The Netherlands, 2017; pp. 354–362. [Google Scholar]
- Pai, Y.C.; Patton, J. Center of mass velocity-position predictions for balance control. J. Biomech. 1997, 30, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-W.; Lu, T.-W.; Lee, W.-C.; Ho, Y.-T.; Wang, J.-H.; Kuo, K.N.; Wang, T.-M. Whole body balance control in Lenke 1 thoracic adolescent idiopathic scoliosis during level walking. PLoS ONE 2020, 15, e0229775. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.W.; Lu, T.W.; Lee, W.C.; Ho, Y.T.; Huang, T.C.; Wang, J.H.; Wang, T.M. Altered balance control in thoracic adolescent idiopathic scoliosis during obstructed gait. PLoS ONE 2020, 15, e0228752. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Galante, M.; Grasso, M.; Schieppati, M. Stance ataxia and delayed leg muscle responses to postural perturbations in cervical spondylotic myelopathy. J. Rehabil. Med. 2008, 40, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, M.; Persson, L.; Magnusson, M. Reduced postural control in patients with chronic cervicobrachial pain syndrome. Gait Posture 1995, 3, 241–249. [Google Scholar] [CrossRef]
- Hong, S.L.; Manor, B.; Li, L. Stance and sensory feedback influence on postural dynamics. Neurosci. Lett. 2007, 423, 104–108. [Google Scholar] [CrossRef]
- Carr, J.H.; Shepherd, R.B.; Nordholm, L.; Lynne, D. Investigation of a new motor assessment scale for stroke patients. Phys. Ther. 1985, 65, 175–180. [Google Scholar] [CrossRef]
- Ho, T.-J.; Chen, S.-C.; Hong, S.-W.; Lu, T.-W.; Lin, J.-G. Influence of Long-Term Tai-Chi Chuan Training on Standing Balance in the Elderly. Biomed. Eng.-Appl. Basis Commun. 2012, 24, 17–25. [Google Scholar] [CrossRef]
- Hsieh, H.-J.; Lu, T.-W.; Chen, S.-C.; Chang, C.-M.; Hung, C. A new device for in situ static and dynamic calibration of force platforms. Gait Posture 2011, 33, 701–705. [Google Scholar] [CrossRef]
- Quijoux, F.; Nicolaï, A.; Chairi, I.; Bargiotas, I.; Ricard, D.; Yelnik, A.; Oudre, L.; Bertin-Hugault, F.; Vidal, P.P.; Vayatis, N. A review of center of pressure (COP) variables to quantify standing balance in elderly people: Algorithms and open-access code. Physiol. Rep. 2021, 9, e15067. [Google Scholar] [CrossRef]
- Chen, S.-C.; Hsieh, H.-J.; Lu, T.-W.; Tseng, C.-H. A method for estimating subject-specific body segment inertial parameters in human movement analysis. Gait Posture 2011, 33, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-W.; O’Connor, J.J. Bone position estimation from skin marker co-ordinates using global optimisation with joint constraints. J. Biomech. 1999, 32, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-L.; Lu, T.-W.; Liu, M.-W. Effects of long-term wearing of high-heeled shoes on the control of the body’s center of mass motion in relation to the center of pressure during walking. Gait Posture 2014, 39, 1045–1050. [Google Scholar] [CrossRef]
- Woltring, H.J. A fortran package for generalized, cross-validatory spline smoothing and differentiation. Adv. Eng. Softw. Workstn. 1986, 8, 104–113. [Google Scholar] [CrossRef]
- Hansen, A.H.; Childress, D.S.; Meier, M.R. A simple method for determination of gait events. J. Biomech. 2002, 35, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Erdfelder, E.; Faul, F.; Buchner, A. GPOWER: A general power analysis program. Behav. Res. Methods Instrum. Comput. 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Gatev, P.; Thomas, S.; Kepple, T.; Hallett, M. Feedforward ankle strategy of balance during quiet stance in adults. J. Physiol. 1999, 514, 915–928. [Google Scholar] [CrossRef]
- Cooper, N.A.; Scavo, K.M.; Strickland, K.J.; Tipayamongkol, N.; Nicholson, J.D.; Bewyer, D.C.; Sluka, K.A. Prevalence of gluteus medius weakness in people with chronic low back pain compared to healthy controls. Eur. Spine J. 2016, 25, 1258–1265. [Google Scholar] [CrossRef]
- Wang, T.Y.; Pao, J.L.; Yang, R.S.; Jang, J.S.R.; Hsu, W.L. The adaptive changes in muscle coordination following lumbar spinal fusion. Hum. Mov. Sci. 2015, 40, 284–297. [Google Scholar] [CrossRef]
- Allum, J.H.J.; Bloem, B.R.; Carpenter, M.G.; Hulliger, M.; Hadders-Algra, M. Proprioceptive control of posture: A review of new concepts. Gait Posture 1998, 8, 214–242. [Google Scholar] [CrossRef]
- Mauritz, K.H.; Dietz, V.; Haller, M. Balancing as a Clinical-Test in the Differential-Diagnosis of Sensory-Motor Disorders. J. Neurol. Neurosurg. Psychiatry 1980, 43, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Patla, A.E.; Frank, J.S. Assessment of Balance Control in Humans. Med. Prog. Through Technol. 1990, 16, 31–51. [Google Scholar]
- Berg, K.O.; Maki, B.E.; Williams, J.I.; Holliday, P.J.; L, S. Wooddauphinee. Clinical and Laboratory Measures of Postural Balance in an Elderly Population. Arch. Phys. Med. Rehabil. 1992, 73, 1073–1080. [Google Scholar]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef] [PubMed]

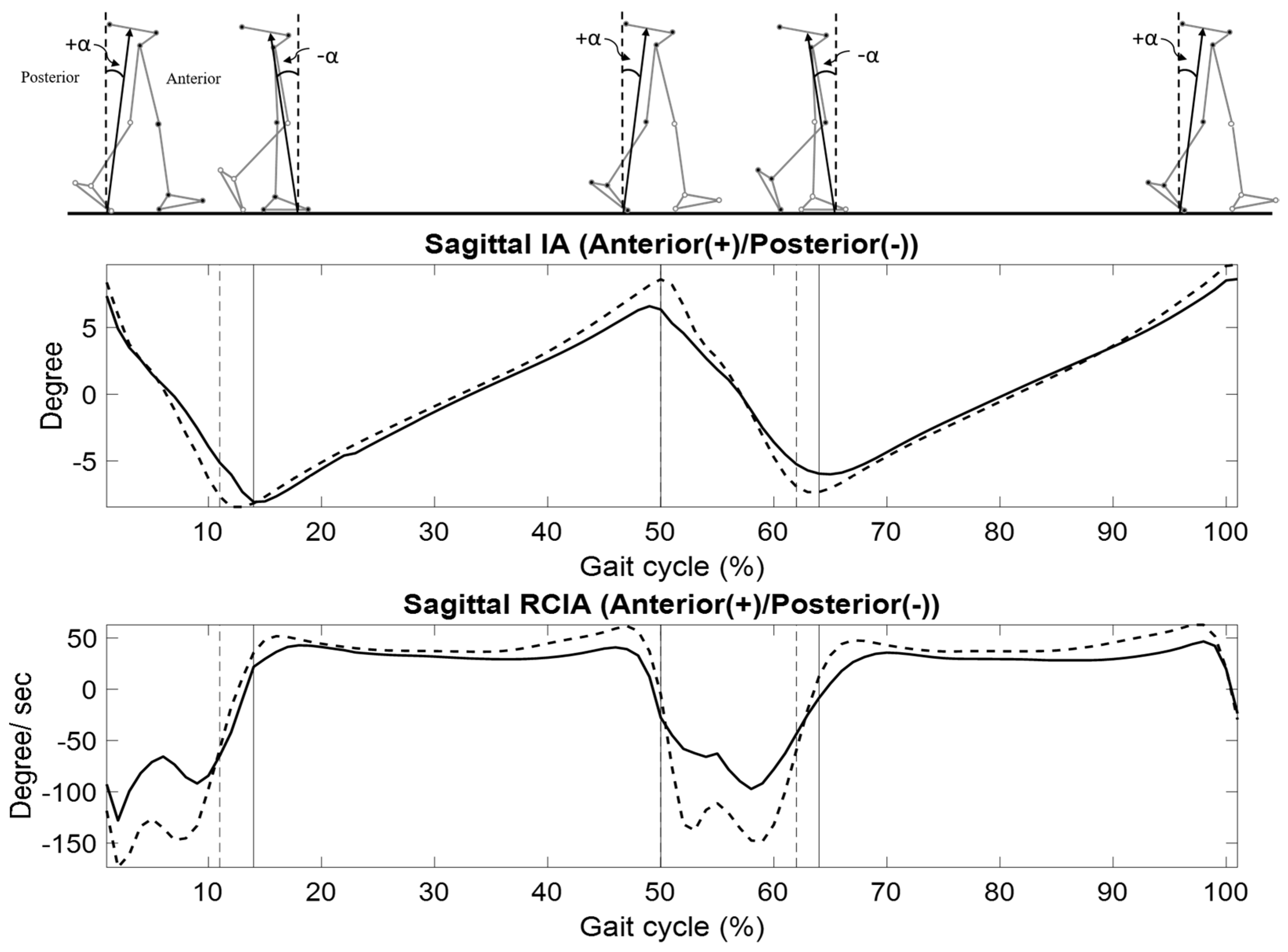
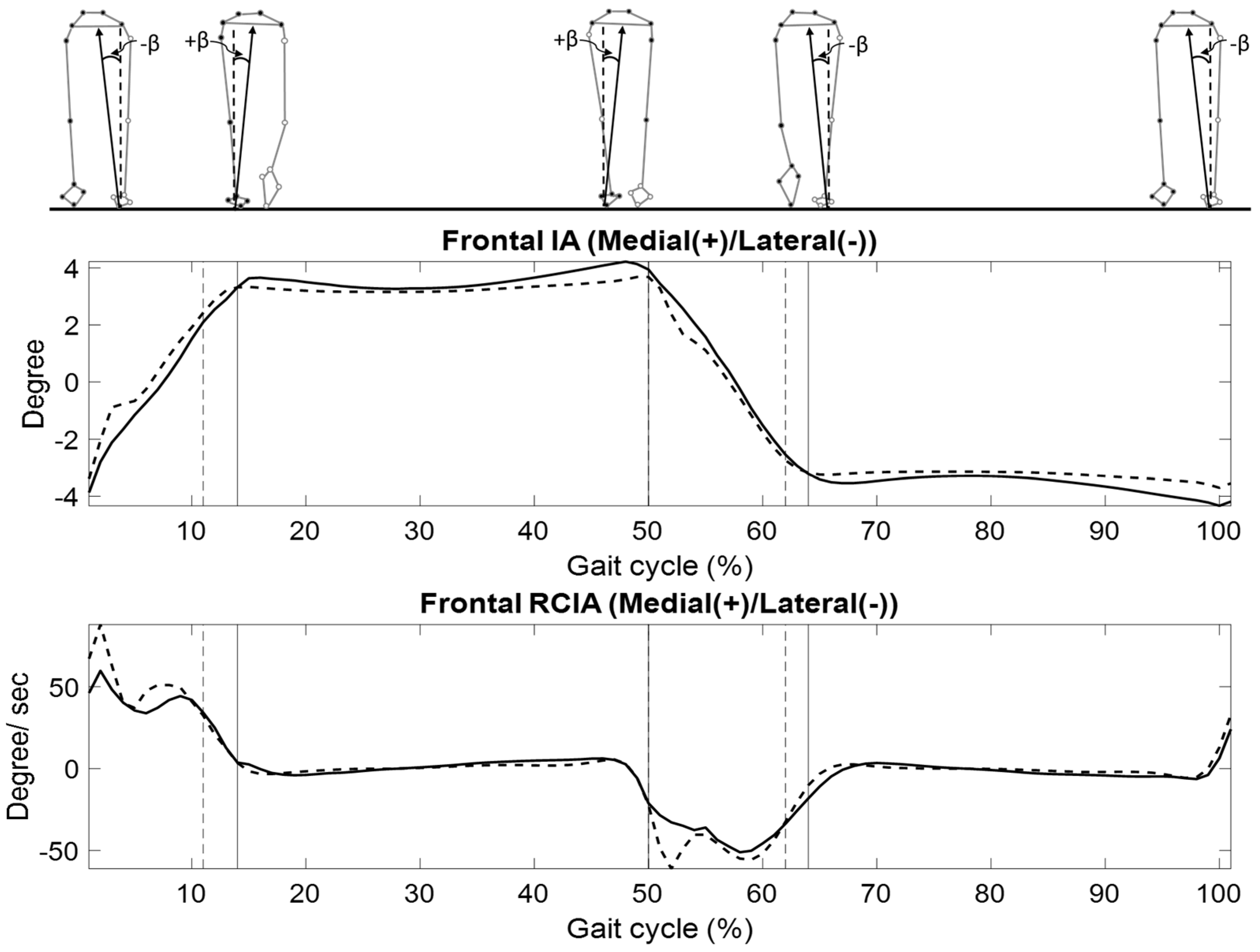
| Variables | LS | Control | p-Value |
|---|---|---|---|
| Stride length (mm) | 1043.5 (88.8) | 1048.8 (37.9) | 0.857 |
| Step length (mm) | 511.3 (47.3) | 507.0 (15.7) | 0.777 |
| Step width (mm) | 105.3 (34.6) | 78.9 (24.5) | 0.052 |
| Cadence (steps/min) | 92.6 (14.5) | 111.6 (8.5) | 0.001 * |
| Gait speed (mm/s) | 811.0 (174.7) | 983.5 (91.6) | 0.009 * |
| Stride time (s) | 1.3 (0.2) | 1.1 (0.1) | 0.001 * |
| DLS duration (%) | 29.2 (6.1) | 23.5 (3.1) | 0.012 * |
| SLS duration (%) | 36.3 (3.0) | 39.9 (1.7) | 0.002 * |
| DCOP (mm) | 3104.0 (870.6) | 2426.7 (518.9) | 0.038 * |
| ACOP (mm2) | 532.4 (619.8) | 124.7 (72.4) | 0.042 * |
| COM–COP Inclination Angle (IA, °) | |||
|---|---|---|---|
| Variables | LS | Control | p-Value |
| Sagittal Plane: Anterior (+)/Posterior (−) | |||
| HS | 7.2 (1.0) | 8.4 (0.7) | 0.004 * |
| TO | −8.1 (1.6) | −7.6 (1.8) | 0.492 |
| Time-averaged values | |||
| DLS | −1.8 (2.1) | 0.5 (2.4) | 0.027 * |
| SLS | −0.2 (0.7) | −0.2 (0.4) | 0.994 |
| Ranges | |||
| DLS | 15.7 (2.7) | 16.3 (1.6) | 0.521 |
| SLS | 16.1 (2.3) | 18.5 (1.8) | 0.012 * |
| Frontal Plane: Medial (+)/Lateral (−) | |||
| HS | −3.7 (0.7) | −3.6 (0.9) | 0.312 |
| TO | −3.6 (0.8) | −3.1 (0.9) | 0.238 |
| Time-averaged values | |||
| DLS | 0.1 (0.6) | −0.1 (0.7) | 0.487 |
| SLS | 3.7 (0.7) | 3.3 (0.8) | 0.304 |
| Ranges | |||
| DLS | 8.0 (1.3) | 6.9 (1.8) | 0.120 |
| SLS | 1.5 (0.3) | 0.9 (0.2) | <0.001 * |
| COM–COP Inclination Angle (RCIA, °/s) | |||
|---|---|---|---|
| Variables | LS | Control | p-Value |
| Sagittal Plane: Anterior (+)/Posterior (−) | |||
| HS | −124.7 (57.0) | −147.7 (64.5) | 0.386 |
| TO | −21.8 (76.6) | −53.7 (34.1) | 0.221 |
| Time-averaged values | |||
| DLS | −90.0 (33.0) | −136.2 (37.7) | 0.006 * |
| SLS | 32.6 (8.1) | 40.3 (4.6) | 0.013 * |
| Ranges | |||
| DLS | 275.4 (69.8) | 264.9 (105.4) | 0.787 |
| SLS | 166.5 (71.2) | 234.5 (66.0) | 0.031 * |
| Frontal Plane: Medial (+)/Lateral (−) | |||
| HS | 60.0 (23.9) | 65.8 (35.7) | 0.661 |
| TO | −23.8 (32.7) | −46.3 (51.9) | 0.238 |
| Time-averaged | |||
| DLS | 47.0 (17.5) | 56.8 (14.9) | 0.172 |
| SLS | 1.0 (1.2) | −0.2 (1.3) | 0.037 * |
| Ranges | |||
| DLS | 109.3 (29.2) | 69.9 (8.8) | 0.001 * |
| SLS | 76.1 (41.0) | 99.7 (72.7) | 0.358 |
| DCOP | p-Value | ACOP | p-Value | |
|---|---|---|---|---|
| Sagittal Plane | ||||
| HS | 0.35 | 0.297 | −0.47 | 0.142 |
| TO | −0.66 | 0.027 * | 0.43 | 0.193 |
| Time-averaged values | ||||
| DLS | −0.63 | 0.038 * | −0.08 | 0.819 |
| SLS | −0.61 | 0.048 * | 0.53 | 0.094 |
| Ranges | ||||
| DLS | 0.67 | 0.024 * | −0.46 | 0.158 |
| SLS | 0.46 | 0.157 | −0.73 | 0.011 * |
| Frontal Plane | ||||
| HS | −0.08 | 0.820 | −0.69 | 0.019 * |
| TO | 0.02 | 0.961 | −0.40 | 0.222 |
| Time-averaged | ||||
| DLS | 0.37 | 0.263 | 0.10 | 0.762 |
| SLS | −0.01 | 0.980 | 0.28 | 0.397 |
| Ranges | ||||
| DLS | 0.12 | 0.725 | 0.55 | 0.078 |
| SLS | 0.33 | 0.320 | 0.43 | 0.182 |
| COM–COP Inclination Angle (RCIA, °/s) of LS | ||||
|---|---|---|---|---|
| Variables | DCOP | p-Value | ACOP | p-Value |
| Sagittal Plane: Anterior (+)/Posterior (-) | ||||
| HS | 0.19 | 0.583 | 0.09 | 0.798 |
| TO | 0.46 | 0.156 | 0.14 | 0.687 |
| Time-averaged | ||||
| DLS | −0.08 | 0.818 | 0.50 | 0.115 |
| SLS | 0.48 | 0.132 | −0.39 | 0.234 |
| Ranges | ||||
| DLS | 0.38 | 0.256 | 0.07 | 0.841 |
| SLS | −0.57 | 0.065 | −0.18 | 0.607 |
| Frontal Plane: Medial (+)/Lateral (-) | ||||
| HS | −0.34 | 0.313 | 0.21 | 0.531 |
| TO | 0.42 | 0.203 | 0.13 | 0.710 |
| Time-averaged | ||||
| DLS | −0.11 | 0.754 | −0.08 | 0.821 |
| SLS | 0.51 | 0.111 | 0.00 | 0.999 |
| Ranges | ||||
| DLS | 0.04 | 0.908 | 0.78 | 0.005 * |
| SLS | −0.53 | 0.094 | 0.22 | 0.509 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.-C.; Huang, H.-P.; Wu, K.-W.; Pao, J.-L.; Chen, C.-K.; Wang, T.-M.; Lu, T.-W. Body’s Center of Mass Motion Relative to the Center of Pressure during Gait, and Its Correlation with Standing Balance in Patients with Lumbar Spondylosis. Appl. Sci. 2022, 12, 12915. https://doi.org/10.3390/app122412915
Huang T-C, Huang H-P, Wu K-W, Pao J-L, Chen C-K, Wang T-M, Lu T-W. Body’s Center of Mass Motion Relative to the Center of Pressure during Gait, and Its Correlation with Standing Balance in Patients with Lumbar Spondylosis. Applied Sciences. 2022; 12(24):12915. https://doi.org/10.3390/app122412915
Chicago/Turabian StyleHuang, Ting-Chun, Hsing-Po Huang, Kuan-Wen Wu, Jwo-Luen Pao, Cheng-Kuang Chen, Ting-Ming Wang, and Tung-Wu Lu. 2022. "Body’s Center of Mass Motion Relative to the Center of Pressure during Gait, and Its Correlation with Standing Balance in Patients with Lumbar Spondylosis" Applied Sciences 12, no. 24: 12915. https://doi.org/10.3390/app122412915
APA StyleHuang, T.-C., Huang, H.-P., Wu, K.-W., Pao, J.-L., Chen, C.-K., Wang, T.-M., & Lu, T.-W. (2022). Body’s Center of Mass Motion Relative to the Center of Pressure during Gait, and Its Correlation with Standing Balance in Patients with Lumbar Spondylosis. Applied Sciences, 12(24), 12915. https://doi.org/10.3390/app122412915






