An Array SPRi Biosensor for Simultaneous VEGF-A and FGF-2 Determination in Biological Samples
Abstract
1. Introduction
2. Reagents, Materials and Methods
2.1. Reagents and Materials
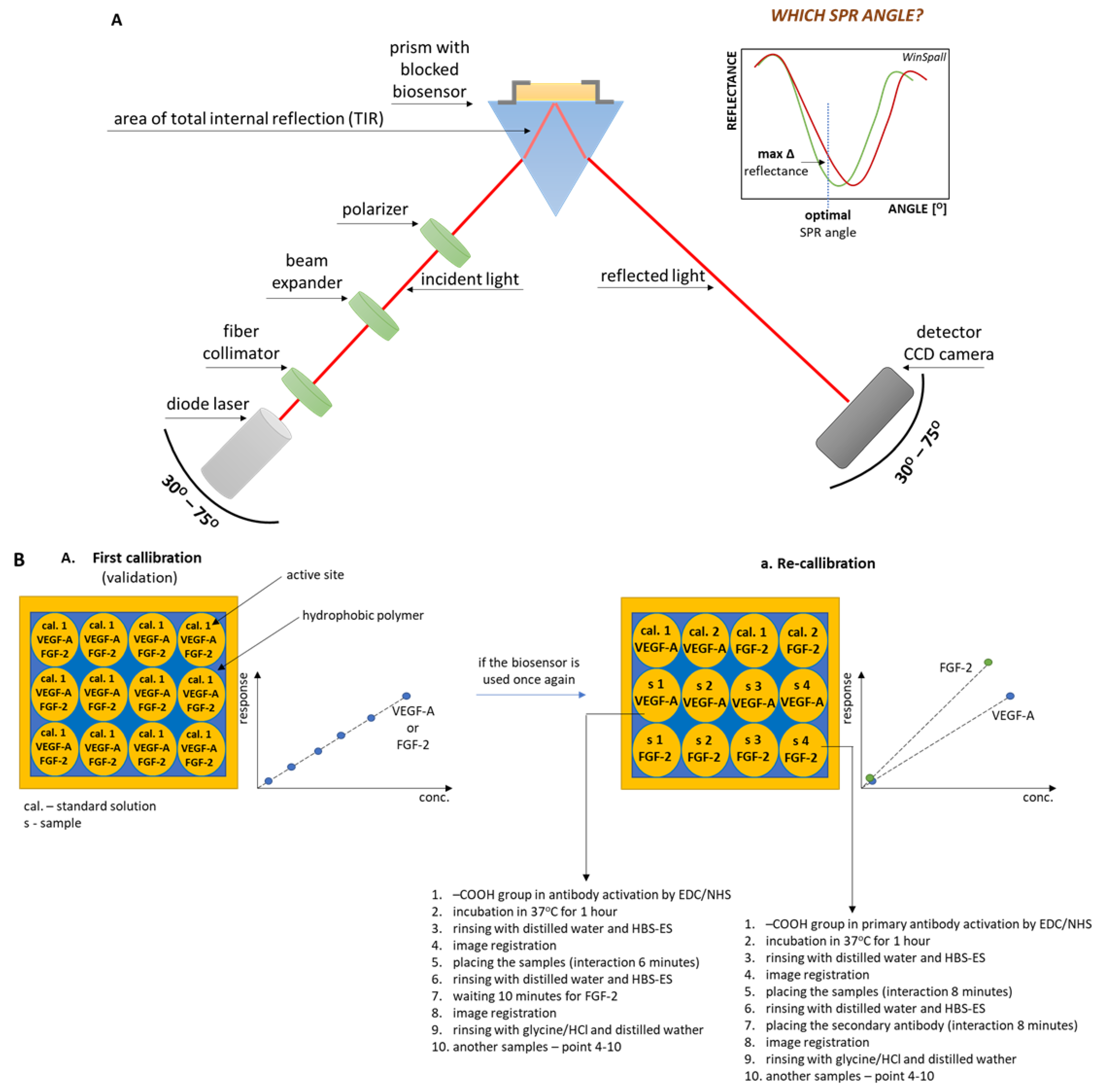
2.2. SPRi and ELISA Apparatus
2.3. Biological Material
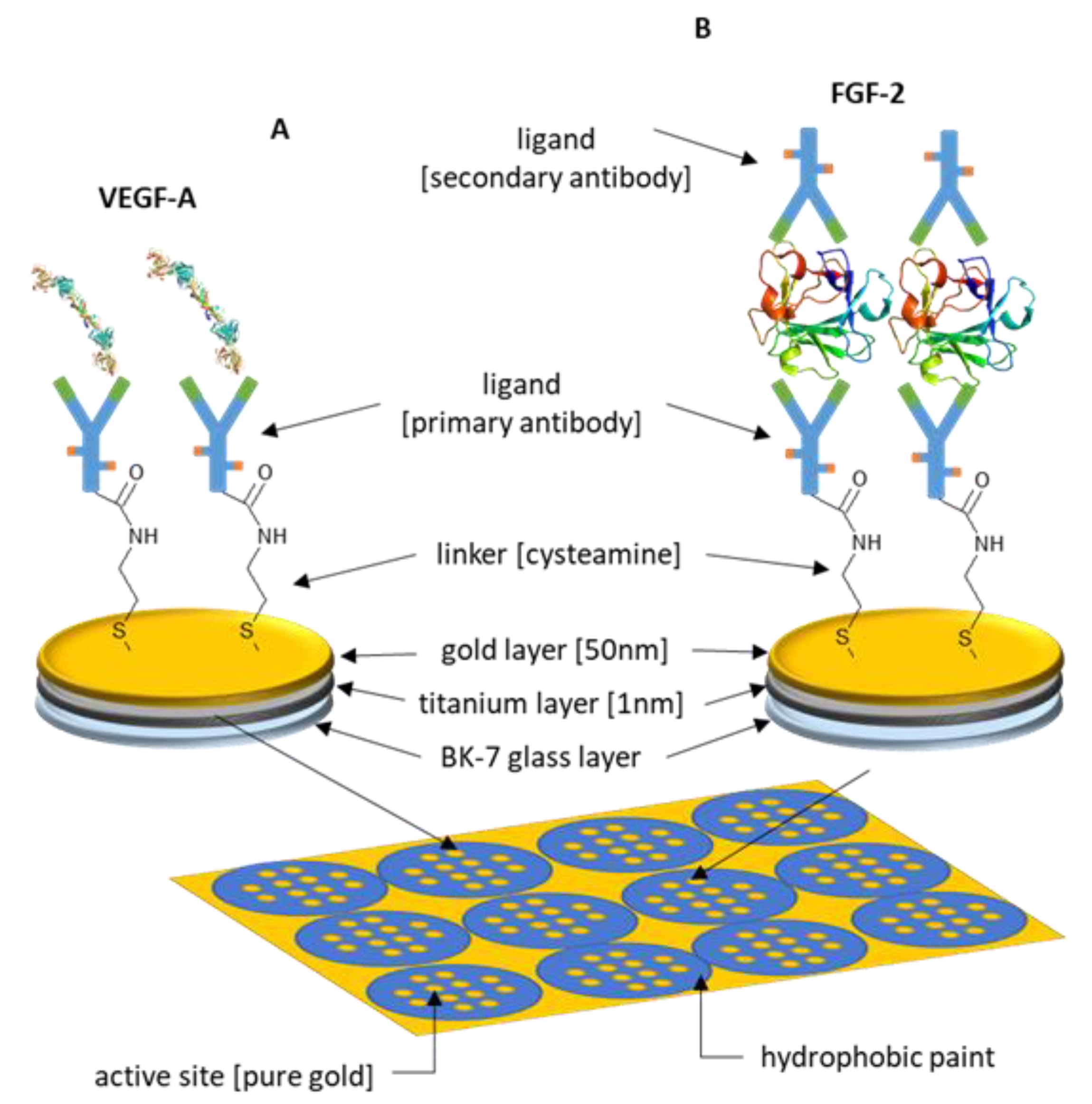
2.4. Chip Preparation
2.5. Data Analysis
3. Results and Discussion of Biosensor Formation and Its Validation
3.1. Formation of Successive Layers of the Biosensor
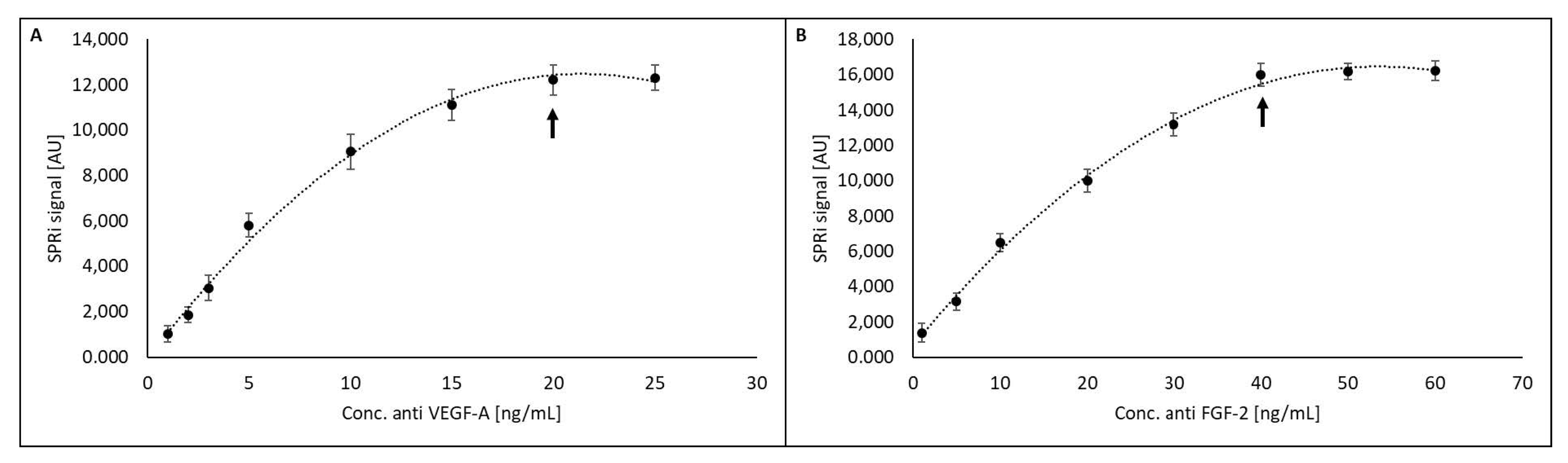
3.2. Saturation of the Biosensor Surface with a Ligand (Receptor)
3.3. Method Calibration
3.4. Limit of detection (LOD) and Limit of Quantification (LOQ)
3.5. Precision
3.6. Robustness
3.7. Recovery
3.8. Selectivity
3.9. Repeatability
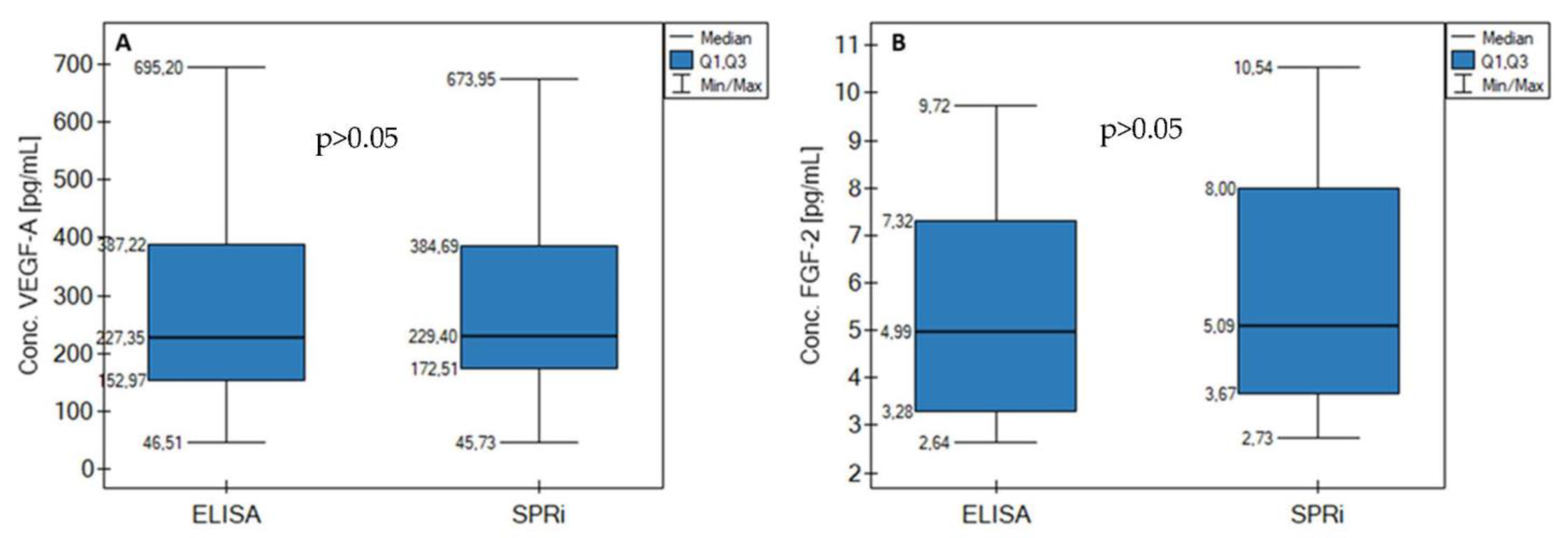
3.10. Comparison to ELISA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukaemia |
| CML | chronic myeloid leukaemia |
| HCL | hairy-cell leukaemia |
| CLL | chronic lymphocytic leukaemia |
| ALL | lymphocytic leukaemia |
| MM | multiple myeloma |
| NHL | non-Hodgkin lymphoma |
| CMML | chronic myelomonocytic leukaemia |
| MDS | myelodysplastic syndromes |
| CRM | CRM is the concentration of reference material obtained from standard solutions. It is not a certified reference material. |
| XC | mean concentration of the analyte |
Appendix A
| [analyte:interferent] | Biomarker | |||||
|---|---|---|---|---|---|---|
| Conc. theoret. VEGF-A [pg/mL] | Conc. VEGF-A [pg/mL] | SD [pg/mL] | Conc. theoret. FGF-2 [pg/mL] | Conc. FGF-2 [pg/mL] | SD [pg/mL] | |
| [1:1] analyte:VEGF-R1 | 50.00 | 50.65 | 0.14 | 10.00 | 10.98 | 0.35 |
| [1:10] analyte:VEGF-R1 | 52.05 | 0.58 | 10.08 | 0.73 | ||
| [1:100] analyte:VEGF-R1 | 50.45 | 0.70 | 10.93 | 0.65 | ||
| [1:1] analyte:VEGF-R2 | 52.53 | 0.65 | 11.46 | 0.89 | ||
| [1:10] analyte:VEGF-R2 | 50.05 | 0.13 | 11.54 | 0.69 | ||
| [1:100] analyte:VEGF-R2 | 52.78 | 0.15 | 11.02 | 0.54 | ||
| [1:1] analyte:albumin | 51.92 | 0.36 | 11.95 | 0.53 | ||
| [1:10] analyte:albumin | 52.15 | 0.29 | 11.91 | 0.72 | ||
| [1:100] analyte:albumin | 51.09 | 0.86 | 11.82 | 0.53 | ||
| [1:1] analyte:FGF-2 | 50.41 | 0.18 | ||||
| [1:10] analyte:FGF-2 | 52.30 | 0.92 | ||||
| [1:100] analyte:FGF-2 | 51.83 | 0.70 | ||||
| [1:1] analyte:VEGF-A | 10.01 | 0.32 | ||||
| [1:10] analyte:VEGF-A | 10.84 | 0.54 | ||||
| [1:100] analyte:VEGF-A | 10.47 | 0.35 | ||||
| Caverage [pg/mL] | VEGF-A | FGF-2 | ||||
| 51.52 ± 0.94 | 11.08 ± 0.67 | |||||
| Recovery [%] | 103.04 | 110.80 | ||||
| CV [%] | 1.82 | 6.05 | ||||
References
- Ribatti, D.; Vacca, A.; Presta, M. The Discovery of Angiogenic Factors: A Historical Review. Gen. Pharmacol. 2000, 35, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T. Molecular Mechanisms of VEGF-A Action during Tissue Repair. J. Investig. Dermatol. Symp. Proc. 2006, 11, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, L.; Zhang, Z.; Wang, Y.; Zuo, C.; Bo, S. A Shorter-Bout of HIIT Is More Effective to Promote Serum BDNF and VEGF-A Levels and Improve Cognitive Function in Healthy Young Men. Front. Physiol. 2022, 13, 898603. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Ç.; Sertoğlu, E.; Fırat Oğuz, E.; Hayran, Y.; Omma, A.; Özgürtaş, T. Serum VEGF-A and VEGFR-1 Levels in Patients with Adult Immunoglobulin A Vasculitis. Int. J. Rheum. Dis. 2021, 24, 789–794. [Google Scholar] [CrossRef]
- Yao, J.H.; Shao, Y.; Wang, J.J.; Li, Y.L.; Yang, H.Q.; Liu, J.; Yang, Y. Evaluation of Diagnostic and Predictive Values of the Serum VEGF-A Level and Systemic Immune-Inflammation Index in Small Cell Lung Cancer. J. Cancer 2021, 12, 1356–1364. [Google Scholar] [CrossRef]
- Larsson, A.; Sköldenberg, E.; Ericson, H. Serum and Plasma Levels of FGF-2 and VEGF in Healthy Blood Donors. Angiogenesis 2002, 5, 107–110. [Google Scholar] [CrossRef]
- Woolard, J.; Bevan, H.S.; Harper, S.J.; Bates, D.O. Molecular Diversity of VEGF-A as a Regulator of Its Biological Activity. Microcirculation 2009, 16, 572–592. [Google Scholar] [CrossRef]
- Badr, Z.; AL-Moosawi, W.; Ali, S. Evaluation of VEGF-A in Relation to Childhood Acute Lymphoblastic Leukemia (in Basrah/Iraq). Iraqi Natl. J. Med. 2021, 3, 39–44. [Google Scholar] [CrossRef]
- Delrieu, I. The High Molecular Weight Isoforms of Basic Fibroblast Growth Factor (FGF-2): An Insight into an Intracrine Mechanism. FEBS Lett. 2000, 468, 6–10. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Cheng, Y.; Kardami, E.; Loh, Y.P. Low and High Molecular Weight FGF-2 Have Differential Effects on Astrocyte Proliferation, but Are Both Protective against Aβ-Induced Cytotoxicity. Front. Mol. Neurosci. 2020, 12, 328. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; He, S.; Hong, B.; Chen, Z.; Peng, D.; Wu, Y.; Wen, H.; Lin, Z.; Fang, Y.; et al. Elevated Serum Levels of FGF-2, NGF and IGF-1 in Patients with Manic Episode of Bipolar Disorder. Psychiatry Res. 2014, 218, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Shain, S.A.; Šarić, T.; Ke, L.D.; Nannen, D.; Yoas, S. Endogenous Fibroblast Growth Factor-1 or Fibroblast Growth Factor-2 Modulate Prostate Cancer Cell Proliferation. Cell Growth Differ. 1996, 7, 573–586. [Google Scholar] [PubMed]
- Folkman, J. Role of Angiogenesis in Tumor Growth and Metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Perez-Atayde, A.R.; Sallan, S.E.; Tedrow, U.; Connors, S.; Allred, E.; Folkman, J. Spectrum of Tumor Angiogenesis in the Bone Marrow of Children with Acute Lymphoblastic Leukemia. Am. J. Pathol. 1997, 150, 815–821. [Google Scholar] [PubMed]
- Vacca, A.; Ribatti, D.; Roncali, L.; Ranieri, G.; Serio, G.; Silvestris, F.; Dammacco, F. Bone Marrow Angiogenesis and Progression in Multiple Myeloma. Br. J. Haematol. 1994, 87, 503–508. [Google Scholar] [CrossRef]
- Vacca, A.; Ribatti, D.; Presta, M.; Minischetti, M.; Iurlaro, M.; Ria, R.; Albini, A.; Bussolino, F.; Dammacco, F. Bone Marrow Neovascularization, Plasma Cell Angiogenic Potential, and Matrix Metalloproteinase-2 Secretion Parallel Progression of Human Multiple Myeloma. Blood 1999, 93, 3064–3073. [Google Scholar] [CrossRef]
- Aguayo, A.; Estey, E.; Kantarjian, H.; Mansouri, T.; Gidel, C.; Keating, M.; Giles, F.; Estrov, Z.; Barlogie, B.; Albitar, M. Cellular Vascular Endothelial Growth Factor Is a Predictor of Outcome in Patients with Acute Myeloid Leukemia. Blood 1999, 94, 3717–3721. [Google Scholar] [CrossRef]
- Bellamy, W.T. Expression of Vascular Endothelial Growth Factor and Its Receptors in Multiple Myeloma and Other Hematopoietic Malignancies. Semin. Oncol. 2001, 28, 551–559. [Google Scholar] [CrossRef]
- Fiedler, W.; Graeven, U.; Ergün, S.; Verago, S.; Kilic, N.; Stockschläder, M.; Hossfeld, D.K. Vascular Endothelial Growth Factor, a Possible Paracrine Growth Factor in Human Acute Myeloid Leukemia. Blood 1997, 89, 1870–1875. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J.; Machalinski, B.; Majka, M.; Marlicz, W.; Carter, A.; Pietrzkowski, Z.; Gewirtz, A.M. Role of Vascular Endothelial Growth Factor (VEGF) and Placenta-Derived Growth Factor (PlGF) in Regulating Human Haemopoietic Cell Growth. Br. J. Haematol. 1998, 103, 969–979. [Google Scholar] [CrossRef]
- Kim, J.G.; Sohn, S.K.; Kim, D.H.; Baek, J.H.; Lee, N.Y.; Suh, J.S.; Chae, S.C.; Lee, K.S.; Lee, K.B. Clinical Implications of Angiogenic Factors in Patients with Acute or Chronic Leukemia: Hepatocyte Growth Factor Levels Have Prognostic Impact, Especially in Patients with Acute Myeloid Leukemia. Leuk. Lymphoma 2005, 46, 885–891. [Google Scholar] [PubMed]
- Aguayo, A.; Kantarjian, H.; Manshouri, T.; Gidel, C.; Estey, E.; Thomas, D.; Koller, C.; Estrov, Z.; O’Brien, S.; Keating, M.; et al. Angiogenesis in Acute and Chronic Leukemias and Myelodysplastic Syndromes. Blood 2000, 96, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, F.; Azzaro, M.P.; Palumbo, G.A.; Bagnato, S.; Giustolisi, G.; Floridia, P.M.; Sortino, G.; Giustolisi, R. Angiogenic Factors in Multiple Myeloma: Higher Levels in Bone Marrow than in Peripheral Blood. Haematologica 2000, 85, 800–805. [Google Scholar] [PubMed]
- Salven, P.; Orpana, A.; Teerenhovi, L.; Joensuu, H. Simultaneous Elevation in the Serum Concentrations of the Angiogenic Growth Factors VEGF and BFGF Is an Independent Predictor of Poor Prognosis in Non-Hodgkin Lymphoma: A Single-Institution Study of 200 Patients. Blood 2000, 96, 3712–3718. [Google Scholar] [CrossRef]
- Aziz, K.A.; Till, K.J.; Chen, H.; Slupsky, J.R.; Campbell, F.; Cawley, J.C.; Zuzel, M. The Role of Autocrine FGF-2 in the Distinctive Bone Marrow Fibrosis of Hairy-Cell Leukemia (HCL). Blood 2003, 102, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Krejčí, P.; Dvořáková, D.; Krahulcová, E.; Pacherník, J.; Mayer, J.; Hampl, A.; Dvořák, P. FGF-2 Abnormalities in B Cell Chronic Lymphocytic and Chronic Myeloid Leukemias. Leukemia 2001, 15, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Loo, J.F.C.; Chen, J.; Yam, Y.; Chen, S.C.; He, H.; Kong, S.K.; Ho, H.P. Recent Advances in Surface Plasmon Resonance Imaging Sensors. Sensors 2019, 19, 1266. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ho, H.P.; Kong, S.K.; Kabashin, A. V Phase-Sensitive Surface Plasmon Resonance Biosensors: Methodology, Instrumentation and Applications. Ann. Phys. 2012, 524, 637–662. [Google Scholar] [CrossRef]
- Szymanska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. An Immunosensor for the Determination of Carcinoembryonic Antigen by Surface Plasmon Resonance Imaging. Anal. Biochem. 2020, 609, 113964. [Google Scholar] [CrossRef]
- Szymanska, B.; Lukaszewski, Z.; Zelazowska-Rutkowska, B.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. An Spri Biosensor for Determination of the Ovarian Cancer Marker He4 in Human Plasma. Sensors 2021, 21, 3567. [Google Scholar] [CrossRef]
- Szymańska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. A Biosensor for Determination of the Circulating Biomarker CA125/MUC16 by Surface Plasmon Resonance Imaging. Talanta 2020, 206, 120187. [Google Scholar] [CrossRef] [PubMed]
- Gorodkiewicz, E.; Sankiewicz, A.; Laudański, P. Surface Plasmon Resonance Imaging Biosensors for Aromatase Based on a Potent Inhibitor and a Specific Antibody: Sensor Development and Application for Biological Material. Cent. Eur. J. Chem. 2014, 12, 557–567. [Google Scholar] [CrossRef]
- Gorodkiewicz, E.; Charkiewicz, R.; Rakowska, A.; Bajko, P.; Chyczewski, L.; Niklinski, J. SPR Imaging Biosensor for Podoplanin: Sensor Development and Application to Biological Materials. Microchim. Acta 2012, 176, 337–343. [Google Scholar] [CrossRef]
- Gorodkiewicz, E.; Lukaszewski, Z. Recent Progress in Surface Plasmon Resonance Biosensors (2016 to Mid-2018). Biosensors 2018, 8, 132. [Google Scholar] [CrossRef] [PubMed]
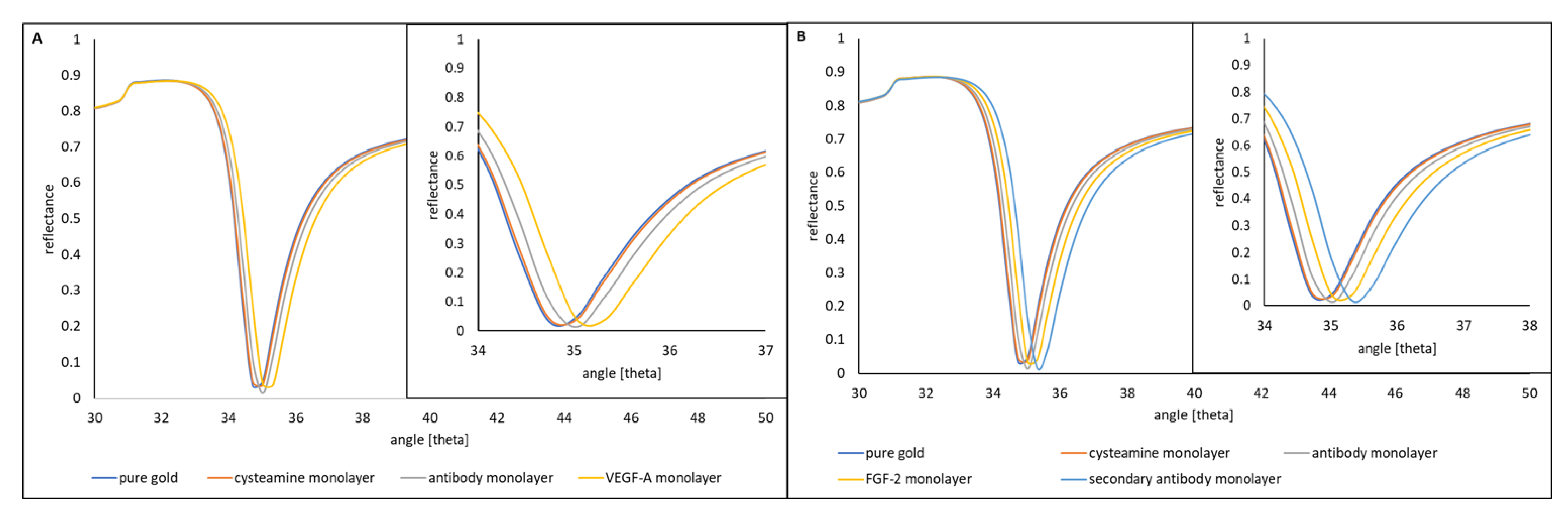
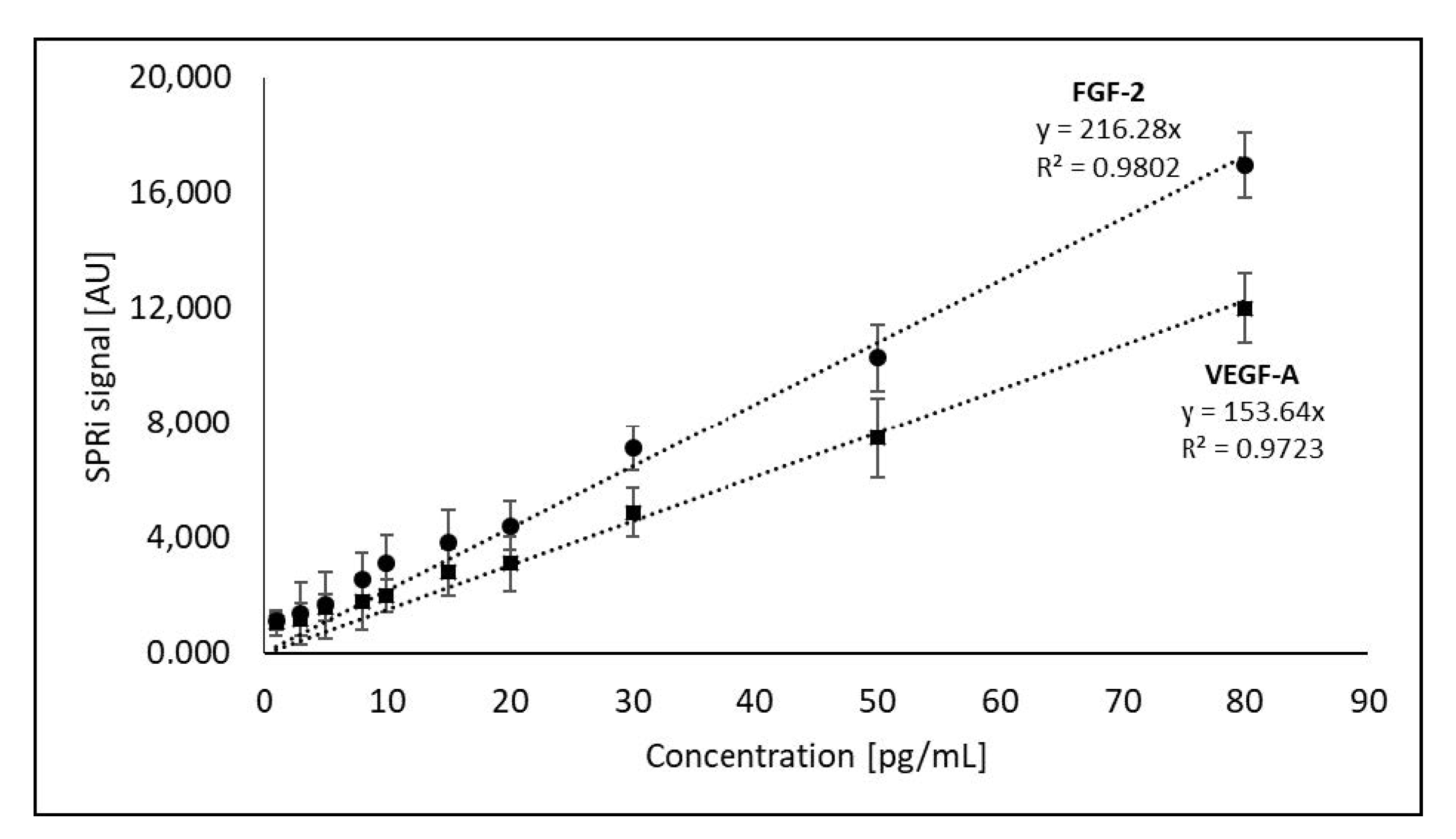
| Biomarker | Description | Ref. |
|---|---|---|
| VEGF-A | Elevated levels in AML patients, worse overall prognosis. | [17] |
| Secretion by all 12 cell lines representing the lymphoma, leukaemia, and multiple myeloma phenotypes. | [18] | |
| Increased expression in blast cells (20 out of 28 patients) with newly diagnosed AML. | [19] | |
| Secretion into the culture medium in leukaemia cell lines and cultured bone marrow cells from CML patients. | [20] | |
| Elevated levels in haematological cancers, such as MM, NHL, CML, CLL, CMML, MDS, and AML. | [21,22,23,24] | |
| FGF-2 | Increased mRNA expression in half of the 12 cell lines tested representing the phenotypes of lymphoma, leukaemia, and multiple myeloma. | [18] |
| Elevated levels of FGF-2 in HCL correlated with elevated levels of fibronectin. | [25] | |
| Elevated levels of FGF-2 in CLL from B lymphocytes and increased levels of FGF-2 in CML. | [26] | |
| Increased FGF-2 levels in ALL and increased bone marrow microvessel density. | [22] |
| Parameter | Biomarker | |||||||
|---|---|---|---|---|---|---|---|---|
| VEGF-A | FGF-2 | |||||||
| CRM [pg/mL] | CRM [pg/mL] | |||||||
| 4 | 20 | 50 | 80 | 5 | 20 | 50 | 80 | |
| XC [pg/mL] | 3.94 | 21.36 | 52.08 | 80.40 | 4.97 | 22.70 | 53.60 | 80.07 |
| SD | 0.47 | 1.19 | 2.36 | 0.80 | 0.52 | 1.74 | 1.75 | 0.87 |
| CV [%] | 11.9 | 5.6 | 4.5 | 1.0 | 10.5 | 7.6 | 3.3 | 1.1 |
| Change in Analytical Procedure | Description of the Change in the Analytical Procedure | Biomarker | |||
|---|---|---|---|---|---|
| VEGF-A | FGF-2 | ||||
| XC [pg/mL] | SD [pg/mL] | XC [pg/mL] | SD [pg/mL] | ||
| - | Real sample (prepared earlier on the day of analysis) | 45.74 | 0.82 | 5.67 | 0.84 |
| T↑ | sample heated in an incubator for 2 h (40 °C) | 31.61 | 0.54 | 3.82 (<LOQ) | 0.39 |
| T↓ | sample chilled in the refrigerator for 2 h (4 °C) | 44.75 | 0.29 | 5.72 | 1.05 |
| pH < 7.40 | pH = 4.99 | 38.07 | 0.98 | 3.02 (<LOQ) | 0.72 |
| pH > 7.40 | pH = 9.50 | 29.06 | 0.44 | 3.15 (<LOQ) | 0.47 |
| shot and gun | sample prepared immediately before the analysis | 45.30 | 0.71 | 5.65 | 0.53 |
| Analysis time (interaction between ligand and analyte) | 30 s | 22.47 | 0.22 | <LOD | - |
| 2 min | 37.21 | 0.87 | <LOD | - | |
| 6 min | 46.12 | 0.76 | 4.97 | 0.19 | |
| 8 min | 45.09 | 0.69 | 5.63 | 0.46 | |
| Parameter | Biomarker | |
|---|---|---|
| VEGF-A | FGF-2 | |
| Creal sample [pg/mL] | 45.62 | 5.75 |
| Cstandard [pg/mL] | 100.00 | 10.00 |
| Cquantified [pg/mL] | 147.32 | 16.03 |
| Cadd * [pg/mL] | 101.7 | 10.28 |
| Recovery [%] | 101.7 | 102.8 |
| Parameter | Biomarker | |||||||
|---|---|---|---|---|---|---|---|---|
| VEGF-A | FGF-2 | |||||||
| CRM [pg/mL] | Real Sample | CRM [pg/mL] | Real Sample | |||||
| 20 | 30 | 50 | 5 | 20 | 50 | |||
| XC [pg/mL] | 21.47 | 30.62 | 51.19 | 45.73 | 4.93 | 19.88 | 51.46 | 5.62 |
| SD [pg/mL] | 0.51 | 0.39 | 0.42 | 0.81 | 0.16 | 0.62 | 0.55 | 0.43 |
| Recovery [%] | 107.35 | 102.07 | 102.98 | - | 98.60 | 99.40 | 102.92 | - |
| CV [%] | 2.38 | 1.27 | 0.82 | 1.77 | 3.25 | 3.12 | 1.07 | 7.65 |
| Sample | Biomarker | |||
|---|---|---|---|---|
| VEGF-A | FGF-2 | |||
| SPRi Biosensor [pg/mL] | ELISA [pg/mL] | SPRi Biosensor [pg/mL] | ELISA [pg/mL] | |
| L1 | 673.95 | 695.20 | 9.02 | 8.77 |
| L2 | 387.54 | 394.12 | 6.15 | 6.09 |
| L3 | 376.12 | 366.50 | 8.62 | 7.73 |
| L4 | 445.29 | 434.31 | 10.54 | 9.72 |
| C1 | 234.59 | 227.35 | 3.45 | 3.23 |
| C2 | 45.73 | 46.51 | 5.29 | 5.67 |
| C3 | 135.71 | 134.40 | 4.89 | 4.30 |
| C4 | 192.23 | 174.52 | 3.96 | 3.43 |
| C5 | 165.93 | 145.78 | 3.57 | 3.11 |
| C6 | 224.21 | 227.35 | 2.73 | 2.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oldak, L.; Leśniewska, A.; Zelazowska-Rutkowska, B.; Latoch, E.; Lukaszewski, Z.; Krawczuk-Rybak, M.; Gorodkiewicz, E. An Array SPRi Biosensor for Simultaneous VEGF-A and FGF-2 Determination in Biological Samples. Appl. Sci. 2022, 12, 12699. https://doi.org/10.3390/app122412699
Oldak L, Leśniewska A, Zelazowska-Rutkowska B, Latoch E, Lukaszewski Z, Krawczuk-Rybak M, Gorodkiewicz E. An Array SPRi Biosensor for Simultaneous VEGF-A and FGF-2 Determination in Biological Samples. Applied Sciences. 2022; 12(24):12699. https://doi.org/10.3390/app122412699
Chicago/Turabian StyleOldak, Lukasz, Anna Leśniewska, Beata Zelazowska-Rutkowska, Eryk Latoch, Zenon Lukaszewski, Maryna Krawczuk-Rybak, and Ewa Gorodkiewicz. 2022. "An Array SPRi Biosensor for Simultaneous VEGF-A and FGF-2 Determination in Biological Samples" Applied Sciences 12, no. 24: 12699. https://doi.org/10.3390/app122412699
APA StyleOldak, L., Leśniewska, A., Zelazowska-Rutkowska, B., Latoch, E., Lukaszewski, Z., Krawczuk-Rybak, M., & Gorodkiewicz, E. (2022). An Array SPRi Biosensor for Simultaneous VEGF-A and FGF-2 Determination in Biological Samples. Applied Sciences, 12(24), 12699. https://doi.org/10.3390/app122412699







