Association between Cough and Ambient Polycyclic Aromatic Hydrocarbons in Patients with Chronic Cough: An Observational Study in Two Regions of Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Surveillance of Daily Cough
2.3. Other Clinical Information
2.4. Ambient Air Sampling
2.5. Measurement of PAHs
2.6. Daily Ambient Concentrations of SO2, NO2, and PM2.5
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Study Patients According to Monitoring Sites
3.2. Characteristics of Study Patients According to the Cough Prevalence
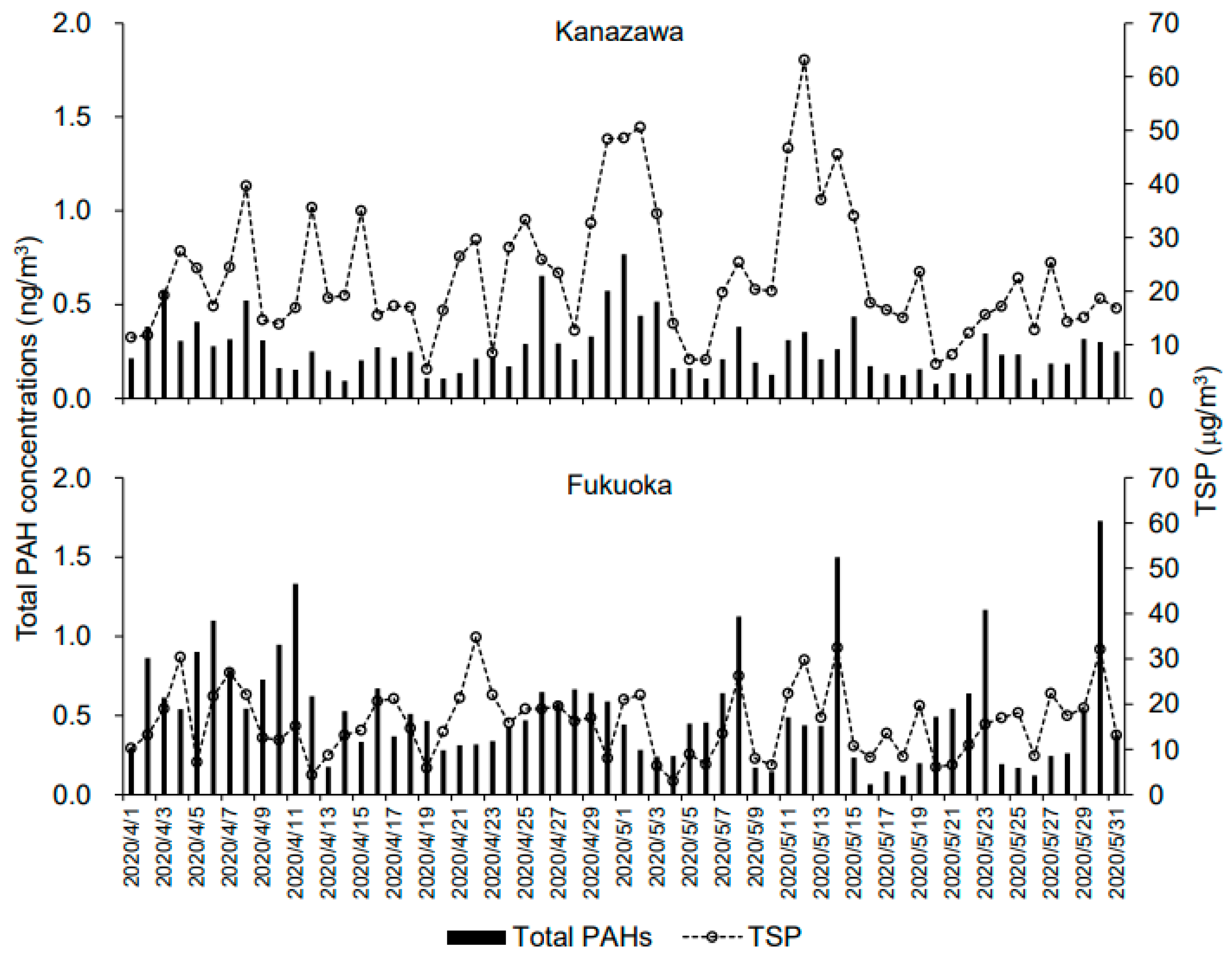
3.3. Daily Ambient Air Pollutant Concentrations in Kanazawa and Fukuoka
3.4. Associations between Ambient PAH and Cough
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morice, A.; Dicpinigaitis, P.; Mcgarvey, L.; Birring, S.S. Chronic Cough: New Insights and Future Prospects. Eur. Respir. Rev. 2021, 30, 210127. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor Air Pollution and Asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Låg, M.; Øvrevik, J.; Refsnes, M.; Holme, J.A. Potential Role of Polycyclic Aromatic Hydrocarbons in Air Pollution-Induced Non-Malignant Respiratory Diseases. Respir. Res. 2020, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Peters, K.O.; Bidad, K.; Strickland, P.T. Polycyclic Aromatic Hydrocarbons and Childhood Asthma. Eur. J. Epidemiol. 2015, 30, 91–101. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of Environmental Exposure to Polycyclic Aromatic Hydrocarbons: A Review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Anyenda, E.O.; Higashi, T.; Kambayashi, Y.; Thi, T.; Nguyen, T.; Michigami, Y.; Fujimura, M.; Hara, J.; Tsujiguchi, H.; Kitaoka, M.; et al. Associations of Cough Prevalence with Ambient Polycyclic Aromatic Hydrocarbons, Nitrogen and Sulphur Dioxide: A Longitudinal Study. Int. J. Environ. Res. Public Health 2016, 13, 800. [Google Scholar] [CrossRef] [PubMed]
- Anyenda, E.O.; Higashi, T.; Kambayashi, Y.; Thao, N.T.T.; Michigami, Y.; Fujimura, M.; Hara, J.; Tsujiguchi, H.; Kitaoka, M.; Asakura, H.; et al. Exposure to Daily Ambient Particulate Polycyclic Aromatic Hydrocarbons and Cough Occurrence in Adult Chronic Cough Patients: A Longitudinal Study. Atmos. Environ. 2016, 140, 34–41. [Google Scholar] [CrossRef]
- Shiue, I. Urinary Polyaromatic Hydrocarbons Are Associated with Adult Emphysema, Chronic Bronchitis, Asthma, and Infections: US NHANES, 2011–2012. Environ. Sci. Pollut. Res 2016, 23, 25494–25500. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; Díaz De León-Martínez, L.; García-Luna, S.; Gómez-Gómez, A.; Karen González-Palomo, A.; Javier Pérez-Vázquez, F.; Díaz-Barriga, F.; Trujillo, J.; Flores-Ramírez, R. Respiratory Health Assessment and Exposure to Polycyclic Aromatic Hydrocarbons in Mexican Indigenous Population. Environ. Sci. Pollut. Res. 2019, 26, 25825–25833. [Google Scholar] [CrossRef]
- Padula, A.M.; Balmes, J.R.; Eisen, E.A.; Mann, J.; Noth, E.M.; Lurmann, F.W.; Pratt, B.; Tager, I.B.; Nadeau, K.; Hammond, S.K. Ambient Polycyclic Aromatic Hydrocarbons and Pulmonary Function in Children HHS Public Access. J. Expo Sci. Environ. Epidemiol. 2015, 25, 295–302. [Google Scholar] [CrossRef]
- Ichinose, M.; Sugiura, H.; Nagase, H.; Yamaguchi, M.; Inoue, H.; Sagara, H.; Tamaoki, J.; Tohda, Y.; Munakata, M.; Yamauchi, K.; et al. Japanese Guidelines for Adult Asthma 2017. Allergol. Int. 2017, 66, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Abo, M.; Ogawa, H.; Nishi, K.; Kibe, Y.; Hirose, T.; Nakatsumi, Y.; Iwasa, K. Importance of atopic cough, cough variant asthma and sinobronchial syndrome as causes of chronic cough in the Hokuriku area of Japan. Respirology 2005, 10, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Nakamura, T.; Takahashi, M.; Ryo, M.; Inoue, S.; Ikeda, Y.; Ohno, M.; Sakata, T.; Fukagawa, K.; Saitoh, Y.; et al. New Criteria for “obesity Disease” in Japan. Circ. J. 2002, 66, 987–992. [Google Scholar] [CrossRef]
- Kuroiwa, M.; Sayuri, H.F.; Shiho, A.; Kime, R.; Endo, T.; Tanaka, R.; Kurosawa, Y.; Hamaoka, T. Impact of Brown Adipose Tissue Vascular Density on Body Adiposity in Healthy Japanese Infants and Children. Obes. Sci. Pract. 2021, 8, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Ikeda, A.; Suzuki, Y.; Maruyama, K.; Wada, H.; Tanigawa, T. The Association between Asthma and Anxiety in Elementary School Students in Japan. Pediatr. Pulmonol. 2020, 55, 2603–2609. [Google Scholar] [CrossRef]
- Pham, K.-O.; Hara, A.; Zhao, J.; Suzuki, K.; Matsuki, A.; Inomata, Y.; Matsuzaki, H.; Odajima, H.; Hayakawa, K.; Nakamura, H. Different Transport Behaviors between Asian Dust and Polycyclic Aromatic Hydrocarbons in Urban Areas: Monitoring in Fukuoka and Kanazawa, Japan. Appl. Sci. 2022, 12, 5404. [Google Scholar] [CrossRef]
- Wide-Area Monitoring System for Air Pollutants by Ministry of the Environment (Soramamekun). Available online: https://soramame.env.go.jp/ (accessed on 29 September 2022).
- Orellano, P.; Quaranta, N.; Reynoso, J.; Balbi, B.; Vasquez, J. Effect of Outdoor Air Pollution on Asthma Exacerbations in Children and Adults: Systematic Review and Multilevel Meta-Analysis. PLoS ONE 2017, 12, e0174050. [Google Scholar] [CrossRef]
- Morice, A.H.; Jakes, A.D.; Faruqi, S.; Birring, S.S.; Mcgarvey, L.; Canning, B.; Smith, J.A.; Parker, S.M.; Chung, K.F.; Lai, K.; et al. Worldwide Survey of Chronic Cough: A Manifestation of Enhanced Somatosensory Response. Eur. Respir. J. 2014, 44, 1149–1155. [Google Scholar] [CrossRef]
- Lätti, A.M.; Pekkanen, J.; Koskela, H.O. Defining the Risk Factors for Acute, Subacute and Chronic Cough: A Cross-Sectional Study in a Finnish Adult Employee Population. BMJ Open 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Yamasaki, A.; Hanaki, K.; Tomita, K.; Watanabe, M.; Hasagawa, Y.; Okazaki, R.; Yamamura, M.; Fukutani, K.; Sugimoto, Y.; Kato, K.; et al. Cough and Asthma Diagnosis: Physicians’ Diagnosis and Treatment of Patients Complaining of Acute, Subacute and Chronic Cough in Rural Areas of Japan. Int. J. Gen. Med. 2010, 3, 101–107. [Google Scholar] [CrossRef][Green Version]
- Niimi, A. Geography and Cough Aetiology. Pulm. Pharmacol. Ther. 2007, 20, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Shinkai, M.; Shinoda, M.; Hara, Y.; Yamaguchi, N.; Rubin, B.K.; Ishigatsubo, Y.; Kaneko, T. Measurement of ENO with Portable Analyser Might Improve the Management of Persistent Cough at Primary Care Practice in Japan. Clin. Respir. J. 2016, 10, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Holinger, L.D.; Sanders, A.D. Chronic Cough in Infants and Children: An Update. Laryngoscope 1991, 101, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Ueki, S.; Tamari, M.; Imoto, Y.; Fujieda, S.; Taniguchi, M. Adult-Onset Eosinophilic Airway Diseases. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 3087–3099. [Google Scholar] [CrossRef]
- Hayakawa, K.; Tang, N.; Xing, W.; Oanh, P.K.; Hara, A.; Nakamura, H. Concentrations and Sources of Atmospheric PM, Polycyclic Aromatic Hydrocarbons and Nitropolycyclic Aromatic Hydrocarbons in Kanazawa, Japan. Atmosphere 2021, 12, 256. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alokail, M.S.; Abd-Alrahman, S.H.; Draz, H.M.; Yakout, S.M.; Clerici, M. Polycyclic Aromatic Hydrocarbon Exposure and Pediatric Asthma in Children: A Case-Control Study. Environ. Health A Glob. Access Sci. Source 2013, 12, 2–7. [Google Scholar] [CrossRef]
- Wang, I.J.; Karmaus, W.J.J.; Yang, C.C. Polycyclic Aromatic Hydrocarbons Exposure, Oxidative Stress, and Asthma in Children. Int. Arch. Occup. Environ. Health 2017, 90, 297–303. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Y.; Cui, X.; Wu, X.; Yuan, J.; Xie, J.; Chen, W. Urinary Polycyclic Aromatic Hydrocarbon Metabolites and Adult Asthma: A Case-Control Study. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Smith, C.J.; Perfetti, T.A.; Rumple, M.A.; Rodgman, A.; Doolittle, D.J. “IARC Group 2A Carcinogens” Reported in Cigarette Mainstream Smoke. Food Chem. Toxicol. 2001, 39, 183–205. [Google Scholar] [CrossRef]
- Seike, K.; Murata, M.; Hirakawa, K.; Deyashiki, Y.; Kawanishi, S. Oxidative DNA Damage Induced by Benz[a]Anthracene Dihydrodiols in the Presence of Dihydrodiol Dehydrogenase. Chem. Res. Toxicol. 2004, 17, 1445–1451. [Google Scholar] [CrossRef]
- Klingbeil, E.C.; Hew, K.M.; Nygaard, U.C.; Nadeau, K.C. Polycyclic Aromatic Hydrocarbons, Tobacco Smoke, and Epigenetic Remodeling in Asthma. Immunol. Res. 2014, 58, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Matsui, E.C.; Hansel, N.N.; Mc Cormack, M.C.; Rusher, R.; Breysse, P.N.; Diette, G.B. Asthma in the Inner City and the Indoor Environment. Immunol. Allergy Clin. N. Am. 2008, 28, 665–686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Perret, J.L.; Chang, A.B.; Idrose, N.S.; Bui, D.S.; Lowe, A.J.; Abramson, M.J.; Haydn Walters, E.; Lodge, C.J.; Dharmage, S.C.; et al. Risk Factors for Chronic Cough in Adults: A Systematic Review and Meta-Analysis. Respirology 2022, 27, 36–47. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 98) | Kanazawa (n = 59) | Fukuoka (n = 39) | p Value | |
|---|---|---|---|---|
| Age, years | 42.0 (9.0, 66.0) | 63.0 (43.0, 70.0) | 9.0 (8.0, 15.0) | <0.001 |
| Men, n | 39 (39.8) | 17 (28.8) | 22 (56.4) | 0.006 |
| Asthma, n | 60 (61.2) | 21 (35.6) | 39 (100) | <0.001 |
| Height, cm | 156.0 (135.8, 162.8) | 157.0 (152.6, 163.8) | 133.5 (126.9, 158.6) | <0.001 |
| Weight, kg | 51.4 (30.3, 62.3) | 56.5 (48.4, 67.0) | 30.3 (26.4, 50.9) | <0.001 |
| Body mass rank (n = 93) | 0.004 | |||
| Lean | 14 | 3 | 11 | |
| Normal | 57 | 38 | 19 | |
| Overweight/obese | 22 | 16 | 6 | |
| Cough prevalence, % | 1.8 (0.0, 37.7) | 8.2 (0.0, 68.5) | 0.0 (0.0, 6.6) | 0.002 |
| Less Cough (n = 55) | Frequent Cough (n = 43) | p Value | |
|---|---|---|---|
| Age, years | 16.0 (9.0, 63.0) | 56.0 (15.0, 70.0) | 0.018 |
| Men, n | 25 (45.5) | 14 (32.6) | 0.196 |
| Asthma, n | 39 (70.9) | 21 (48.8) | 0.026 |
| Height, cm | 153.8 (130.2, 162.6) | 157.0 (150.2, 163.0) | 0.147 |
| Weight, kg | 48.0 (29.0, 60.5) | 53.0 (46.5, 62.5) | 0.127 |
| Body mass rank (n = 93) | 0.504 | ||
| Lean | 9 | 5 | |
| Normal | 28 | 29 | |
| Overweight/obese | 13 | 9 | |
| Cough prevalence, % | 0.0 (0.0, 0.0) | 50.0 (13.1, 87.0) | <0.001 |
| Kanazawa | Fukuoka | p Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Total PAHs (ng/m3) | 0.264 | 0.146 | 0.523 | 0.343 | <0.001 |
| Flt (ng/m3) | 0.053 | 0.037 | 0.100 | 0.084 | <0.001 |
| Pyr (ng/m3) | 0.046 | 0.025 | 0.082 | 0.056 | <0.001 |
| BaA (ng/m3) | 0.007 | 0.002 | 0.013 | 0.008 | <0.001 |
| Chr (ng/m3) | 0.024 | 0.015 | 0.059 | 0.042 | <0.001 |
| BbF (ng/m3) | 0.036 | 0.020 | 0.082 | 0.059 | <0.001 |
| BkF (ng/m3) | 0.012 | 0.007 | 0.026 | 0.019 | <0.001 |
| BaP (ng/m3) | 0.018 | 0.011 | 0.039 | 0.029 | <0.001 |
| BghiP (ng/m3) | 0.039 | 0.019 | 0.062 | 0.034 | <0.001 |
| IcdP (ng/m3) | 0.027 | 0.014 | 0.051 | 0.032 | <0.001 |
| TSP (μg/m3) | 23.016 | 12.360 | 15.946 | 7.531 | <0.001 |
| PM2.5 (μg/m3) | 6.557 | 3.699 | 12.426 | 4.166 | <0.001 |
| SO2 (ppb) | 0.330 | 0.473 | 1.330 | 0.926 | <0.001 |
| NO2 (ppb) | 2.460 | 0.976 | 8.570 | 3.294 | <0.001 |
| Pollutants | Lag | Coefficient (B) | 95% CI | p Value |
|---|---|---|---|---|
| Total PAHs | 0 | −0.011 | −0.250, 0.228 | 0.929 |
| 1 | 0.066 | −0.166, 0.298 | 0.575 | |
| 2 | 0.083 | −0.135, 0.301 | 0.454 | |
| 3 | 0.142 | −0.052, 0.336 | 0.152 | |
| 4 | 0.193 | −0.038, 0.424 | 0.101 | |
| 5 | 0.149 | −0.076, 0.373 | 0.195 | |
| Flt | 0 | 0.219 | −0.776, 1.214 | 0.666 |
| 1 | 0.481 | −0.541, 1.502 | 0.356 | |
| 2 | 0.422 | −0.500, 1.345 | 0.370 | |
| 3 | 0.537 | −0.249, 1.323 | 0.180 | |
| 4 | 0.768 | −0.226, 1.762 | 0.130 | |
| 5 | 0.446 | −0.463, 1.354 | 0.336 | |
| Pyr | 0 | 0.208 | −1.305, 1.720 | 0.788 |
| 1 | 0.766 | −0.765, 2.297 | 0.327 | |
| 2 | 0.775 | −0.619, 2.169 | 0.276 | |
| 3 | 1.054 | −0.153, 2.260 | 0.087 | |
| 4 | 1.386 | −0.088, 2.859 | 0.065 | |
| 5 | 1.007 | −0.397, 2.411 | 0.160 | |
| BaA | 0 | −2.765 | −10.015, 4.485 | 0.455 |
| 1 | −0.535 | −11.288, 10.219 | 0.922 | |
| 2 | 3.190 | −5.982, 12.362 | 0.495 | |
| 3 | 8.696 | −0.554, 17.945 | 0.065 | |
| 4 | 8.407 | 1.260, 15.554 | 0.021 | |
| 5 | 9.406 | 0.678, 18.134 | 0.035 | |
| Chr | 0 | 0.195 | −1.836, 2.227 | 0.851 |
| 1 | 0.656 | −1.393, 2.704 | 0.531 | |
| 2 | 0.716 | −1.113, 2.544 | 0.443 | |
| 3 | 0.984 | −0.605, 2.573 | 0.225 | |
| 4 | 1.396 | −0.570, 3.362 | 0.164 | |
| 5 | 0.640 | −1.245, 2.524 | 0.506 | |
| BbF | 0 | −0.541 | −1.721, 0.639 | 0.369 |
| 1 | −0.018 | −1.187, 1.151 | 0.976 | |
| 2 | 0.315 | −0.871, 1.501 | 0.602 | |
| 3 | 0.499 | −0.584, 1.583 | 0.366 | |
| 4 | 0.704 | −0.381, 1.788 | 0.203 | |
| 5 | 0.761 | −0.415, 1.936 | 0.205 | |
| BkF | 0 | −0.646 | −4.881, 3.590 | 0.765 |
| 1 | 0.827 | −3.354, 5.007 | 0.698 | |
| 2 | 1.056 | −2.764, 4.875 | 0.588 | |
| 3 | 1.816 | −1.629, 5.260 | 0.302 | |
| 4 | 3.123 | −0.923, 7.169 | 0.130 | |
| 5 | 1.769 | −2.171, 5.709 | 0.379 | |
| BaP | 0 | −1.067 | −3.798, 1.663 | 0.444 |
| 1 | −0.056 | −2.787, 2.675 | 0.968 | |
| 2 | 0.048 | −2.349, 2.445 | 0.969 | |
| 3 | 0.935 | −1.388, 3.257 | 0.430 | |
| 4 | 1.964 | −0.642, 4.570 | 0.140 | |
| 5 | 0.743 | −1.902, 3.389 | 0.582 | |
| BghiP | 0 | 0.370 | −2.169, 2.910 | 0.775 |
| 1 | 0.659 | −1.464, 2.782 | 0.543 | |
| 2 | 0.724 | −1.553, 3.000 | 0.533 | |
| 3 | 1.539 | −0.690, 3.769 | 0.176 | |
| 4 | 2.043 | −0.257, 4.342 | 0.082 | |
| 5 | 2.248 | −0.069, 4.565 | 0.057 | |
| IcdP | 0 | −0.841 | −3.504, 1.821 | 0.536 |
| 1 | −0.376 | −2.574, 1.821 | 0.737 | |
| 2 | 0.071 | −2.086, 2.228 | 0.948 | |
| 3 | 0.909 | −1.199, 3.018 | 0.398 | |
| 4 | 0.788 | −1.521, 3.098 | 0.504 | |
| 5 | 1.094 | −1.536, 3.724 | 0.415 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, A.; Odajima, H.; Matsuzaki, H.; Fujimura, M.; Toma, T.; Wada, T.; Ohkura, N.; Zhao, J.; Pham, K.-O.; Suzuki, K.; et al. Association between Cough and Ambient Polycyclic Aromatic Hydrocarbons in Patients with Chronic Cough: An Observational Study in Two Regions of Japan. Appl. Sci. 2022, 12, 12505. https://doi.org/10.3390/app122412505
Hara A, Odajima H, Matsuzaki H, Fujimura M, Toma T, Wada T, Ohkura N, Zhao J, Pham K-O, Suzuki K, et al. Association between Cough and Ambient Polycyclic Aromatic Hydrocarbons in Patients with Chronic Cough: An Observational Study in Two Regions of Japan. Applied Sciences. 2022; 12(24):12505. https://doi.org/10.3390/app122412505
Chicago/Turabian StyleHara, Akinori, Hiroshi Odajima, Hiroshi Matsuzaki, Masaki Fujimura, Tomoko Toma, Taizo Wada, Noriyuki Ohkura, Jiaye Zhao, Kim-Oanh Pham, Keita Suzuki, and et al. 2022. "Association between Cough and Ambient Polycyclic Aromatic Hydrocarbons in Patients with Chronic Cough: An Observational Study in Two Regions of Japan" Applied Sciences 12, no. 24: 12505. https://doi.org/10.3390/app122412505
APA StyleHara, A., Odajima, H., Matsuzaki, H., Fujimura, M., Toma, T., Wada, T., Ohkura, N., Zhao, J., Pham, K.-O., Suzuki, K., Tsujiguchi, H., Takami, A., Hayakawa, K., & Nakamura, H. (2022). Association between Cough and Ambient Polycyclic Aromatic Hydrocarbons in Patients with Chronic Cough: An Observational Study in Two Regions of Japan. Applied Sciences, 12(24), 12505. https://doi.org/10.3390/app122412505






