Analysis of Chemical Components of Fine Particulate Matter Observed at Fukuoka, Japan, in Spring 2020 and Their Transport Paths
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Overview of Results from February to April in 2020
3.2. Correlation Plot
3.3. Days of High Concentration and Transport Paths
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of the Environment (Japan). Reiwagannendo Taikiosenjokyohokokusho (Report of Air Pollution Condition in 2019); Ministry of the Environment, Government of Japan: Tokyo, Japan, 2019; p. 90. (In Japanese)
- Takami, A.; Miyoshi, T.; Shimono, A.; Hatakeyama, S. Chemical composition of fine aerosol measured by AMS at Fukue Island, Japan during APEX period. Atmos. Environ. 2005, 39, 4913–4924. [Google Scholar] [CrossRef]
- Takami, A.; Miyoshi, T.; Shimono, A.; Kaneyasu, N.; Kato, S.; Kajii, Y.; Hatakeyama, S. Transport of anthropogenic aerosols from Asia and subsequent chemical transformation. J. Geophys. Res. 2007, 112, D22S31. [Google Scholar] [CrossRef]
- Sato, K.; Li, H.; Tanaka, Y.; Ogawa, S.; Iwasaki, Y.; Takami, A.; Hatakeyama, S. Long-range transport of particulate polycyclic aromatic hydrocarbons at Cape Hedo remote island site in the East China Sea between 2005 and 2008. J. Atmos. Chem. 2008, 61, 243–257. [Google Scholar] [CrossRef]
- Sato, K.; Takami, A.; Irei, S.; Miyoshi, T.; Ogawa, Y.; Yoshino, A.; Nakayama, H.; Maeda, M.; Hatakeyama, S.; Hara, K.; et al. Transported and local organic aerosols over Fukuoka, Japan. Aerosol Air Qual. Res. 2013, 13, 1263–1272. [Google Scholar] [CrossRef]
- Kaneyasu, N.; Yamamoto, S.; Sato, K.; Shimizu, A.; Hayashi, M.; Hara, K.; Kawamoto, K.; Okuda, T.; Hatakeyama, S. Impact of long-range transport of aerosols on the PM2.5 composition at a major metropolitan area in the northern Kyushu area of Japan. Atmos. Environ. 2014, 97, 416–425. [Google Scholar] [CrossRef]
- Takami, A.; Miyoshi, T.; Irei, S.; Yoshino, A.; Sato, K.; Shimizu, A.; Hayashi, M.; Hara, K.; Kaneyasu, N.; Hatakeyama, S. Analysis of organic aerosol in Fukuoka, Japan using a PMF method. Aerosol Air Qual. Res. 2016, 16, 314–322. [Google Scholar] [CrossRef]
- Yoshino, A.; Takami, A.; Sato, K.; Shimizu, A.; Kaneyasu, N.; Hatakeyama, S.; Hara, K.; Hayashi, M. Influence of trans-boundary air pollution on the urban atmosphere in Fukuoka, Japan. Atmosphere 2016, 7, 51. [Google Scholar] [CrossRef]
- Yoshino, A.; Takami, A.; Hara, K.; Nishita-Hara, C.; Hayashi, M.; Kanyasu, N. Contribution of local and transboundary air pollution to the urban air quality of Fukuoka, Japan. Atmosphere 2021, 12, 431. [Google Scholar] [CrossRef]
- Michikawa, T.; Ueda, K.; Takami, A.; Sugata, S.; Yoshino, A.; Nitta, H.; Yamazaki, S. Japanese nationwide study on the association between short-term exposure to particulate matter and mortality. J. Epidemiol. 2019, 29, 471–477. [Google Scholar] [CrossRef]
- Kojima, S.; Michikawa, T.; Matsui, K.; Ogawa, H.; Yamazaki, S.; Nitta, H.; Takami, A.; Ueda, K.; Tahara, Y.; Yonemoto, N.; et al. Japanese Circulation Society with R (JCS-ReSS) Group, Relationship between fine particulate matter exposure and out-of-hospital cardiac arrest of cardiac origin: A nationwide registry-based Japanese study. SSRN Electron. J. 2019, 3, e203043. [Google Scholar] [CrossRef]
- Integrated Science Assessment for Particulate Matter; USEPA: Washington, DC, USA, 2019.
- Michikawa, T.; Yamazaki, S.; Ueda, K.; Yoshino, A.; Sugata, S.; Saito, S.; Hoshi, J.; Nitta, H.; Takami, A. Effects of exposure to chemical components of fine particulate matter on mortality in Tokyo: A case-crossover study. Sci. Total Environ. 2021, 755, 142489. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, T.; Morokuma, S.; Yamazaki, S.; Takami, A.; Sugata, S.; Yoshino, A.; Takeda, Y.; Nakahara, K.; Saito, S.; Hoshi, J.; et al. Exposure to chemical components of fine particulate matter and ozone, and placenta-mediated pregnancy complications in Tokyo: A register-based study. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.-O.; Hara, A.; Zhao, J.; Suzuki, K.; Matsuki, A.; Inomata, Y.; Matsuzaki, H.; Odajima, H.; Hayakawa, K.; Nakamura, H. Different transport behaviors between Asian Dust and polycyclic aromatic hydrocarbons in urban areas: Monitoring in Fukuoka and Kanazawa, Japan. Appl. Sci. 2022, 12, 5404. [Google Scholar] [CrossRef]
- Yang, L.; Tang, N.; Matsuki, A.; Takami, A.; Hatakeyama, S.; Kaneyasu, N.; Nagato, E.G.; Sato, K.; Yoshino, A.; Hayakawa, K. A comparison of particulate-bound polycyclic aromatic hydrocarbons long-range transported from the Asian continent to the Noto Peninsula and Fukue Island, Japan. Asian J. Atmos. Environ. 2018, 12, 369–376. [Google Scholar] [CrossRef]
- Hayakawa, K.; Tang, N.; Xing, W.; Oanh, P.K.; Hara, A.; Nakamura, H. Concentrations and sources of atmospheric PM, polycyclic aromatic hydrocarbons and nitro polycyclic aromatic hydrocarbons in Kanazawa, Japan. Atmosphere 2021, 12, 256. [Google Scholar] [CrossRef]
- PM2.5 Shitsuryo Nodo Oyobi Seibun Sokutei Kekka (Mass Concentration of PM2.5 and its Chemical Composition Analysis Data), Ministry of the Environment, Japan. Available online: https://www.env.go.jp/air/osen/pm/monitoring.html (accessed on 12 August 2022). (In Japanese).
- Kakono Kisho Data (Past Weather Data), Japan Meteorological Agency. Available online: https://www.data.jma.go.jp/obd/stats/etrn/index.php (accessed on 12 August 2022). (In Japanese).
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Rolph, G.; Stein, A.; Stunder, B. Real-time Environmental Applications and Display sYstem: READY. Environ. Model. Softw. 2017, 95, 210–228. [Google Scholar] [CrossRef]
- Fukuda, K.; Matsunaga, N.; Sakai, S. Behaviors of sea breeze above Fukuoka City. Annu. J. Hydraul. Eng. JSCE 2000, 44, 85–90. (In Japanese) [Google Scholar] [CrossRef]
- Takashima, H.; Hara, K.; Nishita-Hara, C.; Fujiyoshi, Y.; Shiraishi, K.; Hayashi, M.; Yoshino, A.; Takami, A.; Yamazaki, A. Short-term variation in atmospheric constituents associated with local front passage observed by a 3-D coherent Doppler lidar and in-situ aerosol/gas measurements. Atmos. Environ. X 2019, 3, 100043. [Google Scholar] [CrossRef]
- Chatani, S.; Shimadera, H.; Itahashi, S.; Yamaji, K. Comprehensive analyses of source sensitivities and apportionments of PM2.5 and ozone over Japan via multiple numerical techniques. Atmos. Chem. Phys. 2020, 20, 10311–10329. [Google Scholar] [CrossRef]
- Wu, D.; Li, Q.; Ding, X.; Sun, J.; Li, D.; Fu, H.; Teich, M.; Ye, X.; Chen, J. Primary particulate matter emitted from heavy fuel and diesel oil combustion in a typical container ship: Characteristics and toxicity. Environ. Sci. Technol. 2018, 52, 12943–12951. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Miyata, O.; Imai, S.; Takahashi, C.; Niki, Y.; Xu, Z.; Nishio, S. Analysis of toxic substance in emission gas from marine diesel engine. Kaijo Gijutsu Anzen Kenkyu Hokoku 2011, 11, 111–126. (In Japanese) [Google Scholar]

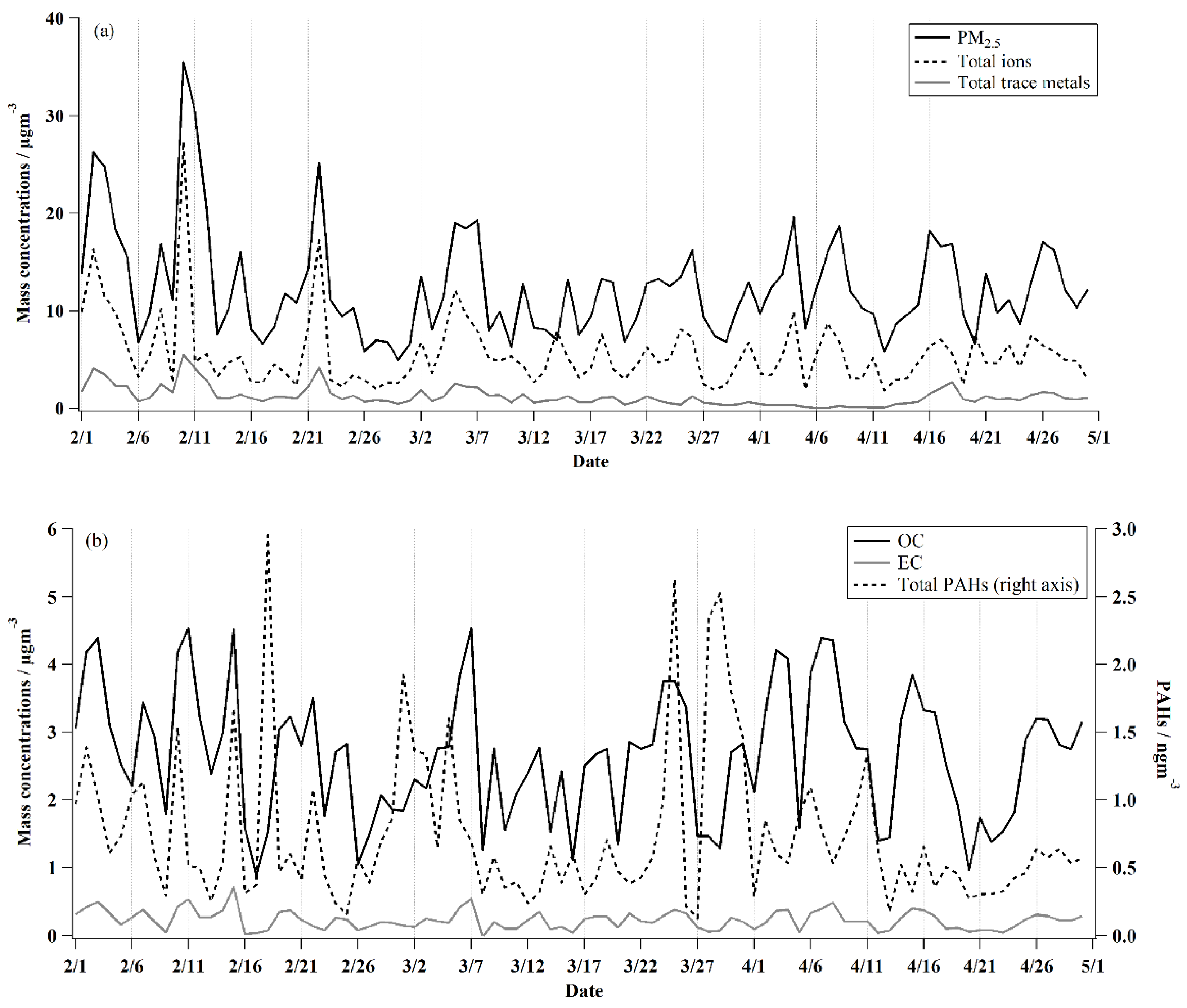



| Date | PM2.5/µgm−3 | Nitrate/µgm−3 | Sulfate/µgm−3 | Total Ions/µgm−3 | Total PAHs/ngm−3 | Total Metals/µgm−3 | OC/µgcm−3 | EC/µgcm−3 | O3/ppb |
|---|---|---|---|---|---|---|---|---|---|
| 1 Feb | 13.8 | 2.81 | 4.21 | 9.90 | 0.97 | 1.72 | 3.05 | 0.31 | 28.0 |
| 2 Feb | 26.3 | 4.60 | 7.08 | 16.3 | 1.39 | 4.11 | 4.18 | 0.42 | 32.1 |
| 3 Feb | 24.8 | 2.71 | 5.38 | 11.5 | 1.03 | 3.49 | 4.38 | 0.50 | 36.4 |
| 4 Feb | 18.3 | 2.78 | 4.20 | 9.76 | 0.61 | 2.27 | 3.10 | 0.33 | 31.8 |
| 5 Feb | 15.5 | 1.95 | 2.44 | 6.31 | 0.74 | 2.26 | 2.52 | 0.17 | 41.6 |
| 7 Feb | 9.70 | 1.60 | 2.12 | 5.34 | 1.13 | 1.04 | 3.44 | 0.38 | 20.2 |
| 8 Feb | 16.9 | 2.83 | 4.51 | 10.2 | 0.58 | 2.47 | 2.93 | 0.21 | 38.2 |
| 10 Feb | 35.5 | 7.52 | 12.3 | 27.3 | 1.54 | 5.49 | 4.18 | 0.42 | 43.8 |
| 11 Feb | 30.4 | 1.28 | 2.16 | 4.81 | 0.51 | 4.10 | 4.53 | 0.53 | 31.4 |
| 12 Feb | 20.5 | 0.62 | 3.36 | 5.56 | 0.50 | 2.88 | 3.23 | 0.27 | 33.2 |
| 15 Feb | 16.0 | 1.40 | 2.27 | 5.30 | 1.68 | 1.42 | 4.52 | 0.71 | 16.9 |
| 18 Feb | 8.40 | 0.81 | 2.42 | 4.49 | 2.96 | 1.16 | 1.53 | 0.08 | 37.5 |
| 20 Feb | 10.8 | 0.90 | 0.74 | 2.30 | 0.60 | 0.99 | 3.23 | 0.37 | 29.4 |
| 21 Feb | 14.3 | 0.36 | 5.58 | 8.47 | 0.42 | 2.22 | 2.80 | 0.24 | 33.0 |
| 22 Feb | 25.2 | 4.71 | 6.23 | 17.2 | 1.08 | 4.15 | 3.51 | 0.14 | 50.3 |
| 1 Mar | 6.60 | 0.84 | 1.98 | 3.84 | 1.93 | 0.76 | 1.84 | 0.15 | 20.6 |
| 2 Mar | 13.5 | 1.55 | 3.17 | 6.79 | 1.36 | 1.87 | 2.31 | 0.13 | 44.0 |
| 3 Mar | 8.10 | 1.16 | 1.32 | 3.64 | 1.34 | 0.70 | 2.17 | 0.26 | 27.9 |
| 5 Mar | 19.0 | 1.64 | 6.66 | 12.1 | 1.60 | 2.50 | 2.78 | 0.19 | 47.1 |
| 6 Mar | 18.5 | 1.96 | 4.71 | 9.60 | 0.85 | 2.22 | 3.83 | 0.41 | 37.4 |
| 7 Mar | 19.3 | 1.94 | 3.64 | 7.91 | 0.70 | 2.14 | 4.53 | 0.54 | 23.7 |
| 14 Mar | 7.00 | 3.39 | 1.84 | 7.92 | 0.66 | 0.84 | 1.54 | 0.09 | 45.6 |
| 19 Mar | 12.9 | 0.66 | 1.82 | 4.04 | 0.71 | 1.18 | 2.75 | 0.28 | 52.9 |
| 24 Mar | 12.5 | 1.07 | 2.11 | 5.08 | 1.01 | 0.50 | 3.75 | 0.30 | 42.2 |
| 25 Mar | 13.5 | 1.75 | 3.80 | 8.13 | 2.63 | 0.38 | 3.76 | 0.38 | 36.9 |
| 26 Mar | 16.2 | 0.01 | 5.28 | 7.24 | 0.22 | 1.26 | 3.37 | 0.33 | 29.9 |
| 28 Mar | 7.40 | 0.37 | 1.05 | 1.91 | 2.34 | 0.46 | 1.47 | 0.06 | - |
| 29 Mar | 6.80 | 0.53 | 1.24 | 2.43 | 2.52 | 0.32 | 1.29 | 0.08 | - |
| 30 Mar | 10.3 | 0.78 | 2.43 | 4.58 | 1.79 | 0.41 | 2.70 | 0.27 | - |
| 31 Mar | 12.9 | 2.25 | 2.60 | 6.74 | 1.46 | 0.62 | 2.83 | 0.21 | 29.7 |
| 3 Apr | 13.8 | 1.24 | 2.30 | 5.41 | 0.60 | 0.33 | 4.22 | 0.36 | 42.6 |
| 4 Apr | 19.6 | 3.25 | 3.47 | 9.91 | 0.53 | 0.33 | 4.09 | 0.38 | 56.4 |
| 6 Apr | 12.3 | 1.68 | 1.95 | 5.58 | 1.09 | 0.07 | 3.88 | 0.33 | 46.3 |
| 7 Apr | 16.0 | 2.99 | 2.98 | 8.78 | 0.78 | 0.05 | 4.39 | 0.39 | 54.8 |
| 8 Apr | 18.7 | 1.75 | 2.74 | 6.83 | 0.53 | 0.27 | 4.36 | 0.48 | 55.8 |
| 11 Apr | 9.70 | 1.47 | 2.02 | 5.20 | 1.33 | 0.08 | 2.75 | 0.22 | 40.2 |
| 13 Apr | 8.60 | 0.45 | 1.33 | 2.94 | 0.18 | 0.42 | 1.45 | 0.08 | 52.9 |
| 15 Apr | 10.6 | 1.08 | 1.97 | 4.69 | 0.32 | 0.66 | 3.85 | 0.40 | 40.9 |
| 16 Apr | 18.2 | 0.50 | 3.73 | 6.39 | 0.65 | 1.51 | 3.33 | 0.37 | 48.1 |
| 17 Apr | 16.6 | 0.31 | 4.51 | 7.06 | 0.36 | 2.08 | 3.30 | 0.29 | 42.1 |
| 18 Apr | 16.9 | 0.73 | 3.10 | 5.59 | 0.50 | 2.64 | 2.52 | 0.10 | 62.9 |
| 20 Apr | 6.60 | 1.82 | 3.56 | 7.68 | 0.27 | 0.67 | 0.97 | 0.06 | 61.5 |
| 21 Apr | 13.8 | 0.98 | 1.67 | 4.71 | 0.30 | 1.26 | 1.74 | 0.08 | 55.2 |
| 24 Apr | 8.70 | 0.73 | 2.05 | 4.26 | 0.43 | 0.83 | 1.81 | 0.13 | 53.9 |
| 25 Apr | 12.9 | 0.56 | 4.56 | 7.48 | 0.46 | 1.35 | 2.89 | 0.24 | 66.7 |
| 26 Apr | 17.1 | 0.64 | 3.74 | 6.47 | 0.64 | 1.68 | 3.20 | 0.31 | 73.0 |
| 27 Apr | 16.2 | 0.64 | 3.28 | 5.84 | 0.57 | 1.56 | 3.19 | 0.29 | 58.2 |
| 28 Apr | 12.2 | 0.56 | 2.76 | 5.02 | 0.64 | 1.01 | 2.81 | 0.23 | 55.0 |
| 29 Apr | 10.3 | 0.59 | 2.57 | 4.86 | 0.53 | 0.90 | 2.75 | 0.23 | 53.4 |
| Average and SD of all days | 12.4 ± 5.50 | 1.20 ± 1.13 | 2.62 ± 1.73 | 5.60 ± 3.71 | 0.75 ± 0.56 | 1.35 ± 1.02 | 2.65 ± 1.34 | 0.24 ± 0.25 | 37.8 ± 16.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshino, A.; Takami, A.; Shimizu, A.; Sato, K.; Hayakawa, K.; Tang, N.; Pham, K.-O.; Hara, A.; Nakamura, H.; Odajima, H. Analysis of Chemical Components of Fine Particulate Matter Observed at Fukuoka, Japan, in Spring 2020 and Their Transport Paths. Appl. Sci. 2022, 12, 11400. https://doi.org/10.3390/app122211400
Yoshino A, Takami A, Shimizu A, Sato K, Hayakawa K, Tang N, Pham K-O, Hara A, Nakamura H, Odajima H. Analysis of Chemical Components of Fine Particulate Matter Observed at Fukuoka, Japan, in Spring 2020 and Their Transport Paths. Applied Sciences. 2022; 12(22):11400. https://doi.org/10.3390/app122211400
Chicago/Turabian StyleYoshino, Ayako, Akinori Takami, Atsushi Shimizu, Kei Sato, Kazuichi Hayakawa, Ning Tang, Kim-Oanh Pham, Akinori Hara, Hiroyuki Nakamura, and Hiroshi Odajima. 2022. "Analysis of Chemical Components of Fine Particulate Matter Observed at Fukuoka, Japan, in Spring 2020 and Their Transport Paths" Applied Sciences 12, no. 22: 11400. https://doi.org/10.3390/app122211400
APA StyleYoshino, A., Takami, A., Shimizu, A., Sato, K., Hayakawa, K., Tang, N., Pham, K.-O., Hara, A., Nakamura, H., & Odajima, H. (2022). Analysis of Chemical Components of Fine Particulate Matter Observed at Fukuoka, Japan, in Spring 2020 and Their Transport Paths. Applied Sciences, 12(22), 11400. https://doi.org/10.3390/app122211400








