Abstract
The relationship between exposure to polycyclic aromatic hydrocarbons and nasal symptoms currently remains unclear. Therefore, we herein examine this relationship in 51 adults living in Ishikawa prefecture, Japan, and conducted a 2 month follow-up survey on these participants. All participants were asked to record daily nasal symptoms in an allergy diary during the study period between 1 April to 31 May 2020. We collected air pollutant samples during the study period and determined the concentrations of PAHs and total suspended particulates by high-performance liquid chromatography. Sulfur dioxide and nitrogen dioxide concentrations were obtained through the Atmospheric Environmental Regional Observation System. We used generalized estimating equations to analyze the association between pollutant and nasal symptoms. After adjustment for confounding factors, the B values of fluoranthene, pyrene, and Benzo[k]fluoranthene were 2.389 (p = 0.026), 3.744 (p = 0.022) and 9.604 (p = 0.041), respectively, with a one-day lag. In contrast, the B value of indeno[1,2,3-cd]pyrene was −6.664 (p = 0.013) with no lag. Collectively, these results suggest ambient PAHs such as Flt, Pyr, and BkF were associated with nasal symptoms in adults. Further studies are needed to elucidate the mechanisms contributing to the relationships between specific PAHs and nasal symptoms.
1. Introduction
With economic development the negative health effects of air pollution are becoming more severe. According to data reported by the World Health Organization (WHO), 99% of the world’s population breathes poor-quality air [1]. According to a report in 2019, air pollution ranked fifth among the global mortality risk factors in 2017 [2]. Therefore, given the survival pressure brought by the deteriorating air environment, it is crucial to understand the mechanism of pollutant-induced disease, which is of great significance for preventing disease and reducing the medical burden. As we know, the respiratory system is susceptible to pollutants [3,4], yet research on the association of pollutants with respiratory diseases is still lacking. Many of the pollutants we are familiar with, such as particulate matter with aerodynamic diameter less than 2.5 µm (PM2.5), nitrogen dioxide (NO2), and sulfur dioxide (SO2), have been widely reported to exert adverse health effects [5,6,7,8,9]. Previous studies revealed a relationship between exposure to air pollution such as PM2.5 and chronic respiratory disease [10], however, limited information is currently available on the relationship between exposure to polycyclic aromatic hydrocarbons (PAHs) as constituents of particulate matter (PM) and respiratory symptoms. Rhinitis is a common respiratory disease, and interestingly, research on its association with air pollution was inconsistent. A longitudinal study demonstrated that exposure to air pollution was not associated with rhinitis in adults [11], while another cohort study showed that only NO2 correlated with rhinitis [10]. In a study conducted in Changsha, China, exposure to traffic air pollution significantly increased the risk of developing allergic rhinitis in the early stages of life [12]. Furthermore, a European cohort study that focused on adults with rhinitis revealed the potential of PM2.5 and NO2 to increase the severity of rhinitis [13].
Nasal symptoms often occur in patients with rhinitis, whether allergic or nonallergic [14] and include nasal congestion, rhinorrhea and sneezing, etc. Many factors can cause nasal symptoms [15], and air pollutants are one of them. However, the mechanism by which air pollutants cause nasal symptoms is unclear. One of the possible explanations is that due to non-allergic rhinitis and allergic rhinitis being associated with inflammation, manifested by an increase in inflammatory cells [16], Air pollutants might be a potential cause of rhinitis because of their role in the onset of inflammation [17,18]. In a study on children, PM2.5 was shown to be associated with nasal allergic inflammation in children with allergic asthma, as eosinophils were significantly increased in their nasal lavage after exposure to PM2.5 [17]. Moreover, after exposure to dose-related NO2, asthmatic patients had increased eosinophils in their sputum, which may exacerbate their airway inflammation [18]. But studies on whether PAHs are associated with airway inflammation are still scarce. Although some studies have found that some PAHs may be related to asthma markers such as Immunoglobulin E (IgE), interleukin (IL)-4 and IL-5 [19], the mechanisms by which exposure to PAHs affects nasal symptoms in adults currently remain unknown.
Polycyclic aromatic hydrocarbons (PAHs) are mainly produced by incomplete combustion of fossil fuels or biomass, and emissions from industrial metal production and vehicle exhaust are also sources of PAHs [20]. PAHs have been proved to have a negative effect on the respiratory system, with one study showing that exposure to PAHs exacerbates chronic obstructive pulmonary disease [21], and a case-control study demonstrating that elevated levels of urinary PAH metabolites increase the risk of asthma in adults [22]. However, there are few reports of PAHs that mentioned an association between PAHs and rhinitis or nasal symptoms. Although some studies have investigated the association between air pollution and rhinitis [11,12,13], these studies did not involve PAHs and their results were inconsistent, so it is still necessary to investigate the direct effects of PAHs on the nose.
To investigate whether PAHs exacerbate pre-existing respiratory diseases as they are also potential causes of disease and to clarify the relationship between exposure to PAHs and nasal symptoms, we conducted a longitudinal study on adult participants with a chronic cough in Ishikawa prefecture, Japan.
2. Materials and Methods
2.1. Participants
Fifty-three participants were recruited at Nanao hospital, Ishikawa Prefecture, Japan between 1 April and 31 May 2020. All participants had received appropriate treatment at the above-mentioned hospitals. Two participants were excluded due to their age (6 and 8 years). All participants lived in Kanazawa city. The measurement site of PAHs and the observation site of the participants were Kanazawa University Hospital in Kanazawa city, and the distances between the observation site and the residence of all participants were no more than 20 km. The screened participants were all adults, and all were older than 30 years. In addition, none of them were smokers and all were diagnosed with at least bronchial asthma, cough variant asthma, or atopic cough by the physician [23]. Among these participants, there were hay fever patients. Also, these patients did not suffer from acute upper respiratory infection and other cardiopulmonary diseases. We collected age, height, weight, and body mass index (BMI) data as basic information. Participants gave their informed consents, and the study was conducted following the principles of the Declaration of Helsinki of the World Medical Association. The protocol was approved by the Medical Ethics Committee of Kanazawa University (Project Identification Code: 981).
2.2. Health Surveys
Participants recorded nasal symptoms in their allergy diary every morning, afternoon, and night from the beginning of the study. Nasal symptoms included the following: 1. nasal congestion 2. rhinorrhea 3. sneezing. Nasal symptoms were considered to be present if any of the above symptoms occurred within 24 h. To prevent missing data, participants were asked to bring their dairies with them when they visited the hospital during the study period. A physician checked if daily symptoms were missing. Participants returned the diaries to the hospital at the end of the study period.
2.3. Air Pollutants Measurement
To obtain data on the total suspended particle (TSP) and PAH, we used a high-volume air sampler (120SL, Kimoto Electric Co., Ltd., Osaka, Japan) equipped with quartz fiber by filters (PALLFLEX Filter, Tokyo Dylec Corp.) at a flow rate of 1000 L/m3, located at Kanazawa University, Ishikawa Prefecture, Japan. After 24 h of collection, the filter was replaced by a blank filter. The collected filter was kept for at least 24 h in a desiccator to keep it dry and was then stored at −30 °C before extraction. Approximately 50 cm2 of the filter was cut from the quartz fiber filter and thoroughly cut into small pieces (approximately 1 × 1 cm2) and loaded into a glass flask. An aliquot of the PAH internal standards (Pyr-d10 and BaP-d12) was added to the flask. A total of 80 mL of ethanol:benzene (v/v 1:4) was used for PAH extraction by sonication. The extract was washed with 5% NaOH, 20% H2SO4 and ultrapure water. The organic phase was evaporated to 100 μL under a gentle nitrogen flow, then reconstituted with 900 μL of acetonitrile (ACN) and filtered into vials. High-performance liquid chromatography (HPLC), which coupled with a fluorescent detector and was utilized with an Inertsil ODS-P16 column (250 × 4.6 mm i.d., 5 μm), was used to calculate the concentration of each PAH, including fluoranthene (Flt), pyrene (Pyr), benz(a)anthracene (BaA), chrysene (Chr), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenz(a,h)anthracene (DahA), benzo(ghi)peryelene (BghiP) and indeno[1,2,3-cd]perylene (IcdP). The temperature of the HPLC was set at 20 °C and the flow rate was 1 mL/min. The limit of detection (LOD) and limit of quantitation (LOQ) of each PAH by HPLC are shown in Table S1, and all undetectable PAHs concentrations were regarded as 0. The sum of these PAHs was used as the total PAH concentration. To make the concentration data obtained more reliable, we used recovery and reproductivity tests. The recovery test was conducted by spiking the internal standard mixture with known concentrations on blank and sample filters, then extracting and analyzing using the method described above. The recovery rate was 110–120%. The reproductivity test was performed by analyzing the same samples using the same method at two facilities at Kanazawa University. The reproductivity range was 90–115%. Data on PM2.5, NO2, and SO2 were obtained from the Atmospheric Environmental Regional Observation System: AEROS provided by the Ministry of the Environment Government of Japan [24].
2.4. Study Period
In our previous study, we found an association between Asian dust and the prevalence of respiratory disease in adult patients suffering from chronic cough [25]. In previous studies, April and May were the most frequent periods of Asian dust [25]. Based on the previous study’s results, we chose April and May as the study period.
2.5. Statistical Analysis
Data on daily nasal symptoms were matched with the daily concentration of air pollution. Given the possibility of delayed onset of nasal symptoms after exposure to air pollutants. lag0 was defined as the occurrence of nasal symptoms on the day of air pollutant measurement. lag1, lag2, lag3, lag4, and lag5 are the effects after exposure one day, two days, three days, four days and five days later, respectively. For the goal of finding if air pollution affects the appearance of nasal symptoms, we used the generalized estimating equation (GEE) model. To clarify whether air pollution is associated with the appearance of nasal symptoms and exclude the effects of other air pollutants, all our models were adjusted for age, sex, BMI, SO2, NO2 and PM2.5. We introduced each individual PAH extracted in the laboratory into the model to investigate their respective associations with nasal symptoms. Considering the different timing of symptoms after exposure to different PAHs, we analyzed the association between symptoms and exposure in lag0-5. Data were analyzed using SPSS software program (IBM), version 19.0. The significance of differences was set at p < 0.05 for all analyses.
3. Results
3.1. Characteristics of Participants and Concentrations of Pollutants
Table 1 shows the descriptive statistics of participants and the concentrations of pollutants in ambient air. The oldest of the participants was 83 years and the youngest was 30 years. The mean age of all participants was 61.17 ± 12.92, and the mean ages of males and females were 66 ± 9.22 and 58.59 ± 13.85, respectively. No significant difference was observed in BMI. The mean BMI of all participants was 24.11 ± 5.21, and the mean BMI of males and females were 23.93 ± 2.32 and 24.19 ± 6.13, respectively.

Table 1.
Participant characteristic.
The mean concentrations of SO2, NO2, and PM2.5 were 0.3 ± 0.5 (ppb), 2 ± 1 (ppb), and 6.56 ± 3.70 (μg/m3), respectively. According to concentration data collected in Kanazawa between 2019 and 2020, the annual mean concentration of SO2 was <1 ppb and close to 0. During the study period, daily concentrations of SO2 and NO2 were always below the 2021 standard WHO of 15 and 13 ppb, respectively; however, the 24-h concentrations of PM2.5 on 1 and 2 May exceeded the WHO standard of 15 μg/m3 [26]. The concentrations of these three substances did not change much during the study period.
Figure 1 shows changes in the 24-h concentrations of TSP and total PAH between 1 April and 31 May 2020. There were no missing data during the study period. The mean concentrations of TSP and total PAH were 23.02 (μg/m3) and (0.26 ng/m3), respectively.
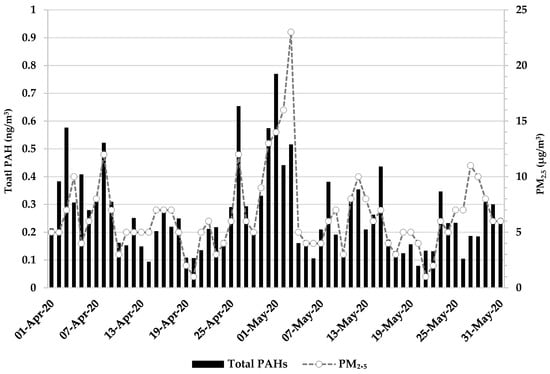
Figure 1.
24-h concentrations of pollutants.
3.2. Relationship between PAHs and Nasal Symptoms
No relationships were observed between nasal symptoms and total PAH, TSP, and PAHs. PAHs correlated with nasal symptoms after adjustments for covariates (age, sex, BMI, SO2, NO2, and PM2.5). After adjustment, Flt, Pyr, and BkF showed significantly positive correlations with B values (the regression slope) of 2.389 [95% Confidence Interval (CI): 0.281 4.496], 3.744 (95% CI: 0.536, 6.951) and 9.604 (95% CI: 0.403, 18.806), respectively, in lag1 only. In contrast, the B value of IcdP was significantly negative with a value of −6.646 (95% CI: −11.914, −1.378) in lag0 (Table 2).

Table 2.
Relationships between PAHs and nasal symptoms.
3.3. Relationships between Covariates and Nasal Symptoms
After analyzing all models of lag0–5, NO2 positively correlated with nasal symptoms in lag5 only. B values adjusted for covariates (sex, age, BMI, SO2, NO2, and PM2.5) are shown in Table S2. In all models of lag5, NO2 was associated with nasal symptoms. However, no other relationships between covariates and nasal symptoms were observed in lag5.
In lag0–4, age, sex, BMI, SO2, NO2, and PM2.5 did not correlate with nasal symptoms.
4. Discussion
The present study demonstrated that exposure to PAHs, even at low levels, was associated with the onset of nasal symptoms. After adjustment, we found that Flt, Pyr and BkF were significantly associated with nasal symptoms. To the best of our knowledge, this is one of only a few longitudinal studies on the relationships between PAHs and nasal symptoms. The results obtained suggest that specific PAHs cause nasal symptoms.
Although exposure to PAHs has been shown to increase the risk of respiratory or cardiovascular diseases among adults [27,28,29,30], limited information is currently available on the relationships between PAHs and rhinitis. One study found that prenatal exposure to PAHs increased the risk of nasal symptoms and cough among children during their first year of life [31]. Difficulties are also associated with finding studies that examined the relationships between nasal symptoms and PAHs. We only found studies on the effects of specific PAHs on the respiratory system. A cohort study in Wuhan and Zhuhai reported correlations between lung function and exposure to Flt and Pyr in adults [negatively correlated with forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1)] [32]. Anyenda et al. showed that after exposure to PAHs, an increased risk of cough was found among chronic cough patients in both lag2 and lag6 [33,34]. These findings suggest that PAHs affect the human respiratory system, which is consistent with the present results. Although the exposure time, exposure level, and population of the present study differed from those in the studies described above, Flt, Pyr, and BkF all contributed to nasal symptoms in adults in the present study. According to the present results, some PAHs appear to be associated with the occurrence of nasal symptoms; however, further studies are warranted to confirm this.
The exact mechanisms by which inhaled PAHs lead to nasal symptoms remain unclear, while the adverse effects of PAHs are well known [35,36,37]. BaP exacerbated nasal congestion in an animal study [38]. Since the present results showing that BaP did not induce significant changes in nasal symptoms did not coincide with the findings of the animal study, its effects on nasal symptoms appear to differ between humans and animals [39,40]. The underlying mechanisms by which PAHs induce rhinitis may be attributed to allergy and/or inflammation [30,31,32]. In a study on children 1-hydroxypyrene, Pyr’s metabolite, was associated with blood IgE levels [41], which plays an important role in allergic airway disease [42]. In another study on children, higher levels of PAHs significantly increased inflammatory cytokines such as IL-10, IL-18 and IL-22 [43]. PAHs may also cause rhinitis through oxidative stress. Cigarette smoke has been shown to promote the production of ROS and play a role in the pathogenesis of rhinitis [44,45]. Therefore, further studies are needed to elucidate the mechanisms contributing to the effects of Flt, Pyr, and BkF on nasal symptoms.
IcdP also showed an association with nasal symptoms in our results, but it was negative. This result is contrary to that of an animal experiment in Taiwan, which found that dose-related exposure to IcdP may increase the risk of allergic airway inflammation in mice [46]. However, the numbers of eosinophils, lymphocytes and neutrophils did not increase significantly when they used the lowest dose of 0.4 μM in their study. The different results may be due to the amount of exposure. Based on our data collected in Kanazawa, the average daily exposure of participants to IcdP was much less than 0.4 μM. Due to the lack of studies on the association of IcdP with the respiratory system, further studies are needed to clarify the reasons for this discrepancy.
We also observed an association between NO2, one of the covariates, and nasal symptoms, although this association was only observed for a delay of 5 days. A previous study demonstrated that no changes in nasal function were observed even in allergic rhinitis patients after exposure to 400 ppb of NO2 for six hours [47], which is consistent with our results. Our results were also in line with another report of short-term exposure to NO2, in which no airway inflammation was found in normal subjects, patients with chronic obstructive pulmonary disease, or patients with asthma [48]. In the previous study, they observed that PAHs, NO2 and SO2 are associated with cough, and their effects on the respiratory system may be delayed [33]. Therefore, our results indicate that NO2 may also require a certain period until a change of nasal function occur.
Our study may be the first longitudinal study targeting the association of specific PAHs with nasal symptoms among adults. An advantage of our study was the use of daily concentrations of different PAHs in combination with nasal symptoms, which allowed us to observe the association of these individual PAHs with nasal symptoms prevalence. In addition, repeated observation of participants over a two-month period was able to better reflect the response of the upper respiratory tract to changes in pollutant concentrations. But there were several limitations that need to be addressed. The number of participants enrolled in the present study was smaller than in previous studies. Furthermore, even though participants were requested to record their daily health status, some diary records were incomplete. Moreover, we were unable to collect information on the previous medical history and current health and mental status of participants; therefore, the absence of this information may have led to inaccurate results. Another limitation is that our participants were not from urban areas. Although they lived close to where data were collected, their exposure to pollution was lower than our measurements. Moreover, the pollen was at a relatively high stage during our study period compared to other months, and we were not able to collect data on pollen. Likewise, ozone can have adverse effects on the human respiratory system, but we have no data on it. In further studies, it is necessary to collect relevant data and take pollen and ozone into consideration as confounding factors. Nevertheless, the results obtained are meaningful because they showed that even exposure to low concentrations of air pollutants may cause nasal symptoms, which was not reported in previous studies. This provides a direction for future research, which will clarify whether PAHs with different molecular weights or isomeric PAHs exert different effects on the human body.
5. Conclusions
In conclusion, our findings suggest that exposure to Flt, Pyr and BkF increases the incidence of nasal symptoms among patients with respiratory disease, whereas exposure to IcdP does the opposite. We also observed that NO2 may have a delayed effect on the onset of nasal symptoms. The results we found can provide evidence that PAHs are potential contributors to nasal symptoms and rhinitis. Further studies are needed to elucidate the exact mechanisms contributing to the relationships between specific PAHs and nasal symptoms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app122412544/s1, Table S1: Test of LOD and LOQ; Table S2: The association between air pollution and nasal symptoms.
Author Contributions
Conceptualization, J.Z., A.H. and H.N.; methodology, J.Z., K.-O.P., K.H., H.T., H.M., H.O., A.T. and H.N.; software, K.S. and H.T.; validation, K.H.; formal analysis, J.Z.; investigation, J.Z.; resources, H.T., H.M., H.O., A.H., H.N. and A.T.; data curation, H.T., A.H. and H.N.; writing—original draft preparation, J.Z.; writing—revising and editing, J.Z., A.H., K.H., K.-O.P. and H.N.; visualization, J.Z. and A.H.; supervision, H.N.; project administration, H.N.; funding acquisition, H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Environment Research and Technology Development Fund (JPMEERF20195051) of the Environmental Restoration and Conservation Agency of Japan (Tokyo, Japan).
Institutional Review Board Statement
This study was approved by the Medical Ethics Committee of Kanazawa University (approval number: 1630).
Informed Consent Statement
Informed consent was obtained from all the participants.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Yuko Katsuragi for her assistance with data management.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Billions of People Still Breathe Unhealthy Air: New WHO Data. Available online: https://www.who.int/news/item/04-04-2022-billions-of-people-still-breathe-unhealthy-air-new-who-data (accessed on 25 July 2022).
- soga_2019_report.pdf. Available online: https://www.stateofglobalair.org/sites/default/files/soga_2019_report.pdf (accessed on 22 August 2022).
- Phung, D.; Hien, T.T.; Linh, H.N.; Luong, L.M.; Morawska, L.; Chu, C.; Binh, N.D.; Thai, P.K. Air pollution and risk of respiratory and cardiovascular hospitalizations in the most populous city in Vietnam. Sci. Total Environ. 2016, 557, 322–330. [Google Scholar] [CrossRef]
- Zhang, H.; Niu, Y.; Yao, Y.; Chen, R.; Zhou, X.; Kan, H. The Impact of Ambient Air Pollution on Daily Hospital Visits for Various Respiratory Diseases and the Relevant Medical Expenditures in Shanghai, China. Int. J. Environ. Res. Public Health 2018, 15, 425. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine Particulate Air Pollution and Hospital Admission for Cardiovascular and Respiratory Diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Yang, H.; Yan, C.; Li, M.; Zhao, L.; Long, Z.; Fan, Y.; Zhang, Z.; Chen, R.; Huang, Y.; Lu, C.; et al. Short term effects of air pollutants on hospital admissions for respiratory diseases among children: A multi-city time-series study in China. Int. J. Hyg. Environ. Health 2021, 231, 113638. [Google Scholar] [CrossRef]
- Qian, Y.; Li, H.; Rosenberg, A.; Li, Q.; Sarnat, J.; Papatheodorou, S.; Schwartz, J.; Liang, D.; Liu, Y.; Liu, P.; et al. Long-Term Exposure to Low-Level NO2 and Mortality among the Elderly Population in the Southeastern United States. Environ. Health Perspect. 2021, 129, 127009. [Google Scholar] [CrossRef]
- Xu, Z.; Xiong, L.; Jin, D.; Tan, J. Association between short-term exposure to sulfur dioxide and carbon monoxide and ischemic heart disease and non-accidental death in Changsha city, China. PLoS ONE 2021, 16, e0251108. [Google Scholar] [CrossRef]
- Orellano, P.; Reynoso, J.; Quaranta, N. Short-term exposure to sulphur dioxide (SO2) and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int. 2021, 150, 106434. [Google Scholar] [CrossRef]
- Kuiper, I.N.; Svanes, C.; Markevych, I.; Accordini, S.; Bertelsen, R.J.; Bråbäck, L.; Christensen, J.H.; Forsberg, B.; Halvorsen, T.; Heinrich, J.; et al. Lifelong exposure to air pollution and greenness in relation to asthma, rhinitis and lung function in adulthood. Environ. Int. 2021, 146, 106219. [Google Scholar] [CrossRef]
- Burte, E.; Leynaert, B.; Bono, R.; Brunekreef, B.; Bousquet, J.; Carsin, A.E.; De Hoogh, K.; Forsberg, B.; Gormand, F.; Heinrich, J.; et al. Association between air pollution and rhinitis incidence in two European cohorts. Environ. Int. 2018, 115, 257–266. [Google Scholar] [CrossRef]
- Deng, Q.; Lu, C.; Yu, Y.; Li, Y.; Sundell, J.; Norbäck, D. Early life exposure to traffic-related air pollution and allergic rhinitis in preschool children. Respir. Med. 2016, 121, 67–73. [Google Scholar] [CrossRef]
- Burte, E.; Leynaert, B.; Marcon, A.; Bousquet, J.; Benmerad, M.; Bono, R.; Carsin, A.E.; de Hoogh, K.; Forsberg, B.; Gormand, F.; et al. Long-term air pollution exposure is associated with increased severity of rhinitis in 2 European cohorts. J. Allergy Clin. Immunol. 2020, 145, 834–842.e6. [Google Scholar] [CrossRef]
- Segboer, C.L.; Holland, C.T.; Reinartz, S.M.; Terreehorst, I.; Gevorgyan, A.; Hellings, P.W.; Van Drunen, C.M.; Fokkens, W.J. Nasal hyper-reactivity is a common feature in both allergic and nonallergic rhinitis. Allergy 2013, 68, 1427–1434. [Google Scholar] [CrossRef]
- Doulaptsi, M.; Steelant, B.; Prokopakis, E.; Ierodiakonou, D.; Tsinaslanidou, Z.; Cools, L.; Pugin, B.; Milioni, A.; Van Gerven, L.; Fokkens, W.J.; et al. Prevalence and impact of nasal hyperreactivity in chronic rhinosinusitis. Allergy 2020, 75, 1768–17471. [Google Scholar] [CrossRef]
- Eifan, A.O.; Durham, S.R. Pathogenesis of rhinitis. Clin. Exp. Allergy 2016, 46, 1139–1151. [Google Scholar] [CrossRef]
- Nikasinovic, L.; Just, J.; Sahraoui, F.; Seta, N.; Grimfeld, A.; Momas, I. Nasal inflammation and personal exposure to fine particles PM2.5 in asthmatic children. J. Allergy Clin. Immunol. 2006, 117, 1382–1388. [Google Scholar] [CrossRef]
- Ezratty, V.; Guillossou, G.; Neukirch, C.; Dehoux, M.; Koscielny, S.; Bonay, M.; Cabanes, P.A.; Samet, J.M.; Mure, P.; Ropert, L.; et al. Repeated Nitrogen Dioxide Exposures and Eosinophilic Airway Inflammation in Asthmatics: A Randomized Crossover Study. Environ. Health Perspect. 2014, 122, 850–855. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alokail, M.S.; Abd-Alrahman, S.H.; Draz, H.M.; Yakout, S.M.; Clerici, M. Polycyclic aromatic hydrocarbon exposure and pediatric asthma in children: A case–control study. Environ. Health 2013, 12, 1. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Qiu, X.; Zhang, H.; Lu, X.; Li, H.; Chen, W.; Zhang, L.; Que, C.; Zhu, T. Association between exposure to polycyclic aromatic hydrocarbons and lipid peroxidation in patients with chronic obstructive pulmonary disease. Sci. Total Environ. 2021, 780, 146660. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Y.; Cui, X.; Wu, X.; Yuan, J.; Xie, J. Urinary polycyclic aromatic hydrocarbon metabolites and adult asthma: A case-control study. Sci. Rep. 2018, 8, 7658. [Google Scholar] [CrossRef]
- Mukae, H.; Kaneko, T.; Obase, Y.; Shinkai, M.; Katsunuma, T.; Takeyama, K.; Terada, J.; Niimi, A.; Matsuse, H.; Yatera, K.; et al. The Japanese respiratory society guidelines for the management of cough and sputum (digest edition). Respir. Investig. 2021, 59, 270–290. [Google Scholar] [CrossRef] [PubMed]
- Wide-Area Monitoring System for Air Pollutants by Ministry of the Environment (Soramamekun). Available online: https://soramame.env.go.jp/ (accessed on 26 July 2022).
- Higashi, T.; Kambayashi, Y.; Ohkura, N.; Fujimura, M.; Nakanishi, S.; Yoshizaki, T.; Saijoh, K.; Hayakawa, K.; Kobayashi, F.; Michigami, Y.; et al. Exacerbation of daily cough and allergic symptoms in adult patients with chronic cough by Asian dust: A hospital-based study in Kanazawa. Atmos. Environ. 2014, 97, 537–543. [Google Scholar] [CrossRef]
- Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 27 July 2022).
- Bortey-Sam, N.; Ikenaka, Y.; Akoto, O.; Nakayama, S.M.; Asante, K.A.; Baidoo, E.; Obirikorang, C.; Saengtienchai, A.; Isoda, N.; Nimako, C.; et al. Oxidative stress and respiratory symptoms due to human exposure to polycyclic aromatic hydrocarbons (PAHs) in Kumasi, Ghana. Environ. Pollut. 2017, 228, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Alhamdow, A.; Zettergren, A.; Kull, I.; Hallberg, J.; Andersson, N.; Ekström, S.; Berglund, M.; Wheelock, C.E.; Essig, Y.J.; Krais, A.M.; et al. Low-level exposure to polycyclic aromatic hydrocarbons is associated with reduced lung function among Swedish young adults. Environ. Res. 2021, 197, 111169. [Google Scholar] [CrossRef] [PubMed]
- Alhamdow, A.; Lindh, C.; Albin, M.; Gustavsson, P.; Tinnerberg, H.; Broberg, K. Early markers of cardiovascular disease are associated with occupational exposure to polycyclic aromatic hydrocarbons. Sci. Rep. 2017, 7, 9426. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hou, J.; Zhou, Y.; Sun, H.; Yin, W.; Zhang, Y.; Wang, X.; Wang, G.; Chen, W.; Yuan, J. Association of polycyclic aromatic hydrocarbons exposure with atherosclerotic cardiovascular disease risk: A role of mean platelet volume or club cell secretory protein. Environ. Pollut. 2018, 233, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, W.; Galas, A.; Pac, A.; Flak, E.; Camman, D.; Rauh, V.; Perera, F. Prenatal Ambient Air Exposure to Polycyclic Aromatic Hydrocarbons and the Occurrence of Respiratory Symptoms over the First Year of Life. Eur. J. Epidemiol. 2005, 20, 775–782. [Google Scholar] [CrossRef]
- Mu, G.; Fan, L.; Zhou, Y.; Liu, Y.; Ma, J.; Yang, S.; Wang, B.; Xiao, L.; Ye, Z.; Shi, T.; et al. Personal exposure to PM2.5-bound polycyclic aromatic hydrocarbons and lung function alteration: Results of a panel study in China. Sci. Total Environ. 2019, 684, 458–465. [Google Scholar] [CrossRef]
- Anyenda, E.O.; Higashi, T.; Kambayashi, Y.; Nguyen, T.T.T.; Michigami, Y.; Fujimura, M.; Hara, J.; Tsujiguchi, H.; Kitaoka, M.; Asakura, H.; et al. Associations of Cough Prevalence with Ambient Polycyclic Aromatic Hydrocarbons, Nitrogen and Sulphur Dioxide: A Longitudinal Study. Int. J. Environ. Res. Public Health 2016, 13, 800. [Google Scholar] [CrossRef]
- Anyenda, E.O.; Higashi, T.; Kambayashi, Y.; Thao, N.T.T.; Michigami, Y.; Fujimura, M.; Hara, J.; Tsujiguchi, H.; Kitaoka, M.; Asakura, H.; et al. Exposure to daily ambient particulate polycyclic aromatic hydrocarbons and cough occurrence in adult chronic cough patients: A longitudinal study. Atmos. Environ. 2016, 140, 34–41. [Google Scholar] [CrossRef]
- Armstrong, B.; Hutchinson, E.; Unwin, J.; Fletcher, T. Lung Cancer Risk after Exposure to Polycyclic Aromatic Hydrocarbons: A Review and Meta-Analysis. Environ. Health Perspect. 2004, 112, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, Y.; Deng, Q.; Chen, Z.; Dai, J.; Li, X.; Zhang, W.; Zhang, X.; He, M.; Wu, T.; et al. Polycyclic aromatic hydrocarbons exposure and lung function decline among coke-oven workers: A four-year follow-up study. Environ. Res. 2016, 150, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Xing, J.; Ji, Q.; Li, Z.; Wang, Y.; Zhao, H.; Wang, Q.; Wang, T.; Yu, L.; Zhang, X.; et al. Declining Pulmonary Function in Populations with Long-term Exposure to Polycyclic Aromatic Hydrocarbons-Enriched PM2.5. Environ. Sci. Technol. 2018, 52, 6610–6616. [Google Scholar] [CrossRef]
- Mizutani, N.; Nabe, T.; Ohtani, Y.; Han, H.Y.; Fujii, M.; Yoshino, S.; Hirayama, T.; Kohno, S. Polycyclic Aromatic Hydrocarbons Aggravate Antigen-Induced Nasal Blockage in Experimental Allergic Rhinitis. J. Pharmacol. Sci. 2007, 105, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Outerbridge, C.A.; Jordan, T.J.M. Current Knowledge on Canine Atopic Dermatitis. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yin, M.; Yang, P.; Li, X.; Di, L.; Wang, W.; Cui, H.; Yan, X.; Liu, J. Effect of Exposure to Cats and Dogs on the Risk of Asthma and Allergic Rhinitis: A Systematic Review and Meta-analysis. Am. J. Rhinol. Allergy 2020, 34, 703–714. [Google Scholar] [CrossRef]
- Wang, I.-J.; Karmaus, W.J.J.; Yang, C.-C. Polycyclic aromatic hydrocarbons exposure, oxidative stress, and asthma in children. Int. Arch. Occup. Environ. Health 2017, 90, 297–303. [Google Scholar] [CrossRef]
- Dullaers, M.; De Bruyne, R.; Ramadani, F.; Gould, H.J.; Gevaert, P.; Lambrecht, B.N. The who, where, and when of IgE in allergic airway disease. J. Allergy Clin. Immunol. 2012, 129, 635–645. [Google Scholar] [CrossRef]
- Cheng, Z.; Huo, X.; Dai, Y.; Lu, X.; Hylkema, M.N.; Xu, X. Elevated expression of AhR and NLRP3 link polycyclic aromatic hydrocarbon exposure to cytokine storm in preschool children. Environ. Int. 2020, 139, 105720. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, J.M.; Yang, H.W.; Kim, T.H.; Lee, S.H.; Lee, H.M.; Cho, J.G.; Park, I.H. Cigarette Smoke Extract Stimulates MMP-2 Production in Nasal Fibroblasts via ROS/PI3K, Akt, and NF-κB Signaling Pathways. Antioxidants 2020, 9, 739. [Google Scholar] [CrossRef]
- Pace, E.; Ferraro, M.; Di Vincenzo, S.; Gerbino, S.; Bruno, A.; Lanata, L.; Gjomarkaj, M. Oxidative stress and innate immunity responses in cigarette smoke stimulated nasal epithelial cells. Toxicol. In Vitro 2014, 28, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.H.; Lee, C.L.; Su, H.H.; Lee, C.L.; Wu, C.C.; Wang, C.C.; Sheu, C.C.; Lai, R.S.; Leung, S.Y.; Lin, C.C.; et al. A prominent air pollutant, Indeno[1,2,3-cd]pyrene, enhances allergic lung inflammation via aryl hydrocarbon receptor. Sci. Rep. 2018, 8, 5198. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Devalia, J.L.; Duddle, J.M.; Hamilton, S.A.; Davies, R.J. Effect of six-hour exposure to nitrogen dioxide on early-phase nasal response to allergen challenge in patients with a history of seasonal allergic rhinitis. J. Allergy Clin. Immunol. 1995, 96, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Vagaggini, B.; Paggiaro, P.L.; Giannini, D.; Franco, A.D.; Cianchetti, S.; Carnevali, S.; Taccola, M.; Bacci, E.; Bancalari, L.; Dente, F.L.; et al. Effect of short-term NO2 exposure on induced sputum in normal, asthmatic and COPD subjects. Eur. Respir. J. 1996, 9, 1852–1857. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).