Abstract
Background: Antiresorptive and antiangiogenic medications can cause a serious adverse effect known as medication-related osteonecrosis of the jaw (MRONJ). In recent years, a new trend of research has emerged emphasizing the potential relation of MRONJ and genetic predisposition. Current evidence-based science of this adverse reaction is associated with poorly performed studies. Additionally, MRONJ research has recently observed a new trend of studies orientated towards the misuse of reviews. This quality meta-review intends to summarize the results of all systematic reviews and meta-analyses that have been published on MRONJ in relation to genetic and pharmacogenomics risk factors. Methods: The research study protocol was registered into the database of the International Network for the Registration of Systematic Reviews and Meta-Analyses (INPLASY) INPLASY202230002. A comprehensive search across several databases (PubMed, EMBASE, MEDLINE, and CINAHL) was conducted to locate multi-language papers published between January 2003 and November 2022. Data were collected from relevant research studies and appraised in accordance with the precise outcomes described in this evaluation. Results: Only five systematic reviews and meta-analyses were analysed in this meta-review. All the reviews included in this research presented qualities mistakes and shortcomings. Two quality assessment tools (Confidence in Evidence from Reviews of Qualitative research (CERQual) and Assessment of Multiple Systematic Reviews 2 (AMSTAR-2)) were used to evaluate each study included in this research. Conclusions: The data evaluated by this meta-review confirmed the poor-quality secondary research underpinning the genetic/pharmacogenomics aspect of MRONJ. Moreover, this study highlighted the many flaws of the current published systematic and meta-analysis studies published so far.
1. Introduction
One of the most serious side effects associated with the use of particular drugs is a condition known as medication-related osteonecrosis of the jaw (MRONJ). This condition is induced primarily by antiresorptive drugs and angiogenesis inhibitors [,]. Commonly, these drugs are used to treat the skeletal signs and symptoms of primary or secondary bone metastases. In addition, these medications are also used to manage chronic benign bone conditions such as osteoporosis or Paget’s disease [,].
Other substances, such as the inhibitors of the tumour necrosis factor-α (TNF-α), have been linked to osteonecrosis of the jaw (ONJ) in a growing number of scientific investigations since 2003 [,,]. Hence, the American Association of Oral and Maxillofacial Surgeons (AAOMS) in 2014 proposed the nomenclature of MRONJ due to the possible association with different drugs and the ONJ []. This positional paper has been recently updated in 2022 [].
The prevalence of MRONJ varies depending on a number of other factors, including a patient’s medical history, pharmacological therapy, length of therapy, and dental therapies []. According to studies, the incidence of MRONJ following a tooth extraction is predicted to range from 1.6–14.8% for cancer patients using intravenous bisphosphonates, with a mean incidence of 7% [,]. This contrasts with an incidence of MRONJ of 0.05% in individuals using oral bisphosphonates and 1.8% in cancer patients receiving denosumab [,,,,]. Furthermore, there is an increased risk of MRONJ development when antiangiogenic medicines are used with antiresorptive medications, with an estimated 16% recurrence rate [].
According to a number of studies, dental extractions are the leading cause of MRONJ, with prevalence rates ranging from 48.5% all the way up to 80% in some cases []. Recently, it was discovered that MRONJ can be triggered by tooth extraction (61.7%), spontaneous onset (14.8%), or ill-fitting dentures (7.4%) in the same proportions regardless of the route of drug administration []. Denosumab-related MRONJ is, however, a subject of limited investigation.
There are many different factors that could cause and increase the severity of osteonecrosis of the jaw. Most of them are not completely understood due to their unclear aetiopathogenesis [,,,].
Since the first study that was ever published detailing ONJ, over the course of the previous two decades, researchers have attempted to understand the underlying molecular process that is responsible for this disease []. Despite the fact that various risk factors have been identified and linked to an increased incidence of MRONJ development, the exact aetiology and pathophysiology of MRONJ are still not fully understood. These risk factors consist of the potency and route of administration of the antiresorptive agent (intravenous bisphosphonate versus oral), the underlying disease (oncology versus osteoporosis), the duration and cumulative dosage of antiresorptive therapy, and the requirement for dentoalveolar surgery or the presence of dental infections [,,].
In recent years, pharmacogenetics represents a promising approach to address the potential predisposition and pathogenesis of MRONJ []. These studies were designed to find out whether or not changes in patients’ genetic makeup can have an effect on how they react to different medications. In order to gain a better understanding of the many forms of genetic differences, a large number of genome-wide association studies (GWAS), candidate gene studies (CGs), and whole-genome studies (WGs) have been conducted [,]. These particular investigations have used single nucleotide polymorphisms (SNPs) to investigate a potential link. It is believed that the human genome has approximately 10 million SNPs, which are often located in the DNA between genes and have low or no effect on the phenotype []. However, because they have the ability to change transcription patterns and the activity of genes, they might potentially be used by forecasters to determine the risk that patients would have when responding to particular medications [,,,,].
The affinity and reliable association of SNPs and MRONJ might allow the development of enhanced preventative strategies, improved diagnostic tests, patient-specific management, and an increased understanding of the MRONJ pathogenic mechanisms. Despite nearly two decades of research, there is still no agreement on how to properly diagnose, prevent, and treat patients with MRONJ [,,,,,,,,,,,,,,].
It has been noticed that MRONJ research has recently observed a new trend of studies orientated towards misuse of reviews contributing little or nothing to current knowledge and exposing clinicians and researchers to incomplete, misdirect or even incorrect conclusions. This ultimately could lead to incorrect general recommendations.
Hence, this meta-review aims to appraise the quality of systematic reviews (SR) and meta-analyses (MA) with regard to the genetic and pharmacogenomic aspects of MRONJ.
2. Materials and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards were followed in order to carry out this more specific investigation []. In order to reduce the likelihood of any potential reporting bias, the protocol for the study was submitted to INPLASY, an international platform of registered systematic review and meta-analysis protocols, and given the registration number INPLASY202230002. In order to reduce the likelihood of important data being overlooked, the search method was carried out in four different databases, namely PubMed, MEDLINE, EMBASE, and CINAHL.
The search was conducted to locate multi-language papers published between January 2003 and November 2022.
The study was conducted using a three-stage screening process to assure accuracy and protect the quality of the findings. Five writers (RS, SO, OA, NS, and VM) independently evaluated the titles and abstracts to remove any unnecessary materials (i.e., reviews, animal studies, and non-clinical studies). Disputes were settled by conversation until a consensus was formed.
The following data screening strategy was used to extract the papers:
- Inclusion and exclusion criteria were used to check the eligibility of the previous studies.
- Quality assessment was performed on the methodology.
- Data were extracted based on characteristics and outcomes for the selected papers. (Figure 1).
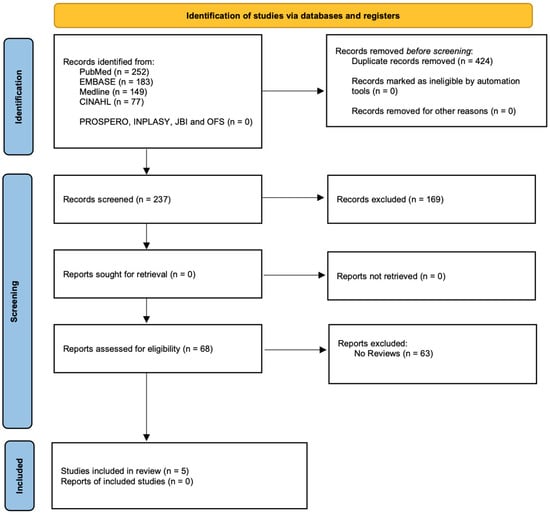
Figure 1.
Prisma study flow diagram.
Direct communication was established with the authors of any studies that were potentially suitable for inclusion in the review but had inadequate information so that the authors might supply more data. The PICO method served as the foundation for the criteria for inclusion in the study [].
PICOS Research Question
What is the present state of knowledge about the pharmacogenomic and genetic aspects of MRONJ?
Population—(P): Patients with MRONJ having any age
Interventions—(I): Any sort of intervention
Comparison—(C): Any sort of comparison
Outcomes—(O): The quality level of pharmacogenomics and genetic studies associated with the MRONJ
Study—S: Systematic Review and/or Meta-Analysis
The search criteria for all the selected databases were followed by searching the MeSH terms and keywords of Osteonecrosis, MRONJ, ONJ, ARONJ, BRONJ, and BONJ. Other MeSH terms used were Pharmacogenetics, Genetic predisposition of MRONJ, Pharmacogenomics and MRONJ or Genetic and MRONJ. The search method made use of appropriate syntax constraints outlined in every repository.
2.1. Inclusion Criteria
2.1.1. Studies Types
This meta-review included every secondary type of research that was published regarding pharmacogenomics and genetics related to MRONJ. The search was carried out without any linguistic limits being placed on it. Excluded from consideration were narrative reviews, animal studies, and studies that involved individuals who had received radiation treatment to the head and neck regions in the past.
2.1.2. Participant Type
Patients affected with MRONJ after treatment with antiangiogenic, antiresorptive, and any other drug linked with the jaw’s osteonecrosis. Restrictions were not put in place on the participants’ sex and ethnicity. Meta-analyses and systematic reviews may incorporate any type of study.
2.1.3. Type of Outcomes Analysed
- (a)
- Primary outcome
Evaluation of the currently published studies associated with MRONJ and its pharmacogenomics and genetic characteristics in SR and MA.
- (b)
- Secondary outcomeEvaluation of the following factors such as:
- The various kinds of studies that were selected in the SR and the MA.
- Different numbers of patients included in the SR and MA.
- Patients’ characteristics included in the meta-review selected studies.
2.1.4. Data Extraction
Five independent review authors selected all the research included articles (RS, SO, CFdeABM, MDCM, and JY). The Data Extraction and Assessment Template from the Cochrane Public Health Group were utilized in order to collect the data. The number of included studies, the studies designed, the total number of patients, the type of outcomes analysed, and the level of evidence suggested, were recorded in the Data Extraction form. A standardised and pre-set Microsoft Excel form was utilised for the process of data collection. Any disagreement was resolved by discussion until a consensus was reached.
In the event of poor or omitted information, the authors of the included studies were notified, and they were given two months to respond. In the event that the information was not provided, the tables and the text both included the notation “Not Reported (NR)”. This study incorporated a total of five different systematic reviews into its findings in Table 1 [,,,,].

Table 1.
Systematic reviews included within this analysis. Systematic review (SR); meta-analysis (MA).
2.1.5. Meta-Review Quality Assessment Criteria
All the studies were evaluated by three separate researchers (NS, MDCM, and RS) for the methodological quality and the evidence quality of the included studies of this meta-review:
- -
- Assessment of Multiple Systematic Reviews 2 (AMSTAR-2) tool
The AMSTAR 2 uses 16 questions (domains) with three possible outcomes: “yes,” “no,” or “partially yes” to assess the methodological quality of any form of review (systematic and meta-analysis reviews). The following marks are indicative of the overall level of confidence in the included studies: low, critically low, moderate, and high [].
- -
- The Confidence in Evidence from Reviews of Qualitative research (CERQual) tool
CERQual tool is recommended by the Grading of Recommendations Assessment, Development and Evaluation Working Group (GRADE) to evaluate the evidence quality []. With the help of the CERQual evaluation tool, the authors were able to assess the studies that were included in the present meta-review according to four key areas:
- (1)
- The limitations of the methodology associated with the individual qualitative studies incorporated in the reviews analysed.
- (2)
- Coherence of the studies and findings in the reviews.
- (3)
- Sufficiency of the data to support a conclusion reached by the reviews.
- (4)
- How strictly the context (perspective or demographic, phenomenon of interest, and setting) that is defined in the research question corresponds to the data from the primary studies that support a specific finding in a review [].
Any points of contention regarding the evaluation of the potential biases were brought to the attention of the third author on the review team (JY), after which they were discussed and settled.
3. Results
The initial consideration for inclusion in this review included 68 studies. In the end, sixty-three publications were deemed ineligible for inclusion in this quality meta-review after a study of the titles and abstracts, as well as an inspection of the full articles. As a result of the data’s wide range of variability, the findings were described only in general terms.
This study comprised a total of five studies. A total of 3663 patients were studied in the included research. Patient evaluations conducted between 2013 and 2020 are described in every published study (Table 2). Systematic reviews (n = 3) and meta-analyses (n = 2) were both included in this quality meta-review. We included all of the accepted reviews in this analysis, including GWAS, CGS, and WGS/WES, as well as reviews that focused on single genes or regions of the genome. The overall characteristics, as well as all of the studies that were analysed and included in the reviews, are provided in Table 2, Table 3 and Table 4.

Table 2.
Summary of genome-wide association studies (GWAS) included in the systematic reviews [,,,,]. BC, breast cancer; BP, bisphosphonate; CC, cervical cancer; MM, multiple myeloma; NR, not reported; OP, osteoporosis; PC, prostate cancer; RA, rheumatoid arthritis; RC, renal cancer; SNP, single nucleotide polymorphism.

Table 3.
Summary of candidate gene studies (CGS) [,,,,]. BC, breast cancer; BP, bisphosphonate; CC, cervical cancer; MM, multiple myeloma; NR, not reported; OP, osteoporosis; PC, prostate cancer; RA, rheumatoid arthritis; RC, renal cancer; SNP, single nucleotide polymorphism; VEGF, vascular endothelial growth factor.

Table 4.
Summary of whole-genome and whole-exome studies (WGS/WES) [,,,,]. BC, breast cancer; BP, bisphosphonate; CC, cervical cancer; MM, multiple myeloma; NR, not reported; OP, osteoporosis; PC, prostate cancer; RA, rheumatoid arthritis; RC, renal cancer; SNP, single nucleotide polymorphism.
All of the reviews that were given in this research project comprised a diverse range of patients, including both oncology and non-oncology patients. All of the studies that were included in the reviews that were suitable for this analysis were retrospective cohort studies with a total of sixteen studies across all the SR and MA selected in this paper [,,,,,,,,,,,,,,,]. Patients who had been diagnosed with MRONJ (group 1), patients who did not have MRONJ (group 2), and healthy patients (group 3) were the primary categories of patients who were evaluated in the reviews included in this study (Table 2, Table 3 and Table 4).
All the patients included in the SR and MA studies were taking bisphosphonates as part of their treatment. The quality of the risk-of-bias assessments in each of the reviews that were incorporated into this meta-review was deemed to be high across the board for each type of bias that was evaluated. Indeed, each and every one of the outcomes that were assessed by the SR and MA studies that were analysed for this review brought attention to the absence of proof or the supply of any definitive advice.
4. Meta-Review Quality Assessment Analysis
4.1. AMSTAR 2 Assessment
Figure 2 summarises the findings of the two study researchers that evaluated each AMSTAR 2 domain for all the SR- and MA-included studies. The average rating was 8.50 (SD ± 3.20).

Figure 2.
AMSTAR2 traffic light plot [,,,,].
The minimum and maximum scores were 6 and 12, respectively. The evaluation criteria were based on:
- -
- Yes—was valued (1 point);
- -
- Partial yes—was valued (0.5 points);
- -
- No—was valued (0 points);
- -
- No information/no meta-analysis conducted—was valued (0 points).
According to the authors, the overall methodology assessment criteria resulted in being critically low in all the SR and MA studies included in this quality meta-review.
4.2. CERQual Assessment
CERQual Qualitative Evidence Profile, which describes the review authors’ assessments, was used to evaluate each CERQual component (Table 5).

Table 5.
CERQual quality assessment evaluation [].
In summary, the authors found that:
Across the board, all of the SR and MA included in this meta-review were considered to have serious deficiencies regarding the quality of the evidence. In addition, serious concerns were identified over the results data in Zhong et al., 2013 [], although moderate concern was expressed in all of the other systematic reviews. Minor concerns were raised regarding the relevance and coherence of the data associated with many of the SR and MA evaluated by this study.
Low confidence was assessed to be appropriate for the overall assessment as a result of a large number of significant concerns.
5. Discussion
According to the findings of our investigation, a large number of studies have been conducted to study the possible link between genetic variations and MRONJ; nevertheless, the conclusions drawn from these investigations have been contradictory thus far.
Only one study included in the reviews analysed found that the CYP2C8 rs1934951 polymorphism was a risk factor for the development of MRONJ; however, other studies have evaluated the association between this polymorphism and susceptibility to MRONJ in cancer patients, but no predisposition was found []. Many other studies included in the reviews analysed revealed rs1152003 and the peroxisome proliferator-activated receptor-gamma (PPARG), but no correlation with the MRONJ group under investigation was found [,]. Additionally, VEGF SNPs (rs3025039, rs699947, and rs2010963) were examined in other research; only for rs3025039 was a significant difference found, whilst differences for the other genotypes were minor (rs2010963 and rs699947) [,]. The candidate genes and SNPs identified through these studies varied and rarely replicated in another.
In recent years, rising evidence has been described to support the role of SNPs with MRONJ. However, these studies have shown either a weak or no connection between the genetic aspects measured and the possibility of MRONJ development [].
Even though the pathophysiology of MRONJ is still a mystery to us, osteoclasts are thought to play an important part in its occurrence. To understand the mechanism, Katz et al., analyzed the SNPs and revealed that osteoclastogenesis and differentiation, bone resorption, bone mineral density, and osteoporosis are all influenced by SNPs in seven genes []. MRONJ was shown to be more common in patients with genetic variation in genes encoding COL1A1 (rs1800012), RANK (rs12458117), MMP2 (rs243865), OPG (rs2073618), and OPN (rs11730582) [].
Furthermore, Nicoletti et al. observed that MRONJ risk was strongly related to RBMS3 rs17024608 in the 53 patients who were using zoledronate for osteoporosis []. In fact, RBMS3 regulates collagen synthesis, contains a significant amount of bone matrix, and is critical for bone resorption. Bone remodelling may be affected and antiresorptive medicines made more potent by RBMS3 mutations, leading to MRONJ []. In addition, farnesyl pyrophosphate synthase is thought to be an additional important participant in the pathophysiology of MRONJ. This theory is based on speculation (FPPs). This is an important enzyme in the process known as the mevalonate pathway. It is responsible for the inhibition and disruption of particular proteins in the cytoskeleton of osteoclasts, which ultimately results in the prevention of bone resorption [].
In order to investigate the prevalence of FDPS rs2297480 in a cohort of patients receiving zoledronate for multiple myeloma, breast cancer, and prostate cancer, Marini et al. designed their study. The study found a strong association between MRONJ and FDPS rs2297480 polymorphism; however, its exact purpose is yet unknown [].
The nicotinamide adenine dinucleotide-dependent deacetylase sirtuin 1 (SIRT1) and E3 ubiquitin-protein ligase (HERC4) are crucial regulators of cell proliferation and differentiation processes in the skeletal system, according to Yang et al. GWAS’s study. This investigation verified that the HERC4 rs3758392 and SIRT1 rs7896005 polymorphisms were substantially linked with MRONJ in cancer patients [].
Previous research highlighted that genetic polymorphism in numerous genes such as TGFb1, MMP2, CYP2C8, VEGF, COL1A1, RANK, OPG, OPN, and PPARG—which are responsible for the differentiation and activation of osteoclast—are considerably linked to MRONJ [,,]. However, despite all the genetic studies (GWAS, WES, and CGS) that have been presented to establish the correct pathological mechanisms, they remain yet to be determined. Furthermore, although potential associations between the development of MRONJ and several genes have been documented in the literature, they have all failed to clarify the responsibility of any genes as the sole risk factor for MRONJ development.
With the goal of highlighting the current state of research quality, the current quality meta-review examined the highest quality evidence currently available that was published in the previous years on genetic and pharmacogenomics studies and MRONJ using the evidence scale developed by the Oxford Centre for Evidence-Based Medicine []. It is found that the authors of the reviews applied stringent inclusion criteria to those who were included in these investigations. The reviews’ research counts, which ranged from 4 [] to 15 articles, make this clear [,,,]. On a very interesting note, the majority of the reviews included in this meta-review were released in 2019, with many of them appearing in the same editorial publication.
All the SR and MA published reviews presented no registration in any public review registry. This indeed increased the chance of reporting bias. The majority of the articles included were low-ranked studies with a moderate to high risk of bias [,,,,]. These deficiencies related to methodology have unquestionably contributed to an increase in the probability of inconsistency in general guidelines that have been issued.
The authors have discovered that most of the reviews feature predominantly retrospective cohort studies with or without multiple arms (quite often without a sample size calculation) [,,,,,,,,,,,,,,,]. That is why the results of these studies and reviews must be interpreted very carefully. Indeed, systematic reviews or meta-analyses will need to provide an outline of a clear and consistent protocol before they can be carried out. This will need to be conducted with the awareness that the results of these types of studies are frequently applied to medical practice in the form of guidance.
Usually, different reviews have a rigorous and transparent protocol, which is strengthened by using the PRISMA checklist. Unfortunately, all of the included reviews did not follow what is currently considered the “gold standard” [,,,,]. Indeed, this shortcoming has affected the adequacy of the evidence published results and conclusions. For better research, it is necessary to conduct analysis and research with excellent quality in the future. Quality research contained randomized control trials that are used to support evidence-based treatments and their protocols with efficiency. The authors suggest the following as some important rules for future studies related to pharmacogenomics and genetics associated with MRONJ:
- It is recommended that a formula for calculating sample size be devised and used for each and every randomised controlled trial (RCT) and cohort study.
- To the best of our ability, big RCTs should be conducted with sufficient depth to enable exact epidemiological and precise findings.
- For every study, risk stratification should be used to reduce the impact of effect modification and confounding variables (e.g., length of drug taken, habits, periodontal status, etc.).
6. Conclusions
According to the finding of this meta-review, there is insufficient data of high quality about pharmacogenomics and genetic aspects to back up various of the presently available guidelines about the MRONJ. This study mainly highlights the availability of low-quality meta-analyses and systematic reviews, which reveal that there are no insightful therapeutic suggestions, risk-predisposition, or criteria that can be implemented to reduce the possibility of developing MRONJ.
We believe that this inconclusive result may suggest that the implication of MRONJ pathophysiology goes beyond genetic aspects and could be potentially explained only by the concomitant association of dysfunctional factors which ultimately promote osteonecrosis. Additionally, it might be possible that genetic predisposition may facilitate MRONJ development but no targeted genetic preventive strategies have been drawn by any of the reviews targeted by this investigation.
Author Contributions
R.S.: Conceptualization, Methodology, Validation, Formal analysis, Writing the original draft. S.O.: Investigation, Validation, Writing the Original draft, Writing—Original draft preparation. N.S.; A.A.; M.D.C.-M.: Methodology, Supervision, Review and editing. C.F.D.A.B.M.; V.M.; O.A.; R.C.G.; J.Y.: Supervision, Review and editing investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data included in the research are available within the article.
Conflicts of Interest
The authors and co-authors have no conflict of interest to declare.
References
- Khan, A.A.; Morrison, A.; Kendler, D.L.; Rizzoli, R.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; et al. Case-Based Review of Osteonecrosis of the Jaw (ONJ) and Application of the International Recommendations for Management From the International Task Force on ONJ. J. Clin. Densitom. 2017, 20, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Eguia, A.; Bagán-Debón, L.; Cardona, F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e71–e83. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P.; Herrstedt, J. ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2014, 25, iii124–iii137. [Google Scholar] [CrossRef] [PubMed]
- Hanley, D.A.; McClung, M.R.; Davison, K.S.; Dian, L.; Harris, S.T.; Miller, P.D.; Lewiecki, E.M.; Kendler, D.L. Western Osteoporosis Alliance Clinical Practice Series: Evaluating the Balance of Benefits and Risks of Long-Term Osteoporosis Therapies. Am. J. Med. 2017, 130, 862.e1–862.e7. [Google Scholar] [CrossRef] [PubMed]
- Marx, E.R. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Sacco, R.; Shah, S.; Leeson, R.; Moraschini, V.; de Almeida Barros Mourão, C.F.; Akintola, O.; Lalli, A. Osteonecrosis and osteomyelitis of the jaw associated with tumour necrosis factor-alpha (TNF-α) inhibitors: A systematic review. Br. J. Oral Maxillofac. Surg. 2020, 58, 25–33. [Google Scholar] [CrossRef]
- Sacco, R.; Ball, R.; Barry, E.; Akintola, O. The role of illicit drugs in developing medication-related osteonecrosis (MRONJ): A systematic review. Br. J. Oral Maxillofac. Surg. 2021, 59, 398–406. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F.; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Yamazaki, T.; Yamori, M.; Ishizaki, T.; Asai, K.; Goto, K.; Takahashi, K.; Nakayama, T.; Bessho, K. Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: A cohort study. Int. J. Oral Maxillofac. Surg. 2012, 41, 1397–1403. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Wouters, K.; Huizing, M.T.; Vermorken, J.B. RANK ligand inhibition in bone metastatic cancer and risk of osteonecrosis of the jaw (ONJ): Non bis in idem? Support Care Cancer 2011, 19, 2035–2040. [Google Scholar] [CrossRef] [PubMed]
- Al-Husein, B.; Abdalla, M.; Trepte, M.; Deremer, D.L.; Somanath, P.R. Antiangiogenic therapy for cancer: An update. Pharmacotherapy 2012, 32, 1095–1111. [Google Scholar] [CrossRef]
- Christodoulou, C.; Pervena, A.; Klouvas, G.; Galani, E.; Falagas, M.E.; Tsakalos, G.; Visvikis, A.; Nikolakopoulou, A.; Acholos, V.; Karapanagiotidis, G.; et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology 2009, 76, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Freitas, M.; Limeres, J. Prevention of medication-related osteonecrosis of the jaws secondary to tooth extractions. A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e250–e259. [Google Scholar] [CrossRef] [PubMed]
- Fliefel, R.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, G.; Beninati, F. Bisphosphonate—Related osteonecrosis of the jaws: The point of view of the oral pathologist. Clin. Cases Miner. Bone Metab. 2007, 4, 53–57. [Google Scholar]
- Otto, S.; Schreyer, C.; Hafner, S.; Mast, G.; Ehrenfeld, M.; Sturzenbaum, S.; Pautke, C. Bisphosphonate-related osteonecrosis of the jaws—Characteristics, risk factors, clinical features, localization and impact on oncological treatment. J. Craniomaxillofac. Surg. 2012, 40, 303–309. [Google Scholar] [CrossRef]
- Mena, A.C.; Pulido, E.G.; Guillen-Ponce, C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: Sunitinib. Anticancer Drugs. 2010, 21, S3–S11. [Google Scholar] [CrossRef]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiødt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef]
- Aghaloo, T.; Hazboun, R.; Tetradis, S. Pathophysiology of Osteonecrosis of the Jaws. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 489–496. [Google Scholar] [CrossRef]
- Saad, F.; Brown, J.E.; Van Poznak, C.; Ibrahim, T.; Stemmer, S.M.; Stopeck, A.T.; Diel, I.J.; Takahashi, S.; Shore, N.; Henry, D.H.; et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: Integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 2012, 23, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Sarasquete, M.E.; García-Sanz, R.; Marín, L.; Alcoceba, M.; Chillón, M.C.; Balanzategui, A.; Santamaria, C.; Rosiñol, L.; de la Rubia, J.; Hernandez, M.T.; et al. Bisphosphonate-related osteonecrosis of the jaw is associated with polymorphisms of the cytochrome P450 CYP2C8 in multiple myeloma: A genome-wide single nucleotide polymorphism analysis. Blood 2008, 112, 2709–2712. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A.; et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 2017, 102, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.T.; Bishop, J.R.; Sangkuhl, K.; Nurmi, E.L.; Mueller, D.J.; Dinh, J.C.; Gaedigk, A.; Klein, T.E.; Caudle, K.E.; McCracken, J.T.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Cytochrome P450 (CYP)2D6 Genotype and Atomoxetine Therapy. Clin. Pharmacol. Ther. 2019, 106, 94–102. [Google Scholar] [CrossRef] [PubMed]
- English, B.C.; Baum, C.E.; Adelberg, D.E.; Sissung, T.M.; Kluetz, P.G.; Dahut, W.L.; Price, D.K.; Figg, W. A SNP in CYP2C8 is not associated with the development of bisphosphonate-related osteonecrosis of the jaw in men with castrate-resistant prostate cancer. Ther. Clin. Risk Manag. 2010, 6, 579–583. [Google Scholar] [PubMed]
- Such, E.; Cervera, J.; Terpos, E.; Bagán, J.V.; Avaria, A.; Gómez, I.; Margaix, M.; Ibañez, M.; Luna, I.; Cordón, L.; et al. CYP2C8 gene polymorphism and bisphosphonate-related osteonecrosis of the jaw in patients with multiple myeloma. Haematologica 2011, 96, 1557–1559. [Google Scholar] [CrossRef][Green Version]
- Khan, A.A.; Sándor, G.K.; Dore, E.; Morrison, A.D.; Alsahli, M.; Amin, F.; Peters, E.; Hanley, D.A.; Chaudry, S.R.; Dempster, D.W.; et al. Canadian consensus practice guidelines for bisphosphonate associated osteonecrosis of the jaw. J. Rheumatol. 2008, 35, 1391–1397. [Google Scholar]
- Dickinson, M.; Prince, H.M.; Kirsa, S.; Zannettino, A.; Gibbs, S.D.; Mileshkin, L.; O’Grady, J.; Seymour, J.F.; Szer, J.; Horvath, N.; et al. Osteonecrosis of the jaw complicating bisphosphonate treatment for bone disease in multiple myeloma: An overview with recommendations for prevention and treatment. Intern. Med. J. 2009, 39, 304–316. [Google Scholar] [CrossRef]
- Terpos, E.; Sezer, O.; Croucher, P.I.; Garcia-Sanz, R.; Boccadoro, M.; San Miguel, J.; Ashcroft, J.; Bladé, J.; Cavo, M.; Delforge, M.; et al. The use of bisphosphonates in multiple myeloma: Recommendations of an expert panel on behalf of the European Myeloma Network. Ann. Oncol. 2009, 20, 1303–1317. [Google Scholar] [CrossRef]
- McLeod, N.M.; Davies, B.J.; Brennan, P.A. Management of patients at risk of bisphosphonate osteonecrosis in maxillofacial surgery units in the UK. Surgeon 2009, 7, 18–23. [Google Scholar] [CrossRef]
- Hellstein, J.W.; Adler, R.A.; Edwards, B.; Jacobsen, P.L.; Kalmar, J.R.; Koka, S.; Migliorati, C.A.; Ristic, H.; American Dental Association Council on Scientific Affairs Expert Panel on Antiresorptive Agents. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: Executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 2011, 142, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.A.; Ahmedzai, S.H.; Ashcroft, J.; D’Sa, S.; Littlewood, T.; Low, E.; Lucraft, H.; Maclean, R.; Feyler, S.; Pratt, G.; et al. Guidelines for supportive care in multiple myeloma 2011. Br. J. Haematol. 2011, 154, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Rhee, Y.; Kwon, Y.D.; Kwon, T.G.; Lee, J.K.; Kim, D.Y. Medication Related Osteonecrosis of the Jaw: 2015 Position Statement of the Korean Society for Bone and Mineral Research and the Korean Association of Oral and Maxillofacial Surgeons. J. Bone Metab. 2015, 22, 151–165. [Google Scholar] [CrossRef]
- Svejda, B.; Muschitz, C.; Gruber, R.; Brandtner, C.; Svejda, C.; Gasser, R.W.; Santler, G.; Dimai, H.P. Position paper on medication-related osteonecrosis of the jaw (MRONJ). Wien. Med. Wochenschr. 2016, 166, 68–74. [Google Scholar] [CrossRef]
- Yoneda, T.; Hagino, H.; Sugimoto, T.; Ohta, H.; Takahashi, S.; Soen, S.; Taguchi, A.; Nagata, T.; Urade, M.; Shibahara, T.; et al. Antiresorptive agent-related osteonecrosis of the jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw. J. Bone Miner. Metab. 2017, 35, 6–19. [Google Scholar] [CrossRef]
- Scottish Dental Clinical Effectiveness Programme (SDCEP). Oral Health Management of Patients at Risk of Medication-Related Osteonecrosis of the Jaw. 2017. Available online: https://www.sdcep.org.uk/media/m0ko0gng/sdcep-oral-health-management-of-patients-at-risk-of-mronj-guidance-full.pdf_ (accessed on 22 September 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 15, 16. [Google Scholar] [CrossRef]
- Zhong, D.N.; Wu, J.Z.; Li, G.J. Association between CYP2C8 (rs1934951) polymorphism and bisphosphonate-related osteonecrosis of the jaws in patients on bisphosphonate therapy: A meta-analysis. Acta Haematol. 2013, 129, 90–95. [Google Scholar] [CrossRef]
- Bastida-Lertxundi, N.; Leizaola-Cardesa, I.O.; Hernando-Vázquez, J.; Muguerza-Iraola, R.; Aguilar-Salvatierra, A.; Gómez-Moreno, G.; Crettaz, J.S. Pharmacogenomics in medication-related osteonecrosis of the jaw: A systematic literature review. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10184–10194. [Google Scholar]
- Guo, Z.; Cui, W.; Que, L.; Li, C.; Tang, X.; Liu, J. Pharmacogenetics of medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 298–309. [Google Scholar] [CrossRef]
- Sandro Pereira da Silva, J.; Pullano, E.; Raje, N.S.; Troulis, M.J.; August, M. Genetic predisposition for medication-related osteonecrosis of the jaws: A systematic review. Int. J. Oral Maxillofac. Surg. 2019, 48, 1289–1299. [Google Scholar] [CrossRef]
- Yang, G.; Singh, S.; Chen, Y.; Hamadeh, I.S.; Langaee, T.; McDonough, C.W.; Holliday, L.S.; Lamba, J.K.; Moreb, J.S.; Katz, J.; et al. Pharmacogenomics of osteonecrosis of the jaw. Bone 2019, 124, 75–82. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Lewin, S.; Glenton, C.; Munthe-Kaas, H.; Carlsen, B.; Colvin, C.J.; Gülmezoglu, M.; Noyes, J.; Booth, A.; Garside, R.; Rashidian, A. Using qualitative evidence in decision making for health and social interventions: An approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual). PLoS Med. 2015, 12, e1001895. [Google Scholar] [CrossRef]
- Nicoletti, P.; Cartsos, V.M.; Palaska, P.K.; Shen, Y.; Floratos, A.; Zavras, A.I. Genomewide pharmacogenetics of bisphosphonate-induced osteonecrosis of the jaw: The role of RBMS3. Oncologist 2012, 17, 279–287. [Google Scholar] [CrossRef]
- Katz, J.; Gong, Y.; Salmasinia, D.; Hou, W.; Burkley, B.; Ferreira, P.; Casanova, O.; Langaee, T.Y.; Moreb, J.S. Genetic polymorphisms and other risk factors associated with bisphosphonate induced osteonecrosis of the jaw. Int. J. Oral Maxillofac. Surg. 2011, 40, 605–611. [Google Scholar] [CrossRef]
- Arduino, P.G.; Menegatti, E.; Scoletta, M.; Battaglio, C.; Mozzati, M.; Chiecchio, A.; Berardi, D.; Vandone, A.M.; Donadio, M.; Gandolfo, S.; et al. Vascular endothelial growth factor genetic polymorphisms and haplotypes in female patients with bisphosphonate-related osteonecrosis of the jaws. J. Oral Pathol. Med. 2011, 40, 510–515. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Arbitrio, M.; Guzzi, P.H.; Leone, E.; Baudi, F.; Piro, E.; Prantera, T.; Cucinotto, I.; Calimeri, T.; Rossi, M.; et al. A peroxisome proliferator-activated receptor gamma (PPARG) polymorphism is associated with zoledronic acid-related osteonecrosis of the jaw in multiple myeloma patients: Analysis by DMET microarray profiling. Br. J. Haematol. 2011, 154, 529–533. [Google Scholar] [CrossRef]
- Marini, F.; Tonelli, P.; Cavalli, L.; Cavalli, T.; Masi, L.; Falchetti, A.; Brandi, M.L. Pharmacogenetics of bisphosphonate-associated osteonecrosis of the jaw. Front. Biosci. 2011, 3, 364–370. [Google Scholar]
- Balla, B.; Vaszilko, M.; Kósa, J.P.; Podani, J.; Takács, I.; Tóbiás, B.; Nagy, Z.; Lazáry, A.; Lakatos, P. New approach to analyze genetic and clinical data in bisphosphonate-induced osteonecrosis of the jaw. Oral Dis. 2012, 18, 580–585. [Google Scholar] [CrossRef] [PubMed]
- La Ferla, F.; Paolicchi, E.; Crea, F.; Cei, S.; Graziani, F.; Gabriele, M.; Danesi, R. An aromatase polymorphism (g.132810C>T) predicts risk of bisphosphonate-related osteonecrosis of the jaw. Biomark. Med. 2012, 6, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, P.; Nkenke, E.; Englbrecht, M.; Schlittenbauer, T.; Wehrhan, F.; Rauh, C.; Beckmann, M.W.; Fasching, P.A.; Kreusch, T.; Mackensen, A.; et al. Major histocompatibility complex class II polymorphisms are associated with the development of anti-resorptive agent-induced osteonecrosis of the jaw. J. Craniomaxillofac. Surg. 2013, 41, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, J.H.; Kim, H.J.; Park, W.; Lee, J.H.; Kim, J.H. Genetic association between VEGF polymorphisms and BRONJ in the Korean population. Oral Dis. 2015, 21, 866–871. [Google Scholar] [CrossRef]
- Kastritis, E.; Melea, P.; Bagratuni, T.; Melakopoulos, I.; Gavriatopoulou, M.; Roussou, M.; Migkou, M.; Eleutherakis-Papaiakovou, E.; Terpos, E.; Dimopoulos, M.A. Genetic factors related with early onset of osteonecrosis of the jaw in patients with multiple myeloma under zoledronic acid therapy. Leuk. Lymphoma 2017, 58, 2304–2309. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, Y.J.; Kim, J.Y.; Oh, Y.; Hwang, J.; Han, S.; Kim, S.; Lee, J.H.; Han, D.H. Genetic investigation of bisphosphonate-related osteonecrosis of jaw (BRONJ) via whole exome sequencing and bioinformatics. PLoS ONE 2015, 10, e0118084. [Google Scholar] [CrossRef]
- Sun, J.; Wen, X.; Jin, F.; Li, Y.; Hu, J.; Sun, Y. Bioinformatics analyses of differentially expressed genes associated with bisphosphonate-related osteonecrosis of the jaw in patients with multiple myeloma. Onco Targets Ther. 2015, 8, 2681–2688. [Google Scholar] [CrossRef]
- Yang, G.; Hamadeh, I.S.; Katz, J.; Riva, A.; Lakatos, P.; Balla, B.; Kosa, J.; Vaszilko, M.; Pelliccioni, G.A.; Davis, N.; et al. SIRT1/HERC4 locus associated with bisphosphonate-Induced osteonecrosis of the jaw: An exome-wide association analysis. J. Bone Miner. Res. 2018, 33, 91–98. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Srivastava, K.; Mansoori, M.N.; Trivedi, R.; Chattopadhyay, N.; Singh, D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: A new candidate in the pathogenesis of osteoporosis. PLoS ONE 2012, 7, e44552. [Google Scholar] [CrossRef]
- Durieux, N.; Vandenput, S.; Pasleau, F. OCEBM levels of evidence system. Rev. Med. Liege 2013, 68, 644–649. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).