Portable X-ray Fluorescence (pXRF) as a Tool for Environmental Characterisation and Management of Mining Wastes: Benefits and Limits
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Strategy and Sample Preparation
2.2. Analytical Determinations by Means of Portable X-ray Fluorescence (pXRF)
2.3. Confirmatory Analyses: Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) and Direct Mercury Analyser (DMA-80)
2.4. Statistical Analysis
3. Results
3.1. Accuracy and Repeatability: pXRF vs. Certified Reference Materials (CRMs)
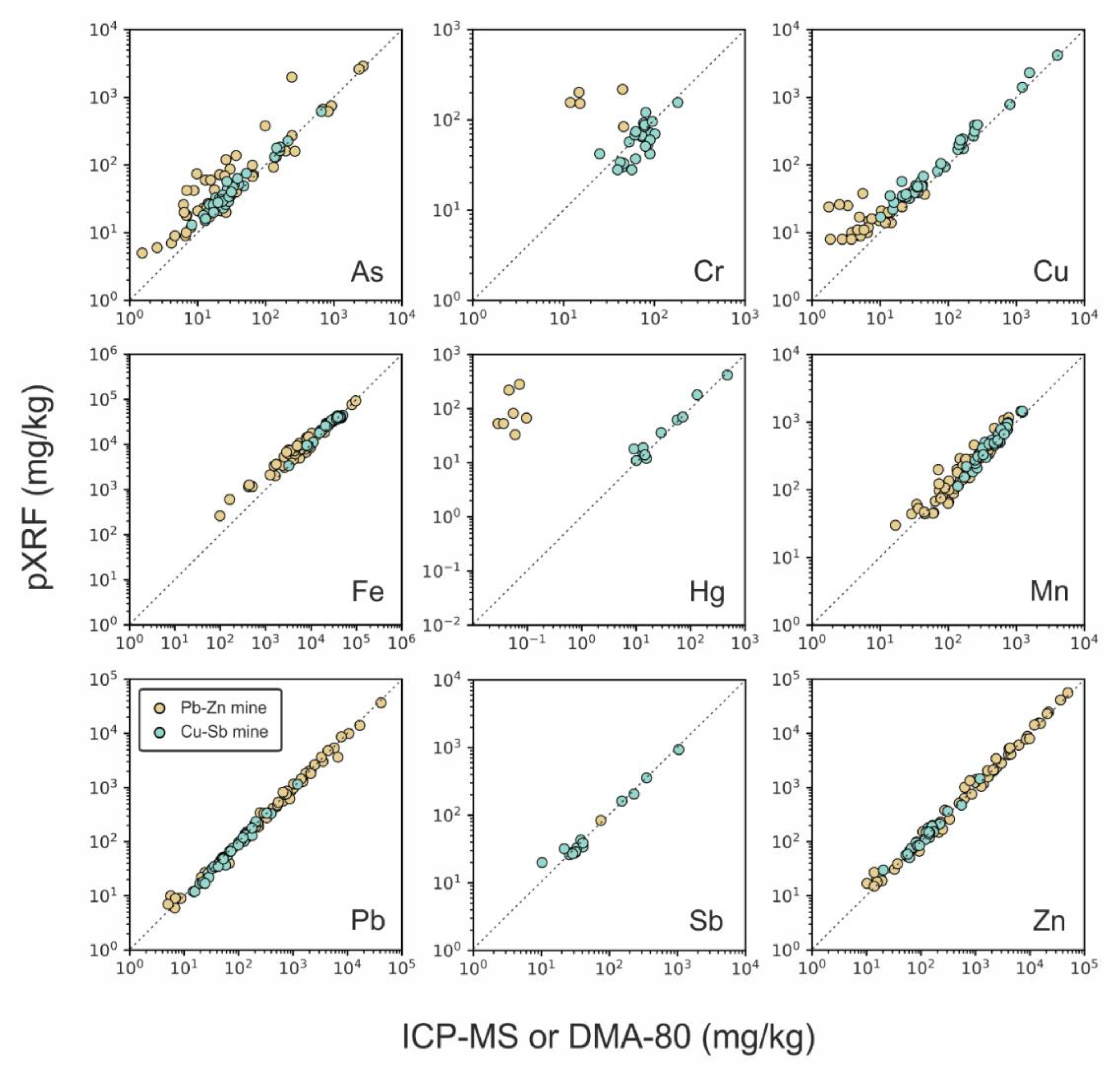
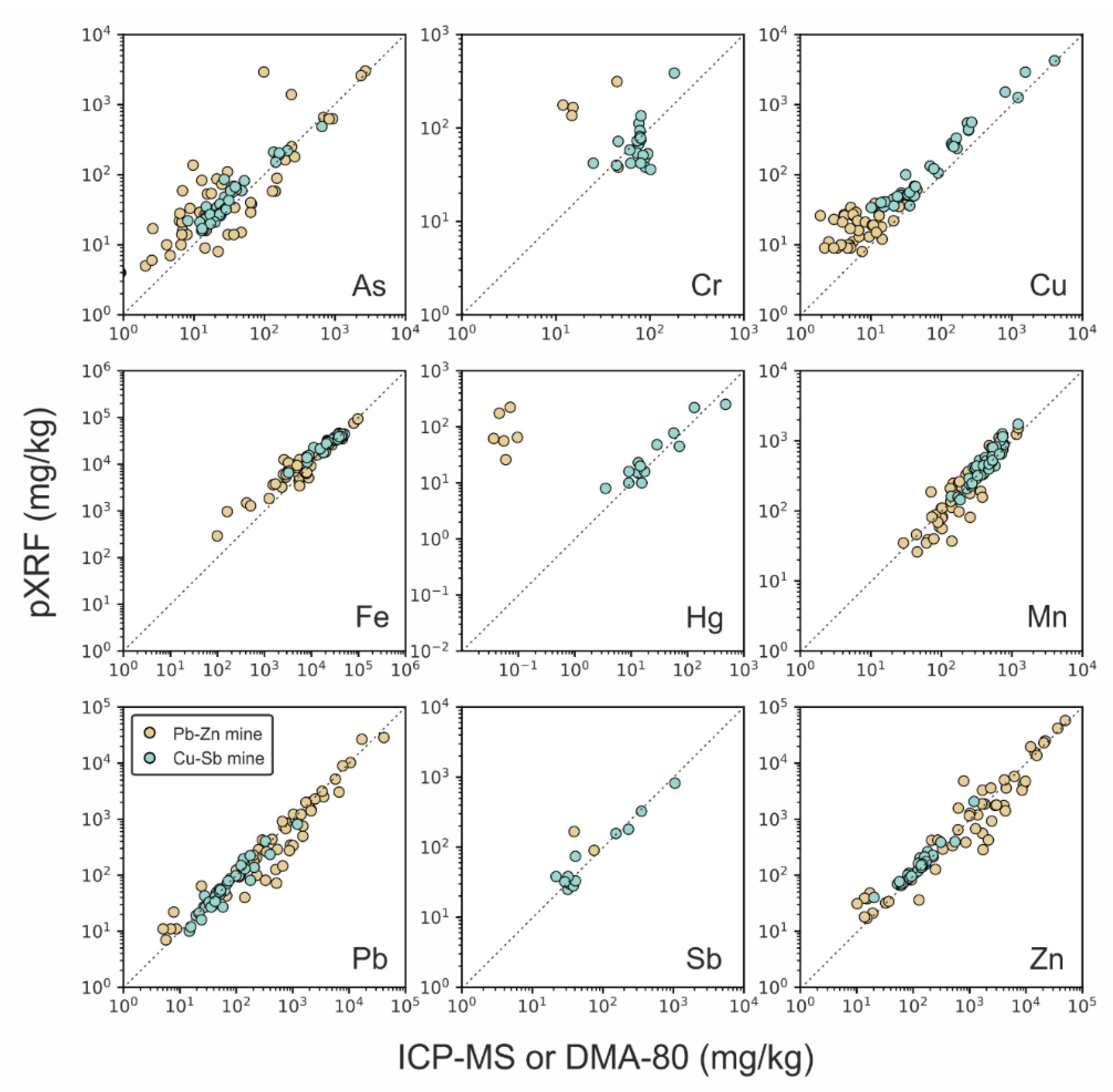
3.2. Validation Assessment: pXRF vs. ICP-MS and DMA-80
3.3. Low Concentration Effect
3.4. Effect of Sample Treatment
4. Conclusions
- -
- Lead (Pb), zinc (Zn), and manganese (Mn): the determination of these base metals by pXRF was often found to be successful (from quantitative to definitive quality levels), and no notable analytical issues related to interferences or concentration effects were observed;
- -
- Arsenic (As): the element met the “quantitative” quality level in both datasets. However, better comparability is found in sites where Pb is not dominant, and As concentrations are elevated enough due to As-K and Pb-L X-ray overlaps, which reduce the accuracy of As determination, so that the traditional laboratory analysis may be preferential. A concentration effect was observed leading to a decrease in comparability for As < 100 mg/kg;
- -
- Chromium (Cr): the metal showed moderate comparability between the two analytical approaches in the Cu-Sb matrix, characterised by relatively higher Cr concentrations and occurrence of false positives by using pXRF in the carbonate-hosted Pb-Zn samples that should be removed by the user via manual spectral interpretation. Overall, Cr never exceeded the “qualitative” level when determined via pXRF;
- -
- Copper (Cu): comparability of pXRF with ICP-MS results was strongly dependent on the element concentration and acceptable for Cu > 100 mg/kg, whereas below such values, Cu concentrations detected via pXRF were false;
- -
- Iron (Fe): similar to Cu, its detectability by pXRF was strongly dependent on the concentration. Comparability between the two analytical approaches was found to be optimal for values >10,000 mg/kg, which are common in many (but not all) natural samples;
- -
- Mercury (Hg) and antimony (Sb): these trace elements were found quantitatively determined by pXRF only when occurring with concentrations > 10–20 mg/kg. Conversely, when the real sample concentrations were below such values, as in the case of Hg, pXRF produced false-positive results, which should be manually removed by the user via spectral interpretation. Unfortunately, this result reveals a serious limitation in using pXRF, as the operator may be precluded from detecting low concentrations of Hg in soils that exceed those threshold levels specified by national regulations that define the use of uncontaminated soil. This is the case with the Italian legislation, for instance, where the highest threshold level, defined by major land use, i.e., for commercial and industrial purposes, is fixed at 5 mg kg−1, whereas for public, private, and residential use, it is 1 mg kg−1 (Italian Legislative Decree 152/06 [9]).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Crosera, M.; Baracchini, E.; Prenesti, E.; Giacomello, A.; Callegher, B.; Oliveri, P.; Adami, G. Elemental Characterization of Surface and Bulk of Copper-Based Coins from the Byzantine-Period by Means of Spectroscopic Techniques. Microchem. J. 2019, 147, 422–428. [Google Scholar] [CrossRef]
- Pavoni, E.; Crosera, M.; Petranich, E.; Faganeli, J.; Klun, K.; Oliveri, P.; Covelli, S.; Adami, G. Distribution, Mobility and Fate of Trace Elements in an Estuarine System Under Anthropogenic Pressure: The Case of the Karstic Timavo River (Northern Adriatic Sea, Italy). Estuaries Coasts 2021, 44, 1831–1847. [Google Scholar] [CrossRef]
- Pavoni, E.; Crosera, M.; Petranich, E.; Adami, G.; Faganeli, J.; Covelli, S. Partitioning and Mixing Behaviour of Trace Elements at the Isonzo/Soča River Mouth (Gulf of Trieste, Northern Adriatic Sea). Mar. Chem. 2020, 223, 103800. [Google Scholar] [CrossRef]
- Sibilia, M.; Stani, C.; Gigli, L.; Pollastri, S.; Migliori, A.; D’Amico, F.; Schmid, C.; Licen, S.; Crosera, M.; Adami, G.; et al. A Multidisciplinary Study Unveils the Nature of a Roman Ink of the I Century AD. Sci. Rep. 2021, 11, 7231. [Google Scholar] [CrossRef] [PubMed]
- Lemière, B. A Review of PXRF (Field Portable X-ray Fluorescence) Applications for Applied Geochemistry. J. Geochem. Explor. 2018, 188, 350–363. [Google Scholar] [CrossRef]
- Higueras, P.; Oyarzun, R.; Iraizoz, J.M.; Lorenzo, S.; Esbrí, J.M.; Martínez-Coronado, A. Low-Cost Geochemical Surveys for Environmental Studies in Developing Countries: Testing a Field Portable XRF Instrument under Quasi-Realistic Conditions. J. Geochem. Explor. 2012, 113, 3–12. [Google Scholar] [CrossRef]
- Kalnicky, D.J.; Singhvi, R. Field Portable XRF Analysis of Environmental Samples. J. Hazard. Mater. 2001, 83, 93–122. [Google Scholar] [CrossRef]
- Horta, A.; Malone, B.; Stockmann, U.; Minasny, B.; Bishop, T.F.A.; McBratney, A.B.; Pallasser, R.; Pozza, L. Potential of Integrated Field Spectroscopy and Spatial Analysis for Enhanced Assessment of Soil Contamination: A Prospective Review. Geoderma 2015, 241–242, 180–209. [Google Scholar] [CrossRef]
- Lgs, D. Decreto Legislativo n. 152 “Norme in Materia Ambientale”. 88 Gazzetta Ufficiale Repubblica Italiana. 152/06, 14 April 2006. [Google Scholar]
- Laiho, J.V.; Perämäki, P. Evaluation of Portable X-ray Fluorescence (PXRF) Sample Preparation Methods. Spec. Pap.-Geol. Surv. Finl. 2005, 38, 73. [Google Scholar]
- US EPA Environmental Technology Verification Report, Field Portable X-ray Fluorescence Analyzer; Metorex X-MET 920-P; United States Environmental Protection Agency: Washington, DC, USA, 1998; EPA/600/R-97/146.
- Pavoni, E.; Petranich, E.; Adami, G.; Baracchini, E.; Crosera, M.; Emili, A.; Lenaz, D.; Higueras, P.; Covelli, S. Bioaccumulation of Thallium and Other Trace Metals in Biscutella Laevigata Nearby a Decommissioned Zinc-Lead Mine (Northeastern Italian Alps). J. Environ. Manag. 2017, 186, 214–224. [Google Scholar] [CrossRef]
- Hall, G.E.M.; Bonham-Carter, G.F.; Buchar, A. Evaluation of Portable X-ray Fluorescence (PXRF) in Exploration and Mining: Phase 1, Control Reference Materials. Geochem. Explor. Environ. Anal. 2014, 14, 99–123. [Google Scholar] [CrossRef]
- Knight, R.D.; Kjarsgaard, B.A.; Russell, H.A.J. An Analytical Protocol for Determining the Elemental Chemistry of Quaternary Sediments Using a Portable X-ray Fluorescence Spectrometer. Appl. Geochem. 2021, 131, 105026. [Google Scholar] [CrossRef]
- Migaszewski, Z.; Gałuszka, A.; Dołęgowska, S. The Use of FPXRF in the Determinations of Selected Trace Elements in Historic Mining Soils in the Holy Cross Mts., South-Central Poland. Geol. Q. 2015, 59, 248–256. [Google Scholar] [CrossRef]
- Rouillon, M.; Taylor, M.P. Can Field Portable X-ray Fluorescence (PXRF) Produce High Quality Data for Application in Environmental Contamination Research? Environ. Pollut. Barking Essex 1987 2016, 214, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Rincheval, M.; Cohen, D.R.; Hemmings, F.A. Biogeochemical Mapping of Metal Contamination from Mine Tailings Using Field-Portable XRF. Sci. Total Environ. 2019, 662, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Spearman, S.; Bartrem, C.; Sharshenova, A.; Salymbekova, K.; Isirailov, M.; Gaynazarov, S.; Gilmanov, R.; von Lindern, I.; von Braun, M.; Möller, G. Comparison of X-ray Fluorescence (XRF) and Atomic Absorption Spectrometry (AAS) Results for an Environmental Assessment at a Mercury Site in Kyrgyzstan. Appl. Sci. 2022, 12, 1943. [Google Scholar] [CrossRef]
- Lemière, B.; Melleton, J.; Auger, P.; Derycke, V.; Gloaguen, E.; Bouat, L.; Mikšová, D.; Filzmoser, P.; Middleton, M. PXRF Measurements on Soil Samples for the Exploration of an Antimony Deposit: Example from the Vendean Antimony District (France). Minerals 2020, 10, 724. [Google Scholar] [CrossRef]
- Brigo, L.; Cerrato, P. Trace Element Distribution of Middle-Upper Triassic Carbonate-Hosted Lead-Zinc Mineralizations: The Example of the Raibl Deposit (Eastern Alps, Italy). In Sediment-Hosted Zn-Pb Ores; Fontboté, L., Boni, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 179–197. ISBN 978-3-662-03054-7. [Google Scholar]
- Barago, N.; Covelli, S.; Mauri, M.; Oberti di Valnera, S.; Forte, E. Prediction of Trace Metal Distribution in a Tailings Impoundment Using an Integrated Geophysical and Geochemical Approach (Raibl Mine, Pb-Zn Alpine District, Northern Italy). Int. J. Environ. Res. Public. Health 2021, 18, 1157. [Google Scholar] [CrossRef]
- Henjes-Kunst, E.; Raith, J.G.; Boyce, A.J. Micro-Scale Sulfur Isotope and Chemical Variations in Sphalerite from the Bleiberg Pb-Zn Deposit, Eastern Alps, Austria. Ore Geol. Rev. 2017, 90, 52–62. [Google Scholar] [CrossRef]
- Giorno, M.; Barale, L.; Bertok, C.; Frenzel, M.; Looser, N.; Guillong, M.; Bernasconi, S.M.; Martire, L. Sulfide-Associated Hydrothermal Dolomite and Calcite Reveal a Shallow Burial Depth for Alpine-Type Zn-(Pb) Deposits. Geology 2022, 50, 853–858. [Google Scholar] [CrossRef]
- Brigo, L.; Dulski, P.; Möller, P.; Schneider, H.-J.; Wolter, R. Strata-Bound Mineralizations in the Carnic Alps/Italy. In Mineral Deposits within the European Community; Boissonnas, J., Omenetto, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 485–498. ISBN 978-3-642-51860-7. [Google Scholar]
- Brigo, L.; Camana, G.; Rodeghiero, F.; Potenza, R. Carbonate-Hosted Siliceous Crust Type Mineralization of Carnic Alps (Italy-Austria). Ore Geol. Rev. 2001, 17, 199–214. [Google Scholar] [CrossRef]
- Brigo, L.; Kostelka, L.; Omenetto, P.; Schneider, H.-J.; Schroll, E.; Schulz, O.; Štrucl, I. Comparative Reflections on Four Alpine Pb-Zn Deposits. In Time- and Strata-Bound Ore Deposits; Klemm, D.D., Schneider, H.-J., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 273–293. ISBN 978-3-642-66808-1. [Google Scholar]
- Ross, P.-S.; Bourke, A.; Fresia, B. Improving Lithological Discrimination in Exploration Drill-Cores Using Portable X-ray Fluorescence Measurements: (1) Testing Three Olympus Innov-X Analysers on Unprepared Cores. Geochem. Explor. Environ. Anal. 2014, 14, 171–185. [Google Scholar] [CrossRef]
- US EPA Method 3052; Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; United States Environmental Protection Agency: Washington, DC, USA, 1996.
- US EPA Method 7473 (SW-846); Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry; United States Environmental Protection Agency: Washington, DC, USA, 1998.
- Al-Musawi, M.; Kaczmarek, S. A New Carbonate-Specific Quantification Procedure for Determining Elemental Concentrations from Portable Energy-Dispersive X-ray Fluorescence (PXRF) Data. Appl. Geochem. 2020, 113, 104491. [Google Scholar] [CrossRef]
- US EPA Method 6200; Field Portable X-ray Fluorescence Spectrometry for the Determination of Elemental Concentrations in Soils and Sediment; United States Environmental Protection Agency: Washington, DC, USA, 2007.
- Durance, P.; Jowitt, S.M.; Bush, K. An Assessment of Portable X-ray Fluorescence Spectroscopy in Mineral Exploration, Kurnalpi Terrane, Eastern Goldfields Superterrane, Western Australia. Appl. Earth Sci. 2014, 123, 150–163. [Google Scholar] [CrossRef]
- Sarala, P. Comparison of Different Portable XRF Methods for Determining till Geochemistry. Geochem. Explor. Environ. Anal. 2016, 16, 181–192. [Google Scholar] [CrossRef]
- Casari, L. Tetrahedrite-Tennantite Series in the Carnic Chain (Eastern Alps, Italy). N. Jb. Miner. Mh. 1996, 5, 193–200. [Google Scholar]

| Data Quality Level | Statistical Parameter |
|---|---|
| Definitive | r2 = 0.85 to 1.0. The repeatability (RSD%) must be less than or equal to 10%, and inferential statistics indicate the two data sets are statistically similar. |
| Quantitative screening | r2 = 0.70 to 1.0. The RSD% must be less than 20%, but the inferential statistics indicate that the data sets are statistically different. |
| Qualitative screening | r2 = less than 0.70. The RSD% is greater than 20%. The data must have less than a 10 percent false-negative rate. |
| Element | CRM Value (mg/kg) | Mean Accuracy—Recovery (%) | Mean Repeatability—RSD (%) | |||
|---|---|---|---|---|---|---|
| PACS-3 | MESS-4 | PACS-3 | MESS-4 | PACS-3 | MESS-4 | |
| As | 30.3 | 21.7 | 164% | 121% | 3.9% | 6.1% |
| Cr | 90.6 | 94.3 | 120% | 106% | 7.4% | 4.1% |
| Cu | 356 | 32.9 | 104% | 160% | 3.5% | 2.4% |
| Fe | 41060 | 37900 | 107% | 102% | 0.6% | 1.4% |
| Hg | 2.98 | 0.09 | n.d. | n.d. | n.d. | n.d. |
| Mn | 432 | 298 | 107% | 103% | 3.7% | 3.4% |
| Pb | 188 | 21.5 | 93% | 93% | 3.4% | 13.0% |
| Sb | 14.7 | 1.07 | n.d. | n.d. | n.d. | n.d. |
| Zn | 376 | 147 | 109% | 102% | 2.0% | 3.3% |
| Matrix Type | Element | Data Quality a | n | Concentration Range ICP-MS—DMA | Concentration Range pXRF | RSD% | r2 | t-Test | Slope z-Test | Y-Int z-Test |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/kg) | (mg/kg) | p-Value | ||||||||
| Cu-Sb | As | Quantitative | 37 | 8.20–654 | 13–1057 | 7% | 0.963 * | 0.000 | 0.527 | 0.185 |
| Cr | Qualitative | 25 | 6.91–182 | 28–156 | 12% | 0.523 * | 0.021 | 0.721 | 0.720 | |
| Cu | Quantitative | 37 | 10.1–4019 | 17–7739 | 8% | 0.979 * | 0.000 | 0.641 | 0.201 | |
| Fe | Quantitative | 37 | 3248–49,918 | 3405–44,504 | 1% | 0.970 * | 0.000 | 0.731 | 0.410 | |
| Hg | Quantitative | 11 | 0.04–473 | 11–720 | 14% | 0.959 * | 0.127 | 0.802 | 0.637 | |
| Mn | Quantitative | 37 | 138–1227 | 113–1452 | 3% | 0.929 * | 0.004 | 0.605 | 0.458 | |
| Pb | Definitive | 37 | 14.7–1216 | 12–1156 | 6% | 0.988 * | 0.760 | 0.770 | 0.524 | |
| Sb | Definitive | 15 | 1.64–1049 | 20–1627 | 9% | 0.970 * | 0.576 | 0.704 | 0.589 | |
| Zn | Quantitative | 37 | 20.3–1204 | 30–1462 | 4% | 0.969 * | 0.007 | 0.849 | 0.709 | |
| Pb-Zn | As | Quantitative | 52 | 0.91–2693 | 5–2886 | 10% | 0.856 * | 0.000 | 0.383 | 0.051 |
| Cr | Qualitative | 5 | 2.04–50.0 | 84–218 | 7% | 0.100 | 0.007 | 0.035 | 0.000 | |
| Cu | Qualitative | 34 | 0.69–45.0 | 8–50 | 16% | 0.358 * | 0.000 | 0.024 | 0.001 | |
| Fe | Quantitative | 53 | 99–96,674 | 263–92,931 | 1% | 0.952 * | 0.000 | 0.201 | 0.003 | |
| Hg | Qualitative | 7 | 0.03–0.12 | 33–280 | 11% | 0.050 | 0.000 | 0.537 | 0.017 | |
| Mn | Quantitative | 57 | 16.6–1239 | 30–1449 | 7% | 0.895 * | 0.003 | 0.917 | 0.747 | |
| Pb | Definitive | 58 | 5.05–41,436 | 6–36,853 | 5% | 0.993 * | 0.370 | 0.789 | 0.718 | |
| Sb | Qualitative | 2 | 0.05–75.0 | 21–84 | - | - | - | - | - | |
| Zn | Definitive | 58 | 10.2–49,752 | 15–56,400 | 4% | 0.992 * | 0.053 | 0.887 | 0.711 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barago, N.; Pavoni, E.; Floreani, F.; Crosera, M.; Adami, G.; Lenaz, D.; Larese Filon, F.; Covelli, S. Portable X-ray Fluorescence (pXRF) as a Tool for Environmental Characterisation and Management of Mining Wastes: Benefits and Limits. Appl. Sci. 2022, 12, 12189. https://doi.org/10.3390/app122312189
Barago N, Pavoni E, Floreani F, Crosera M, Adami G, Lenaz D, Larese Filon F, Covelli S. Portable X-ray Fluorescence (pXRF) as a Tool for Environmental Characterisation and Management of Mining Wastes: Benefits and Limits. Applied Sciences. 2022; 12(23):12189. https://doi.org/10.3390/app122312189
Chicago/Turabian StyleBarago, Nicolò, Elena Pavoni, Federico Floreani, Matteo Crosera, Gianpiero Adami, Davide Lenaz, Francesca Larese Filon, and Stefano Covelli. 2022. "Portable X-ray Fluorescence (pXRF) as a Tool for Environmental Characterisation and Management of Mining Wastes: Benefits and Limits" Applied Sciences 12, no. 23: 12189. https://doi.org/10.3390/app122312189
APA StyleBarago, N., Pavoni, E., Floreani, F., Crosera, M., Adami, G., Lenaz, D., Larese Filon, F., & Covelli, S. (2022). Portable X-ray Fluorescence (pXRF) as a Tool for Environmental Characterisation and Management of Mining Wastes: Benefits and Limits. Applied Sciences, 12(23), 12189. https://doi.org/10.3390/app122312189








