Molecular and Phytochemical Characterizations of Cichorium intybus L. in Diverse Ecogeographical Regions of Kashmir Himalaya
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey and Sample Collection
2.2. Phytochemical Analysis
2.2.1. Preparation of Extract
2.2.2. GC-MS Analysis
2.2.3. Identification of the Compounds
2.3. Collection and Analysis of Soil Samples
2.4. Genetic Diversity Analysis
2.4.1. DNA Extraction
2.4.2. ISSR Analysis and PCR Amplification
2.5. Statistical Analysis
3. Results
3.1. Distribution
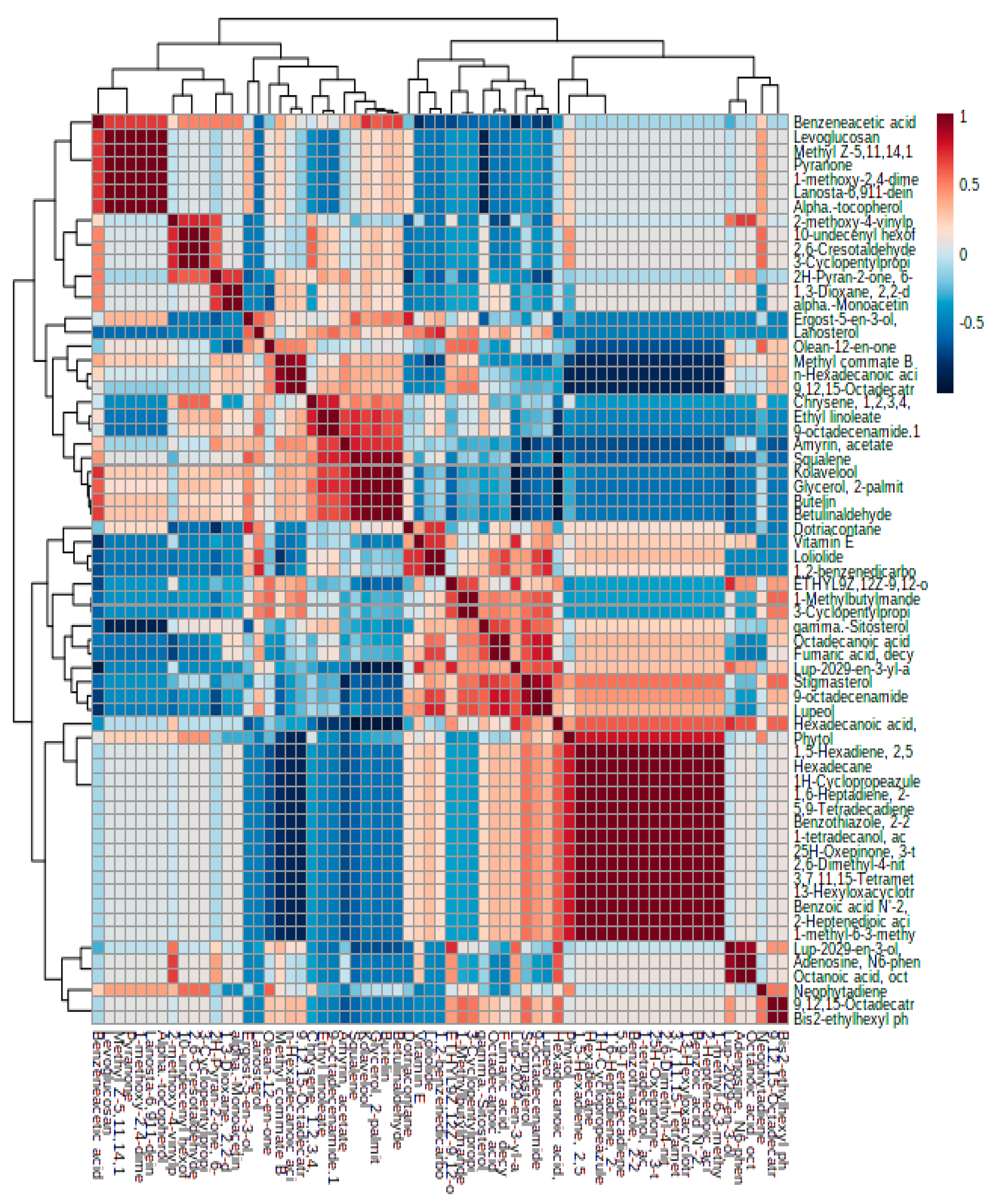
3.2. Identification of the Altitude-Responsive Metabolites in Chicory Leaves
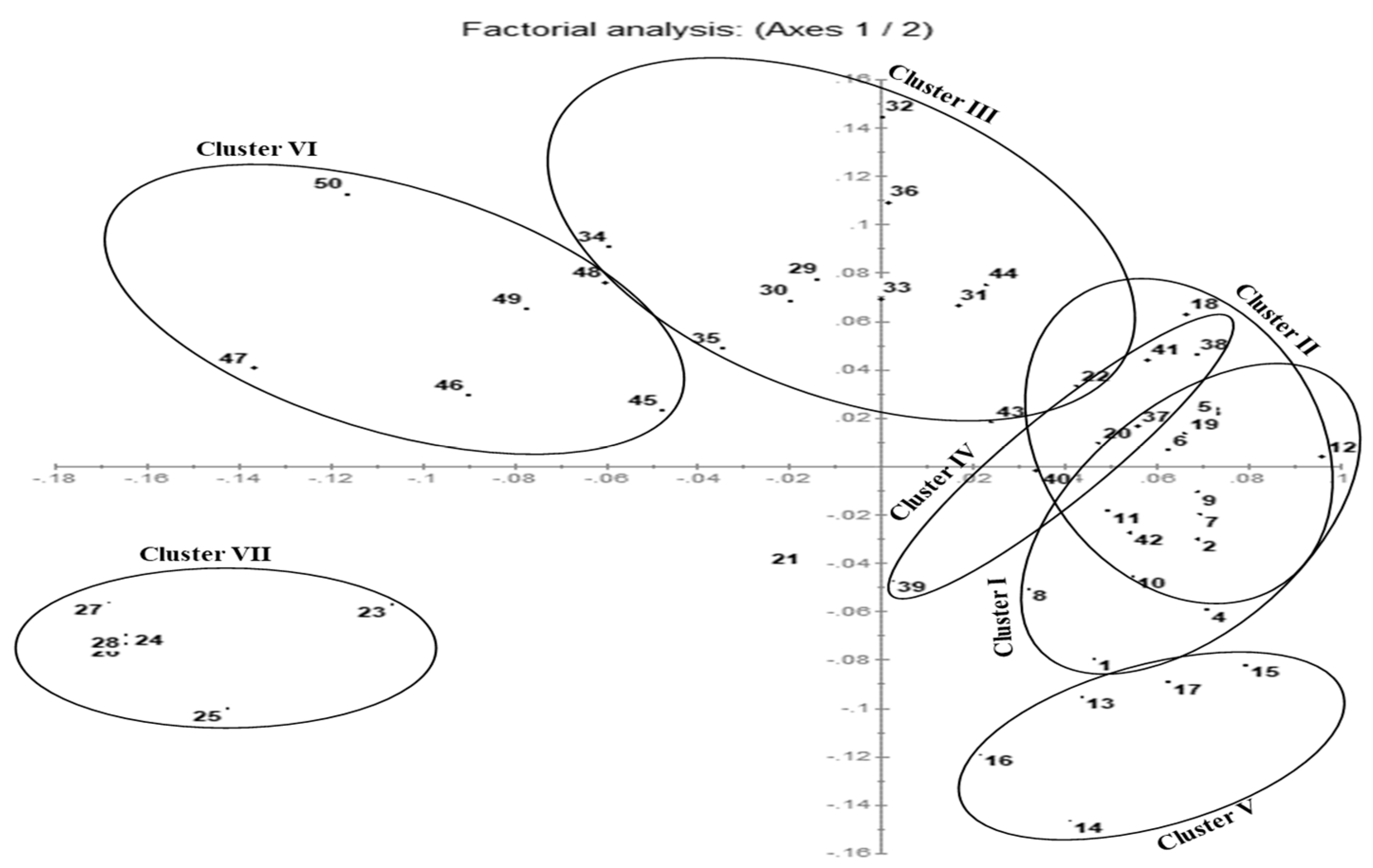
3.3. Multivariate Metabolomics Data Analysis
3.4. Soil Analysis
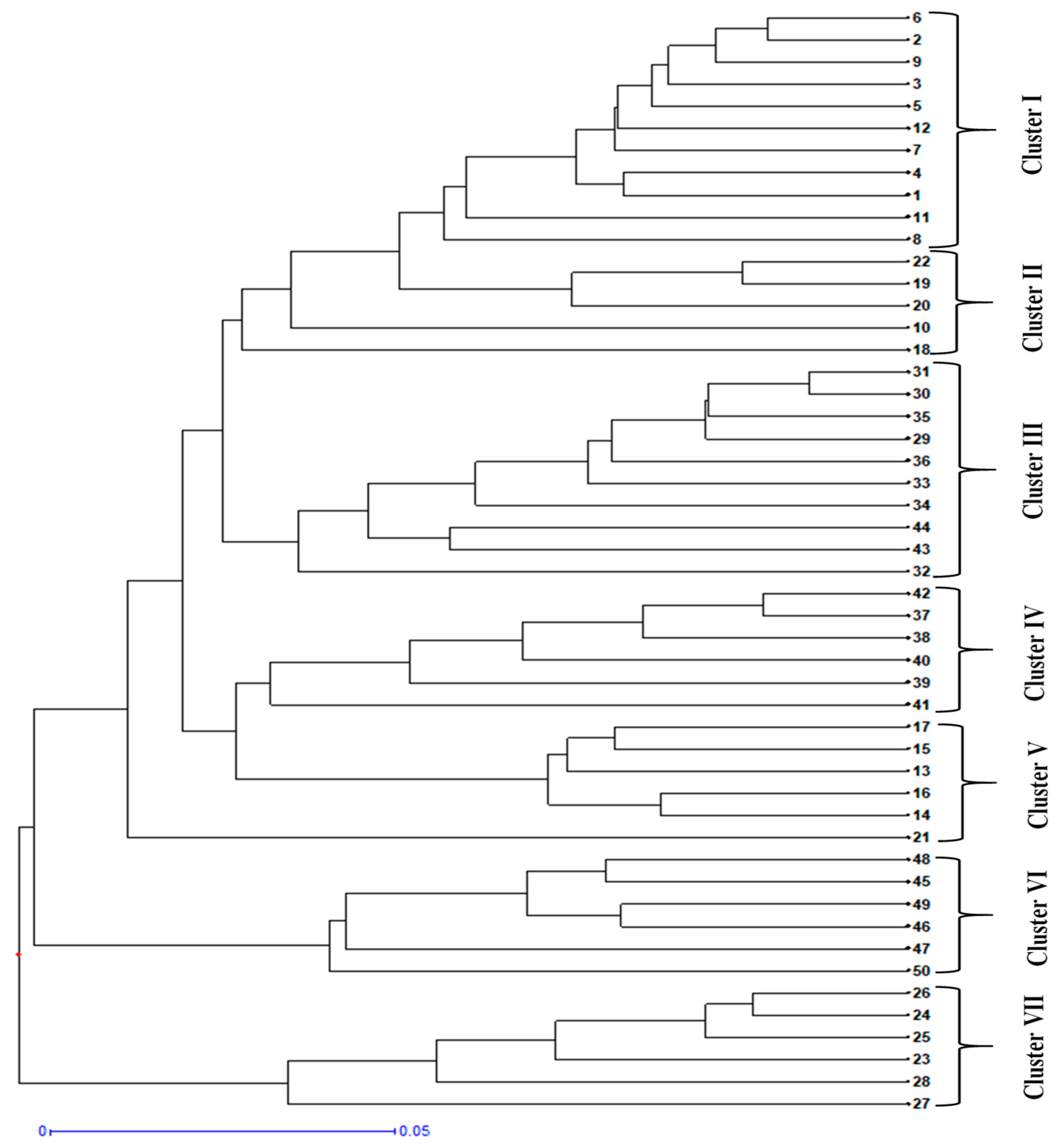
3.5. Genetic Diversity Analysis
4. Discussion
4.1. Distribution of Chicory in the Kashmir Region
4.2. Altitude Variation in Secondary Metabolite Profiling
4.3. Soil Analysis
4.4. Genetic Diversity of Chicory in the Kashmir Region
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bais, H.P.; Ravishankar, G.A. Cichorium intybus L.—Cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J. Sci. Food Agric. 2001, 81, 467–484. [Google Scholar] [CrossRef]
- Hauser, T.P.; Jørgensen, R.B.; Toneatto, F. Reduced sexual compatibility between cultivated and wild chicory and their F 1 hybrids. Genet. Resour. Crop Evol. 2012, 59, 783–791. [Google Scholar] [CrossRef]
- Nandagopal, S.; Kumari, B.D.R. Phytochemical and Antibacterial Studies of Chicory (Cichorium intybus L.)—A Multipurpose Medicinal Plant. Adv. Biol. Res. 2007, 1, 17–21. [Google Scholar]
- Ivarsson, E.; Frankow-Lindberg, B.E.; Andersson, H.K.; Lindberg, J.E. Growth performance, digestibility and faecal coliform bacteria in weaned piglets fed a cereal-based diet including either chicory (Cichorium intybus L.) or ribwort (Plantago lanceolata L.) forage. Animal 2011, 5, 558–564. [Google Scholar] [CrossRef]
- Lee, K.T.; Kim, J.I.; Park, H.J.; Yoo, K.O.; Han, Y.N.; Miyamoto, K.I. Differentiation-inducing effect of magnolialide, a 1β-hydroxyeudesmanolide isolated from Cichorium intybus, on human leukemia cells. Biol. Pharm. Bull. 2000, 23, 1005–1007. [Google Scholar] [CrossRef]
- Mehmood, N.; Zubair, M.; Rizwan, K.; Rasool, N.; Shahid, M.; Ahmad, V.U. Antioxidant, antimicrobial and phytochemical analysis of Cichorium intybus seeds extract and various organic fractions. Iran. J. Pharm. Res. 2012, 11, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Schmidt, B.M.; Ilic, N.; Poulev, A.; Dey, M.; Kurmukov, A.G.; Raskin, I. Anti-inflammatory effects of a sesquiterpene lactone extract from chicory (Cichorium intybus L.) Roots. Nat. Prod. Commun. 2007, 2, 717–722. [Google Scholar] [CrossRef]
- Karim, A.; Nouman Sohail, M.; Munir, S.; Sattar, S.; Abbas, R.J.; Abdel Gadir, E.H.; Gadir, W.S.A.; Adam, S.E.I.; Abdel Gadir, E.H.; Gadir, W.S.A.; et al. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. Int. J. Pharmacol. 2010, 7, 278–282. [Google Scholar]
- Miller, M.C.; Duckett, S.K.; Andrae, J.G. The effect of forage species on performance and gastrointestinal nematode infection in lambs. Small Rumin. Res. 2011, 95, 188–192. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol. 2006, 107, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N. Alloxan diabetes-induced oxidative stress and impairment of oxidative defense system in rat brain: Neuroprotective effects of cichorium intybus. Int. J. Diabetes Metab. 2009, 17, 105–109. [Google Scholar] [CrossRef]
- Das, S.; Vasudeva, N.; Sharma, S. Cichorium intybus: A concise report on its ethnomedicinal, botanical, and phytopharmacological aspects. Drug Dev. Ther. 2016, 7, 1. [Google Scholar] [CrossRef]
- Gürbüz, I.; Üstün, O.; Yeşilada, E.; Sezik, E.; Akyürek, N. In vivo gastroprotective effects of five Turkish folk remedies against ethanol-induced lesions. J. Ethnopharmacol. 2002, 83, 241–244. [Google Scholar] [CrossRef]
- Gilani, A.H.; Janbaz, K.H. Evaluation of the liver protective potential of Cichorium intybus seed extract on Acetaminophen and CCl4-induced damage. Phytomedicine 1994, 1, 193–197. [Google Scholar] [CrossRef]
- Kim, J.H.; Mun, Y.J.; Woo, W.H.; Jeon, K.S.; An, N.H.; Park, J.S. Effects of the ethanol extract of Cichorium intybus on the immunotoxicity by ethanol in mice. Int. Immunopharmacol. 2002, 2, 733–744. [Google Scholar] [CrossRef]
- Behnam-Rassouli, M.; Aliakbarpour, A.; Hosseinzadeh, H.; Behnam-Rassouli, F.; Chamsaz, M. Investigating the effect of aqueous extract of Chicorium intybus L. leaves on offspring sex ratio in rat. Phyther. Res. 2010, 24, 1417–1421. [Google Scholar] [CrossRef]
- Süntar, I.; Küpeli Akkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D.; Baykal, T. Comparative evaluation of traditional prescriptions from Cichorium intybus L. for wound healing: Stepwise isolation of an active component by in vivo bioassay and its mode of activity. J. Ethnopharmacol. 2012, 143, 299–309. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Medicinal importance of Cichorium intybus—A review. IOSR J. Pharm. 2016, 6, 41–56. [Google Scholar]
- Douglas, J.A.; Poll, J.T.K. A preliminary assessment of chicory (Cichorium intybus) as an energy crop. N. Z. J. Exp. Agric. 1986, 14, 223–225. [Google Scholar] [CrossRef]
- Madrigal, L.; Sangronis, E. Inulin and derivates as key ingredients in functional foods. Arch. Latinoam. Nutr. 2007, 57, 387–396. [Google Scholar]
- Slavin, J.; Feirtag, J. Chicory inulin does not increase stool weight or speed up intestinal transit time in healthy male subjects. Food Funct. 2011, 2, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Sinkovic, L.; Hribar, J.; Vidrih, R. Influence of Cultivar and Storage of Chicory (Cichorium intybus L.) Plants on Polyphenol Composition and Antioxidative Potential. Czech J. Food Sci. 2014, 32, 10–15. [Google Scholar] [CrossRef]
- Kim, M.; Shin, H.K. The water-soluble extract of chicory reduces glucose uptake from the perfused jejunum in rats. J. Nutr. 1996, 126, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.M.; van Amerongen, A. Sesquiterpene lactones in chicory (Cichorium intybus L.): Distribution in chicons and effect of storage. Food Res. Int. 1996, 29, 439–444. [Google Scholar] [CrossRef]
- Mares, D.; Romagnoli, C.; Tosi, B.; Andreotti, E.; Chillemi, G.; Poli, F. Chicory extracts from Cichorium intybus L. as potential antifungals. Mycopathologia 2005, 160, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Monde, K.; Oya, T.; Shirata, A.; Takasugi, M. A guaianolide phytoalexin, cichoralexin, from Cichorium intybus. Phytochemistry 1990, 29, 3449–3451. [Google Scholar] [CrossRef]
- Şanli, A.; Karadoğan, T. Geographical Impact on Essential Oil Composition of Endemic Kundmannia Anatolica Hub.-Mor. (Apiaceae). African J. Tradit. Complement. Altern. Med. AJTCAM 2017, 14, 131–137. [Google Scholar] [CrossRef]
- Mishra, R.; Gupta, A.K.; Kumar, A.; Lal, R.K.; Saikia, D.; Chanotiya, C.S. Genetic diversity, essential oil composition, and in vitro antioxidant and antimicrobial activity of Curcuma longa L. germplasm collections. J. Appl. Res. Med. Aromat. Plants 2018, 10, 75–84. [Google Scholar] [CrossRef]
- Mahdavi, M.; Vahid, B.R. The effects of ecologic and habitational factors on the essence quality of Stachys lavandulifolia Vahl. in north Khorassan province. Int. J. Farming Allied Sci. 2015, 4, 448–456. [Google Scholar]
- Zhang, C.; Qi, M.; Shao, Q.; Zhou, S.; Fu, R. Analysis of the volatile compounds in Ligusticum chuanxiong Hort. using HS-SPME-GC-MS. J. Pharm. Biomed. Anal. 2007, 44, 464–470. [Google Scholar] [CrossRef]
- AA, T. Influence of ecological factors on the chemical composition of the essential oil of Stachys lavandulifolia (Lamiaceae). Calodema 2012, 228, 1–4. [Google Scholar]
- Bertoni, B.W.; Telles, M.P.d.C.; Malosso, M.G.; Torres, S.C.Z.; Pereira, J.O.; Lourenço, M.V.; França, S.d.C.; Pereira, A.M.S. Genetic diversity in natural populations of Jacaranda decurrens Cham. determined using RAPD and AFLP markers. Genet. Mol. Biol. 2010, 33, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.L.; Rajewski, J.F.; Baenziger, P.S.; Gill, K.S.; Eskridge, K.M.; Dweikat, I. Assessment of genetic diversity and relationship among a collection of US sweet sorghum germplasm by SSR markers. Mol. Breed. 2008, 21, 497–509. [Google Scholar] [CrossRef]
- Kiers, A.M.; Mes, T.H.; van der Meijden, R.; Bachmann, K. A search for diagnostic AFLP markers in Cichorium species with emphasis on endive and chicory cultivar groups. Genome 2000, 43, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.; Morgante, M.; Andre, C.; Hanafey, M.; Vogel, J.; Tingey, S.; Rafalski, A. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol. Breed. 1996, 2, 225–238. [Google Scholar] [CrossRef]
- Dávila, J.A.; Loarce, Y.; Ramsay, L.; Waugh, R.; Ferrer, E. Comparison of RAMP and SSR markers for the study of wild barley genetic diversity. Hereditas 1999, 131, 5–13. [Google Scholar] [CrossRef]
- Qian, W.; Ge, S.; Hong, D.Y. Genetic variation within and among populations of a wild rice Oryza granulata from China detected by RAPD and ISSR markers. Theor. Appl. Genet. 2001, 102, 440–449. [Google Scholar] [CrossRef]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef]
- Zoratti, L.; Palmieri, L.; Jaakola, L.; Häggman, H. Genetic diversity and population structure of an important wild berry crop. AoB Plants 2015, 7, plv117. [Google Scholar] [CrossRef]
- Dhar, P.; Tayade, A.B.; Kumar, J.; Chaurasia, O.P.; Srivastava, R.B.; Singh, S.B. Nutritional profile of phytococktail from trans-Himalayan plants. PLoS ONE 2013, 8, e83008. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1988, 12, 13–15. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 2, W486–W494. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The mathematical theory of communication, University of Illinois Press. Urbana 1949, 27, 379–423. [Google Scholar]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenALEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Lewontin, R.C. The apportionment of human diversity. Concept Race Nat. Soc. Sci. 2014, 7–15. [Google Scholar] [CrossRef]
- Slatkin, M. A Measure of Population Subdivision Based on Microsatellite Allele Frequencies. Genetics 1995, 139, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Perrier, X.; Jacquemoud-collet, J. DARwin software. Nat. Methods 2006, 5, 1005–1010. [Google Scholar]
- Singh, A.K.; Smartt, J.; Simpson, C.E.; Raina, S.N. Genetic variation vis-a-vis molecular polymorphism in groundnut, Arachis hypogaea L. Genet. Resour. Crop Evol. 1998, 45, 119–126. [Google Scholar] [CrossRef]
- Dar, F.A.; Verma, S.; Rehman, R.U. Genetic Diversity Assessment of Phaseolus vulgaris L. in Two Himalayan Districts of India. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2018, 88, 165–173. [Google Scholar] [CrossRef]
- Abbott RJ, B.A. Altitudinal gradients, plant hybrid zones and evolutionary novelty. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 1648. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Romero, C.; Guzmán-Reyna, R.R.; Rehfeldt, G.E. Altitudinal genetic variation among Pinus oocarpa populations in Michoacán, Mexico. Implications for seed zoning, conservation, tree breeding and global warming. For. Ecol. Manag. 2006, 229, 340–350. [Google Scholar] [CrossRef]
- Van Zonneveld, M.; Jarvis, A.; Dvorak, W.; Lema, G.; Leibing, C. Climate change impact predictions on Pinus patula and Pinus tecunumanii populations in Mexico and Central America. For. Ecol. Manag. 2009, 257, 1566–1576. [Google Scholar] [CrossRef]
- Ohsawa, T.; Ide, Y. Global patterns of genetic variation in plant species along vertical and horizontal gradients on mountains. Glob. Ecol. Biogeogr. 2008, 17, 152–163. [Google Scholar] [CrossRef]
- Frei, E.R.; Ghazoul, J.; Matter, P.; Heggli, M.; Pluess, A.R. Plant population differentiation and climate change: Responses of grassland species along an elevational gradient. Glob. Chang. Biol. 2014, 20, 441–455. [Google Scholar] [CrossRef]
- Knoop, W.T.; Walker, B.H. Interactions of Woody and Herbaceous Vegetation in a Southern African Savanna. J. Ecol. 1985, 73, 235. [Google Scholar] [CrossRef]
- Zidorn, C.; Schubert, B.; Stuppner, H. Altitudinal differences in the contents of phenolics in flowering heads of three members of the tribe Lactuceae (Asteraceae) occurring as introduced species in New Zealand. Biochem. Syst. Ecol. 2005, 33, 855–872. [Google Scholar] [CrossRef]
- Bahrami, Y.; Franco, C.M.M.; Benkendorff, K. Acetylated triterpene glycosides and their biological activity from holothuroidea reported in the past six decades. Mar. Drugs 2016, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Ododo, M.M.; Choudhury, M.K.; Dekebo, A.H. Structure elucidation of β-sitosterol with antibacterial activity from the root bark of Malva parviflora. Springerplus 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.A.; Sáenz, M.T.; García, M.D.; Fernández, M.A. Study of the topical anti-inflammatory activity of Achillea ageratum on chronic and acute inflammation models. Z. Naturforsch.-Sect. C J. Biosci. 1999, 54, 937–941. [Google Scholar] [CrossRef]
- Vohra, A.; Kaur, H.P. Chemical investigation of medicinal plant Ajuga bractaeosa. J. Nat. Prod. Plant Resour. 2011, 1, 37–45. [Google Scholar]
- Alakurtti, S.; Mäkelä, T.; Koskimies, S.Y.-K. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Djachuk, G.I.; Sergeev, D.V.; Pozharitskaya, O.N.; Esaulenko, E.V.; Kosman, V.M.; Makarov, V.G. Birch bark extract as therapy for chronic hepatitis C—A pilot study. Phytomedicine 2011, 18, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Chue, K.T.; Chang, M.S.; Ten, L.N. Synthesis and antibacterial activity of betulin esters. Chem. Nat. Compd. 2011, 47, 583–586. [Google Scholar] [CrossRef]
- Haque, S.; Nawrot, D.A.; Alakurtti, S.; Ghemtio, L.; Yli-Kauhaluoma, J.; Tammela, P. Screening and characterisation of antimicrobial properties of semisynthetic betulin derivatives. PLoS ONE 2014, 9, e102696. [Google Scholar] [CrossRef]
- Sousa, M.C.; Varandas, R.; Santos, R.C.; Santos-Rosa, M.; Alves, V.; Salvador, J.A.R. Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: Synergistic effects with miltefosine. PLoS ONE 2014, 9, e89939. [Google Scholar] [CrossRef]
- Tang, J.; Jones, S.A.; Jeffery, J.L.; Miranda, S.R.; Galardi, C.M.; Irlbeck, D.M.; Brown, K.W.; McDanal, C.B.; Han, N.; Gao, D.; et al. Synthesis and Biological Evaluation of Macrocyclized Betulin Derivatives as a Novel Class of Anti-HIV-1 Maturation Inhibitors. Open Med. Chem. J. 2014, 8, 23–27. [Google Scholar] [CrossRef][Green Version]
- Chawengrum, P.; Boonsombat, J.; Kittakoop, P.; Mahidol, C.; Ruchirawat, S.; Thongnest, S. Cytotoxic and antimicrobial labdane and clerodane diterpenoids from Kaempferia elegans and Kaempferia pulchra. Phytochem. Lett. 2018, 24, 140–144. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Ložienė, K.; Taraškevičius, R.; Butkienė, R. Variation of essential oil composition of Thymus pulegioides in relation to soil chemistry. Ind. Crops Prod. 2017, 95, 422–433. [Google Scholar] [CrossRef]
- Weiss, U.; Edwards, J.M. The Biosynthesis of Aromatic Compounds. Biochem. Educ. 1980, 9, 323–343. [Google Scholar] [CrossRef]
- Azevedo, A.L.S.; Costa, P.P.; Machado, M.A.; de Paula, C.M.P.; Sobrinho, F.S. High degree of genetic diversity among genotypes of the forage grass Brachiaria ruziziensis (Poaceae) detected with ISSR markers. Genet. Mol. Res. 2011, 10, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Morgante, M.; Olivieri, A.M. PCR-amplified microsatellites as markers in plant genetics. Plant J. 1993, 3, 175–182. [Google Scholar] [CrossRef]
- Liang, X.Y.; Zhang, X.Q.; Bai, S.Q.; Huang, L.K.; Luo, X.M.; Ji, Y.; Jiang, L.F. Genetic diversity and relationship of chicory (Cichorium intybus L.) using sequence-related amplified polymorphism markers. Genet. Mol. Res. 2014, 13, 7736–7746. [Google Scholar] [CrossRef]
- Alansi, S.; Tarroum, M.; Al-Qurainy, F.; Khan, S.; Nadeem, M. Use of ISSR markers to assess the genetic diversity in wild medicinal Ziziphus spina-christi (L.) Willd. collected from different regions of Saudi Arabia. Biotechnol. Biotechnol. Equip. 2016, 30, 942–947. [Google Scholar] [CrossRef]
- Oliveira, L.A.R.; Machado, C.A.; Cardoso, M.N.; Oliveira, A.C.A.; Amaral, A.L.; Rabbani, A.R.C.; Silva, A.V.C.; Ledo, A.S. Genetic diversity of Saccharum complex using ISSR markers. Genet. Mol. Res. 2017, 16, gmr16039788. [Google Scholar] [CrossRef][Green Version]
- Yuan, C.Y.; Wang, P.; Chen, P.P.; Xiao, W.J.; Zhang, C.; Hu, S.; Zhou, P.; Chang, H.P.; He, Z.; Hu, R.; et al. Genetic diversity revealed by morphological traits and ISSR markers in 48 Okras (Abelmoschus escullentus L.). Physiol. Mol. Biol. Plants 2015, 21, 359–364. [Google Scholar] [CrossRef]
- Hamouda, M. Molecular analysis of genetic diversity in population of Silybum marianum (L.) Gaertn in Egypt. J. Genet. Eng. Biotechnol. 2019, 17, 12. [Google Scholar] [CrossRef]
- McDermott, J.M.; McDonald, B.A. Gene flow in plant pathosystems. Annu. Rev. Phytopathol. 1993, 31, 353–373. [Google Scholar] [CrossRef]
- Li, K.; Chen, L.; Feng, Y.; Yao, J.; Li, B.; Xu, M.; Li, H. High genetic diversity but limited gene flow among remnant and fragmented natural populations of Liriodendron chinense Sarg. Biochem. Syst. Ecol. 2014, 54, 230–236. [Google Scholar] [CrossRef]
- Chung, M.Y.; Chung, M.G. Large effective population sizes and high levels of gene flow between subpopulations of Lilium cernuum (Liliaceae). Biochem. Syst. Ecol. 2014, 54, 354–361. [Google Scholar] [CrossRef]
- Jena, S.N.; Verma, S.; Nair, K.N.; Srivastava, A.K.; Misra, S.; Rana, T.S. Genetic diversity and population structure of the mangrove lime (Merope angulata) in India revealed by AFLP and ISSR markers. Aquat. Bot. 2015, 120, 260–267. [Google Scholar] [CrossRef]
- Pereira, D.d.A.; Corrêa, R.X.; Oliveira, A.C. de Molecular genetic diversity and differentiation of populations of “somnus” passion fruit trees (Passiflora setacea DC): Implications for conservation and pre-breeding. Biochem. Syst. Ecol. 2015, 59, 12–21. [Google Scholar] [CrossRef]
- Santosh, K.; Kumar, S.; Baranwal, D.K.; Chatterjee, A.; Solankey, S.S. Genetic Diversity Based on Cluster and Principal Component Analyses for Yield and Quality Attributes in Ginger (Zingiber officinale Roscoe). Int. J. Plant Breed. Genet. 2013, 7, 159–168. [Google Scholar] [CrossRef]
- De Magalhães Bertini, C.H.C.; Schuster, I.; Sediyama, T.; de Barros, E.G.; Moreira, M.A. Characterization and genetic diversity analysis of cotton cultivars using microsatellites. Genet. Mol. Biol. 2006, 29, 321–329. [Google Scholar] [CrossRef]
- Reddy, M.P.; Sarla, N.; Siddiq, E.A. Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica 2002, 128, 9–17. [Google Scholar] [CrossRef]
- Sethy, N.K.; Shokeen, B.; Edwards, K.J.; Bhatia, S. Development of microsatellite markers and analysis of intraspecific genetic variability in chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2006, 112, 1416–1428. [Google Scholar] [CrossRef]
- He, Q.; Li, X.W.; Liang, G.L.; Ji, K.; Guo, Q.G.; Yuan, W.M.; Zhou, G.Z.; Chen, K.S.; van de Weg, W.E.; Gao, Z.S. Genetic Diversity and Identity of Chinese Loquat Cultivars/Accessions (Eriobotrya japonica) Using Apple SSR Markers. Plant Mol. Biol. Rep. 2011, 29, 197–208. [Google Scholar] [CrossRef]
- Baraket, G.; Chatti, K.; Saddoud, O.; Abdelkarim, A.B.; Mars, M.; Trifi, M.; Hannachi, A.S. Comparative Assessment of SSR and AFLP Markers for Evaluation of Genetic Diversity and Conservation of Fig, Ficus carica L.; Genetic Resources in Tunisia. Plant Mol. Biol. Rep. 2011, 29, 171–184. [Google Scholar] [CrossRef]
- Sharma, S.S.; Negi, M.S.; Sinha, P.; Kumar, K.; Tripathi, S.B. Assessment of Genetic Diversity of Biodiesel Species Pongamia pinnata Accessions using AFLP and Three Endonuclease-AFLP. Plant Mol. Biol. Rep. 2011, 29, 12–18. [Google Scholar] [CrossRef]
- Kong, Q.; Li, X.; Xiang, C.; Wang, H.; Song, J.; Zhi, H. Genetic Diversity of Radish (Raphanus sativus L.) Germplasm Resources Revealed by AFLP and RAPD Markers. Plant Mol. Biol. Rep. 2011, 29, 217–223. [Google Scholar] [CrossRef]
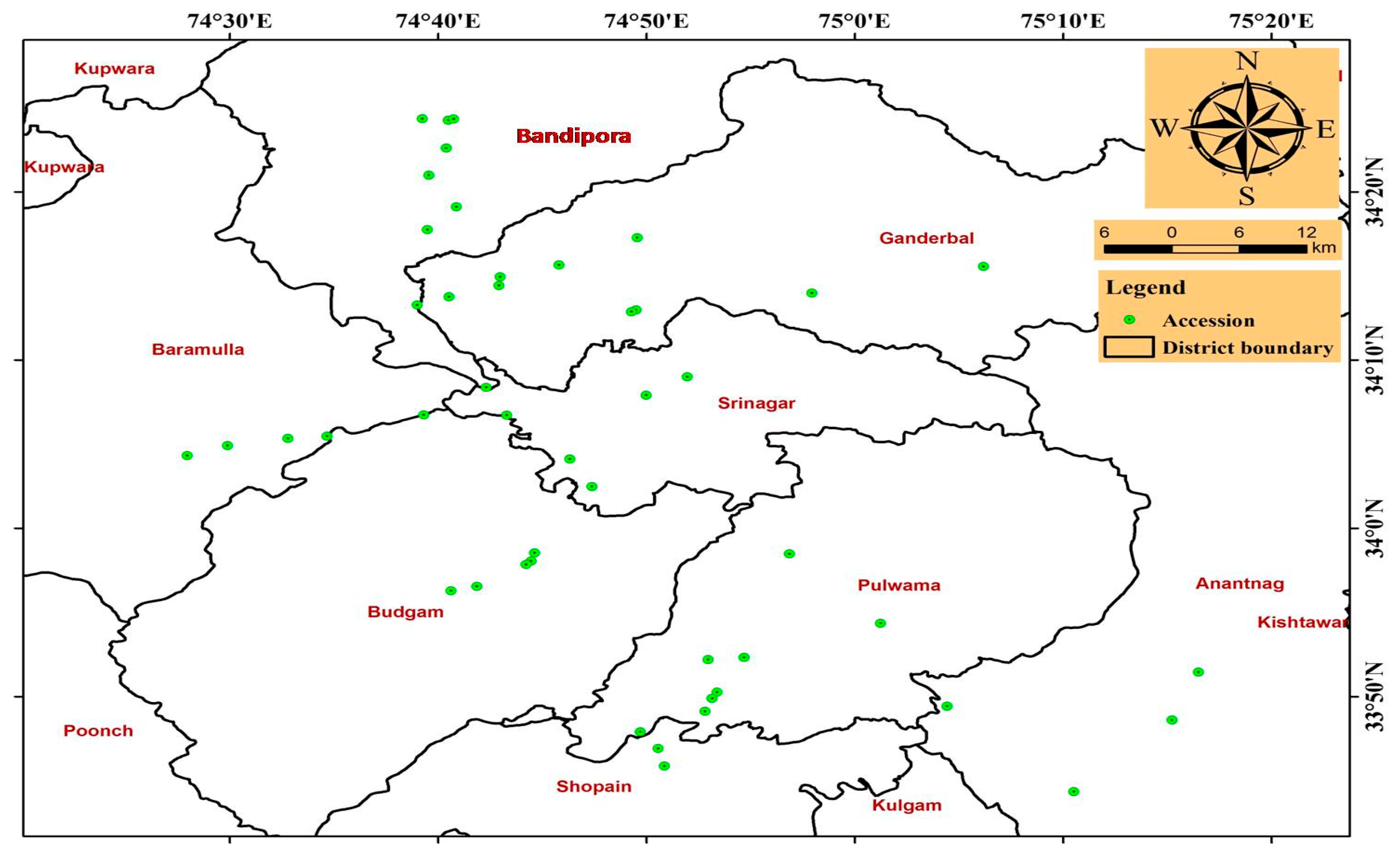
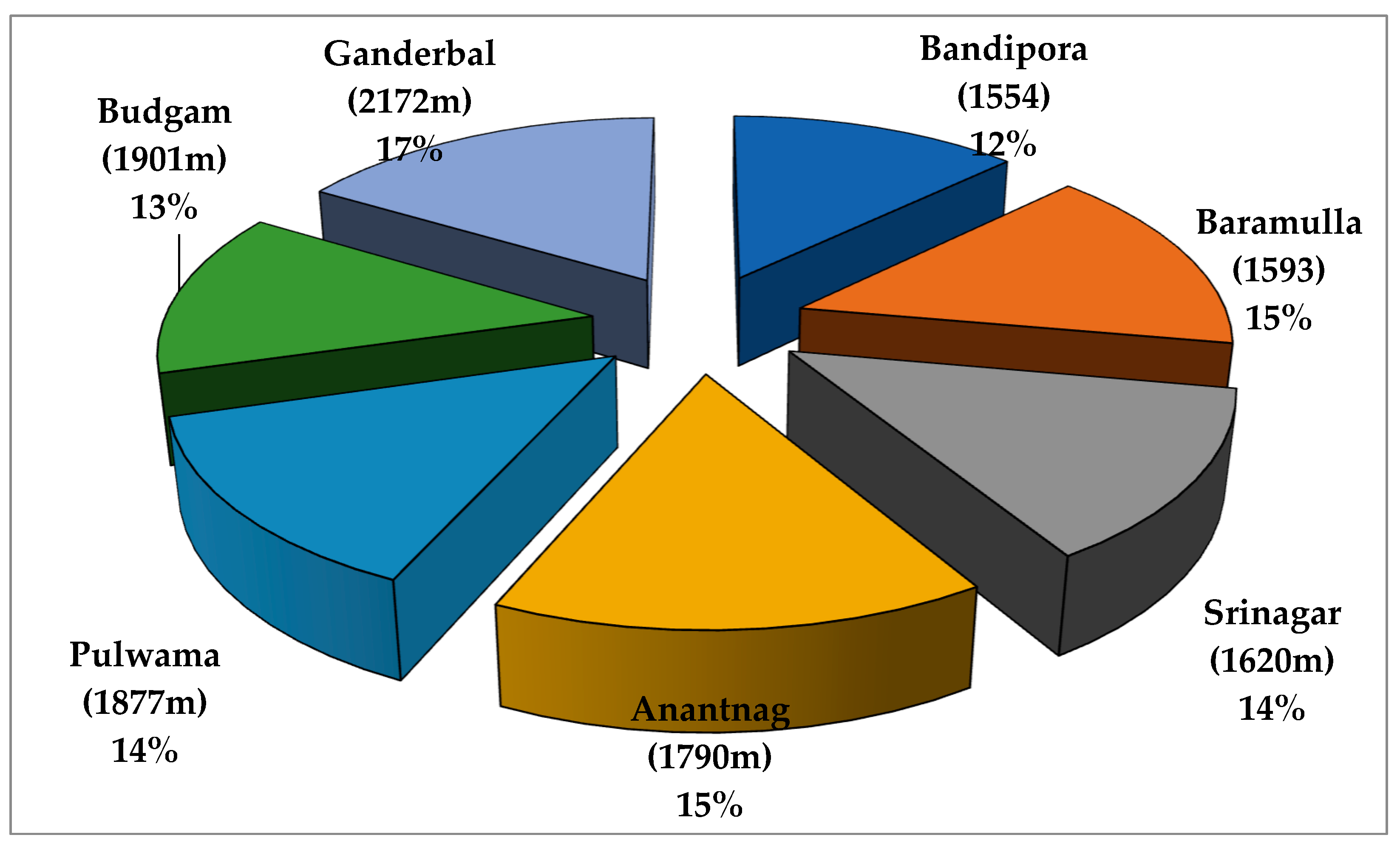

| S. No. | Collection Site | Population Code | No. of Accessions | Latitude (N) | Longitude (E) | Mean Altitude (amsl) m |
|---|---|---|---|---|---|---|
| 1. | Kashmir, Dist. Bandipora | CIN—BD | 1–12 | 34°41′ | 74°65′ | 1554 |
| 2. | Kashmir, Dist. Baramulla | CIN—BM | 13–17 | 34° 12′ | 74°20′ | 1593 |
| 3. | Kashmir, Dist. Anantnag | CIN—AN | 18–22 | 33° 43′ | 75°80′ | 1790 |
| 4. | Kashmir, Dist. Srinagar | CIN—SR | 23–28 | 34° 50′ | 74°47′ | 1620 |
| 5. | Kashmir, Dist. Ganderbal | CIN—GA | 29–36 | 34°12′ | 74°46′ | 2172 |
| 6. | Kashmir, Dist. Pulwama | CIN—PU | 37–44 | 33°87′ | 74°89′ | 1877 |
| 7. | Kashmir, Dist. Budgam | CIN—BU | 45–50 | 34°02′ | 74°72′ | 1901 |
| S. No. | Retention Index (RI) | Name of the Compound | 1554 m | 1593 m | 1620 m | 1790 m | 1877 m | 1901 m | 2172 m | Average (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Area (%) | ||||||||||
| 1. | 1656 | 1-methylbutylmandelate | 3.76 | - | - | - | - | - | 2.58 | 3.17 |
| 2. | 2781 | Neophytadiene | 3.76 | 2.69 | 2.83 | 3.99 | 2.19 | 3.79 | 4.79 | 3.43 |
| 3. | 1968 | n-Hexadecanoic acid | 4.86 | 2.27 | 3.4 | 2.19 | 4.58 | 2.79 | 5.52 | 3.65 |
| 4. | 2101 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z) | 1.04 | - | - | - | - | - | - | 1.04 |
| 5. | 2134 | Phytol | 2.25 | 2.08 | 2.87 | 4.48 | 1.24 | 3.29 | 7.98 | 3.45 |
| 6. | 2191 | 9,12,15-Octadecatrienoic-acid, (Z,Z,Z)- | 6.69 | 2.78 | 3.2 | 2.78 | 5.84 | 2.55 | 8.42 | 4.60 |
| 7. | 2167 | Octadecanoic acid | 1.45 | - | 1.8 | - | 1.19 | - | 2.18 | 1.65 |
| 8. | 1474 | 3-Cyclopentylpropionic acid, 2-dimethylaminoethyl ester | 1.23 | - | - | - | - | - | 1.48 | 1.35 |
| 9. | 2168 | Fumaric acid, decyl neopentyl ester | 1.3 | - | 2.3 | - | 1.33 | - | 3.4 | 2.08 |
| 10. | 2498 | Hexadecanoic acid, 2-hydroxy-1- (hydroxymethyl)ethyl ester | 1.55 | 1.69 | 1.64 | - | - | - | - | 1.62 |
| 11. | 2906 | Bis (2-ethylhexyl) phthalate | 4.29 | - | - | - | - | - | - | 4.29 |
| 12. | 2713 | Ethyl (9Z,12Z)-9,12-octadecadienoate | 2.02 | 2.12 | - | - | - | - | 2.19 | 2.11 |
| 13. | 2228 | 9-Octadecenamide | 1.2 | - | 1.6 | - | - | - | 1.48 | 1.42 |
| 14. | 2739 | Stigmasterol | 3.45 | 1.77 | 4.39 | 2.26 | 1.77 | 1.64 | 4.46 | 2.82 |
| 15. | 2735 | γ-Sitosterol | 8.2 | 2.79 | 6.45 | 4.99 | 4.85 | 7.2 | 9.3 | 6.25 |
| 16. | 2869 | Olean-12-en-3-one | 2.44 | 1.1 | - | 1.25 | - | 1.73 | 3.5 | 2.0 |
| 17. | 2848 | Lupeol | 7.3 | - | 9.74 | - | - | - | 14.2 | 10.41 |
| 18. | 2994 | Methyl commate B | 9.17 | 8.23 | 7.56 | 6.3 | 9.53 | 9.66 | 10.14 | 8.65 |
| 19. | 2987 | Lup-20(29)-en-3-yl-acetate | 5.2 | 7.32 | 5.6 | - | - | - | 6.75 | 6.21 |
| 20. | 2842 | Lup-20 (29)-en-3-ol, acetate, (3.beta) | 5.32 | 27.99 | - | - | - | - | - | 16.65 |
| 21. | 1293 | 2-methoxy-4-vinylphenol | - | 1.82 | - | 2.09 | - | - | - | 1.95 |
| 22 | 1528 | 2-dimethylaminoethyl ester | 2.18 | - | - | - | - | - | - | 2.18 |
| 23. | 1779 | Octanoic acid, octyl ester | - | 1.04 | - | - | - | - | - | 1.04 |
| 24. | 2058 | 2H-Pyran-2-one, 6-[2-E-(3-tolyl) ethenyl]-4-methoxy- | - | 1.22 | - | 2.4 | 3.23 | - | - | 2.28 |
| 25. | 3149 | Vitamin E | - | 2.49 | 2.5 | - | - | - | 4.9 | 3.29 |
| 26. | 2873 | Amyrin, acetate | 1.2 | 1.39 | 1.4 | 1.46 | 3.2 | 3.18 | 4.8 | 2.37 |
| 27. | 1612 | Hexadecane | - | - | 1.09 | - | - | - | - | 1.09 |
| 28. | 1386 | 1H-Cycloprop[e]azulene, 1a,2,3,4,4a,5,6,7b-octahydro-1,1,4,7 | - | - | 1.24 | - | - | - | - | 1.24 |
| 29. | 1574 | 1-tetradecanol, acrylate | - | - | 3.47 | - | - | - | - | 3.47 |
| 30. | 1852 | 5,9-Tetradecadienedioic acid, 5,6,9,10-tetramethyl-, dimethyl ester | - | - | 2.65 | - | - | - | - | 2.65 |
| 31. | 1759 | Loliolide | - | - | 1.08 | - | - | - | 2.29 | 1.68 |
| 32. | 2082 | 2-heptenedioic acid, 4-cyclopropyl-, dimethyl ester, (E) | - | - | 1.81 | - | - | - | - | 1.81 |
| 33. | 2045 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | - | - | 1.49 | - | - | - | - | 1.49 |
| 34. | 3036 | Benzothiazole, 2-(2-hydroxyethylthio)- | - | - | 2.75 | - | - | - | - | 2.75 |
| 35. | 2117 | 1,6-Heptadiene, 2-methyl-6-phenyl | - | - | 2.47 | - | - | - | - | 2.47 |
| 36. | 2048 | 1,5-Hexadiene, 2,5-bis(4-methylphenyl)- | - | - | 2.3 | - | - | - | - | 2.3 |
| 37. | 2325 | 13-hexyloxacyclotridec-10-en-2-one | - | - | 1.43 | - | - | - | - | 1.43 |
| 38. | 1518 | 1-methyl-6-(3-methyl-buta-1,3-dienyl)-7-oxa-bicyclo[4.1.0]heptane | - | - | 1.78 | - | - | - | - | 1.78 |
| 39. | 2333 | Benzoic acid N′-(2,2,7,7-tetramethyl)-tetrahydro-bis[1,3]dioxolo | - | - | 2.09 | - | - | - | - | 2.09 |
| 40. | 1556 | 2(5H)-Oxepinone, 3-tert-butyl-7-phenyl | - | - | 2.84 | - | - | - | - | 2.84 |
| 41. | 1334 | 2,6-Dimethyl-4-nitro-3-phenyl-cyclohexanone | - | - | 2.99 | - | - | - | - | 2.99 |
| 42. | 2704 | 1,2-benzenedicarboxylic acid | - | - | 1.28 | - | - | - | - | 1.28 |
| 43. | 2625 | Dotriacontane | - | - | 1.89 | - | - | 2.3 | 4.7 | 2.96 |
| 44. | 1316 | 2,6-Cresotaldehyde | - | - | - | 2.89 | - | - | - | 2.89 |
| 45. | 1470 | Benzeneacetic acid, 3-hydroxy- | - | - | - | 1.74 | 1.79 | 2.98 | - | 2.17 |
| 46. | 1510 | 10-undecenyl hexofuranoside | - | - | - | 1.6 | - | - | - | 1.6 |
| 47. | 1474 | 3-Cyclopentylpropionic acid | - | - | - | 1.69 | - | - | - | 1.69 |
| 48. | 3090 | Chrysene, 1,2,3,4,4a,4b,5,6,10,10a, 10b,11-dodecahydro- | - | - | - | 1.34 | - | - | 1.34 | 1.34 |
| 49. | 2681 | Glycerol, 2-palmitate | - | - | - | 1.57 | 1.09 | 1.19 | 1.98 | 1.45 |
| 50. | 3081 | Ethyl linoleate | - | - | - | 1.59 | 1.13 | - | 2.6 | 1.77 |
| 51. | 2228 | 9-octadecenamide | - | - | - | 1.9 | 1.27 | - | 4.2 | 2.45 |
| 52. | 2914 | Squalene | - | - | - | 1.05 | 1.15 | 1.17 | 3.3 | 1.66 |
| 53. | 3090 | Betulin | - | - | - | 20.68 | 17.95 | 25.16 | 30.35 | 23.53 |
| 54. | 2904 | Kolavelool | - | - | - | 6.73 | 7.45 | 6.97 | 8.4 | 7.38 |
| 55. | 2407 | Betulinaldehyde | - | - | - | 7.3 | 5.48 | 7.47 | 8.6 | 7.21 |
| 56. | 2632 | Ergost-5-en-3-ol, (3.beta) | - | - | - | - | - | 5.29 | 9.4 | 7.34 |
| 57. | 2882 | Lanosterol | - | - | - | - | - | - | 2.9 | 2.9 |
| 58. | 1091 | α-Monoacetin | - | - | - | - | 1.5 | - | - | 1.5 |
| 59. | 1269 | Pyranone | - | - | - | - | - | 1.19 | - | 1.19 |
| 60. | 1364 | 1-methoxy-2,4-dimethylbenzene | - | - | - | - | - | 2.54 | - | 2.54 |
| 61. | 1404 | Levoglucosan | - | - | - | - | - | 1.08 | - | 1.08 |
| 62. | 3036 | α-Tocopherol | - | - | - | - | - | 1.61 | - | 1.61 |
| 63. | 1470 | Benzeneacetic acid, 4-hydroxy | - | - | - | - | - | - | - | 5.69 |
| 64. | 1936 | Styrene, 2,3,5,6-tetraethyl-4-vinyl | - | - | - | - | - | - | 1.04 | 1.04 |
| Total number of metabolites | 21 | 17 | 32 | 23 | 20 | 21 | 31 | 3.29 | ||
| S. No. | Collection Site | Altitude (m) | pH | SOC (%) | N (%) | P (mg/kg) | K (mg/kg) |
|---|---|---|---|---|---|---|---|
| 1. | Bandipora | 1554 | 8.21 | 2.334 | 0.221 | 210 | 54 |
| 2. | Baramullah | 1593 | 7.2 | 2.437 | 0.426 | 280 | 55 |
| 3. | Srinagar | 1620 | 6.9 | 2.611 | 0.660 | 315 | 60 |
| 4. | Anantnag | 1790 | 7.5 | 2.891 | 0.373 | 355 | 65 |
| 5. | Pulwama | 1877 | 6.49 | 3.210 | 0.432 | 380 | 70 |
| 6. | Budgam | 1901 | 7.21 | 3.253 | 0.353 | 395 | 72 |
| 7. | Ganderbal | 2172 | 7 | 4.792 | 0.432 | 440 | 96 |
| S. No. | Primer Used | Nucleotide Sequence (5′–3′) | Annealing Temperature (°C) | Number of Bands | Number of Polymorphic Bands | Percentage of Polymorphism (%) |
|---|---|---|---|---|---|---|
| 1. | UBC827 | ACACACACACACACACG | 53.0 | 7 | 5 | 71.42 |
| 2. | UBC834 | AGAGAGAGAGAGAGAGYT | 49.2 | 8 | 6 | 75 |
| 3. | UBC840 | GAGAGAGAGAGAGAGAYT | 47.4 | 8 | 4 | 50 |
| 4. | UBC841 | GAGAGAGAGAGAGAGAYC | 48.5 | 9 | 7 | 77.77 |
| 5. | UBC842 | GAGAGAGAGAGAGAGAYG | 48.8 | 10 | 7 | 70 |
| 6. | UBC855 | ACACACACACACACACYT | 53.1 | 8 | 5 | 62.5 |
| 7. | UBC856 | ACACACACACACACACYA | 52.8 | 5 | 4 | 80 |
| 8. | UBC857 | ACACACACACACACACYG | 54.3 | 5 | 3 | 60 |
| 9. | UBC861 | ACCACCACCACCACCACC | 60.6 | 6 | 4 | 66.66 |
| 10. | UBC880 | GGAGAGGAGAGGAGA | 47.9 | 5 | 2 | 40 |
| 11. | UBC884 | HBHAGAGAGAGAGAGAG | 46.1 | 7 | 5 | 71.42 |
| 12. | UBC888 | BDBCACACACACACACA | 52.4 | 8 | 6 | 75 |
| Average | 7.16 | 4.83 | 67.45 | |||
| Total | 86 | 58 | 67.44 |
| Accessions | PPB% | *H (mean) | *I (mean) | *H (SE) | *I (SE) |
|---|---|---|---|---|---|
| CIN-BD | 56.98 | 0.159 | 0.254 | 0.017 | 0.026 |
| CIN-BM | 31.40 | 0.115 | 0.173 | 0.019 | 0.028 |
| CIN-AN | 47.67 | 0.177 | 0.265 | 0.021 | 0.031 |
| CIN-SR | 38.37 | 0.136 | 0.206 | 0.020 | 0.029 |
| CIN-GA | 45.35 | 0.144 | 0.223 | 0.019 | 0.028 |
| CIN-PU | 68.60 | 0.222 | 0.343 | 0.018 | 0.027 |
| CIN-BU | 44.19 | 0.165 | 0.246 | 0.021 | 0.031 |
| Mean | 47.51 | 0.160 | 0.244 | 0.007 | 0.011 |
| HT | HS | Proportion of Diversity within Groups of Accessions | Proportion of Diversity among Groups of Accessions (GST) | Gene Flow (Nm) |
|---|---|---|---|---|
| 0.296 | 0.160 | 0.541 | 0.459 | 0.589 |
| Source of Variation | Df | SS | MSD | VC | % Variation | p |
|---|---|---|---|---|---|---|
| Among groups of accessions | 6 | 321.138 | 53.523 | 6.470 | 44 | <0.01 |
| Within groups of accessions | 43 | 348.342 | 8.101 | 8.101 | 56 | <0.01 |
| Total | 49 | 669.480 | 14.571 | 100 |
| Pop ID | CIN-BD | CIN-BM | CIN-AN | CIN-SR | CIN-GA | CIN-PU | CIN-BU |
|---|---|---|---|---|---|---|---|
| CIN-BD | 0.000 | ||||||
| CIN-BM | 0.579 | 0.000 | |||||
| CIN-AN | 0.352 | 0.586 | 0.000 | ||||
| CIN-SR | 0.649 | 0.686 | 0.607 | 0.000 | |||
| CIN-GA | 0.590 | 0.698 | 0.482 | 0.634 | 0.000 | ||
| CIN-PU | 0.378 | 0.390 | 0.285 | 0.577 | 0.386 | 0.000 | |
| CIN-BU | 0.618 | 0.690 | 0.555 | 0.587 | 0.637 | 0.462 | 0.000 |
| Pop ID | CIN-BD | CIN-BM | CIN-AN | CIN-SR | CIN-GA | CIN-PU | CIN-BU |
|---|---|---|---|---|---|---|---|
| CIN-BD | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| CIN-BM | 0.483 | 0.000 | 0.011 | 0.006 | 0.005 | 0.001 | 0.010 |
| CIN-AN | 0.286 | 0.479 | 0.000 | 0.001 | 0.001 | 0.001 | 0.004 |
| CIN-SR | 0.538 | 0.580 | 0.492 | 0.000 | 0.001 | 0.001 | 0.008 |
| CIN-GA | 0.490 | 0.588 | 0.392 | 0.530 | 0.000 | 0.001 | 0.002 |
| CIN-PU | 0.300 | 0.308 | 0.217 | 0.453 | 0.305 | 0.000 | 0.001 |
| CIN-BU | 0.506 | 0.570 | 0.439 | 0.481 | 0.523 | 0.356 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, B.; Dar, F.A.; Pirzadah, T.B.; Zari, A.; Zari, T.A.; Alharby, H.F.; Hakeem, K.R.; Rehman, R.U. Molecular and Phytochemical Characterizations of Cichorium intybus L. in Diverse Ecogeographical Regions of Kashmir Himalaya. Appl. Sci. 2022, 12, 12061. https://doi.org/10.3390/app122312061
Malik B, Dar FA, Pirzadah TB, Zari A, Zari TA, Alharby HF, Hakeem KR, Rehman RU. Molecular and Phytochemical Characterizations of Cichorium intybus L. in Diverse Ecogeographical Regions of Kashmir Himalaya. Applied Sciences. 2022; 12(23):12061. https://doi.org/10.3390/app122312061
Chicago/Turabian StyleMalik, Bisma, Fayaz Ahmad Dar, Tanveer Bilal Pirzadah, Ali Zari, Talal A. Zari, Hesham F. Alharby, Khalid Rehman Hakeem, and Reiaz Ul Rehman. 2022. "Molecular and Phytochemical Characterizations of Cichorium intybus L. in Diverse Ecogeographical Regions of Kashmir Himalaya" Applied Sciences 12, no. 23: 12061. https://doi.org/10.3390/app122312061
APA StyleMalik, B., Dar, F. A., Pirzadah, T. B., Zari, A., Zari, T. A., Alharby, H. F., Hakeem, K. R., & Rehman, R. U. (2022). Molecular and Phytochemical Characterizations of Cichorium intybus L. in Diverse Ecogeographical Regions of Kashmir Himalaya. Applied Sciences, 12(23), 12061. https://doi.org/10.3390/app122312061









