Featured Application
The combination of natural plant products and probiotics contribute to regulating the gut microbiota and alleviating liver damage.
Abstract
Epigallocatechin gallate (EGCG) is a main active ingredient in tea, but it is difficult for it to be absorbed and utilized by the body, resulting in limited bioactivity. Therefore, we explored the role of probiotics in enhancing the physiological activity of EGCG in a mice model of liver injury. Mice were methodically treated with either a single ingredient or a combination of EGCG and Lactiplantibacillus plantarum P101 (LP.P101) for 21 days, and then administrated with intraperitoneal injection of carbon tetrachloride (CCl4) on the last day. As a result, the antioxidative genes were activated and pro-inflammatory genes were suppressed, reducing the oxidative and inflammatory injury of mice, which indicated a better preventive effect of the combination of EGCG and LP.P101 than the single ingredient. Furthermore, 16S rRNA high-throughput sequencing revealed the role of gut microbiota played in liver injury mitigation. The combination of EGCG and LP.P101 increased the relative abundance of Lactobacillus, Akkermansia and other beneficial bacteria that negatively correlated with inflammation and positively correlated with antioxidation. In conclusion, the combination of EGCG and LP.P101 was more effective than the single ingredient in alleviating liver damage caused by CCl4. Altered gut microbiota may be an important cause.
1. Introduction
Tea is a traditional drink around the world, rich in various active ingredients, mainly catechins. Epigallocatechin gallate (EGCG) is a main active monomer in catechins, well-known for its anti-inflammatory, antioxidant and anti-obesity properties. Li et al. [1] found that EGCG alleviated liver lipid accumulation by regulating gut microbiota. However, these natural active nutrients seem to be difficult to absorb and utilize [2]. In fact, Goldin [3] stated that the probiotics in the digestive tract or in foods could enhance the bioavailability of nutrients. The roles of probiotics in the prevention and treatment of diseases, such as liver and cardiovascular disease, have been reported. In Liu et al. ’s study [4], the probiotics rich in selenium-glutathione alleviated carbon tetrachloride (CCl4)-induced liver fibrosis by attenuating oxidative damage, endoplasmic reticulum stress and inflammation. Recently, studies have found that the natural plant ingredients worked better when combined with probiotics. For example, the combination of hull-less barley β-glucan and Lactobacillus plantarum S58 exhibited a greater effect on ameliorating obesity by activating lipid metabolism pathway and regulating gut microbiota [5]. Therefore, we speculated that EGCG combined with probiotics would have a better bioactivity.
The largest metabolic organ, liver, performs an antioxidant function, substance transformation and an immune function [6]. The liver shares the same embryological origin and has many anatomical and functional connections with intestine [7], and would be greatly affected by gut microbiota. Gut microbiota is an important mediator in regulating the physiological homeostasis and interacting with various organs [8]. However, the imbalance of gut microbiota promotes the occurrence and development of various diseases such as inflammatory bowel disease, obesity and liver diseases [9]. Tilg et al. [10] showed that the alteration of gut microbiota promoted the progression of liver diseases, and prebiotics or probiotics could prevent liver injuries through regulating gut microbiota, which served as effective therapeutics. The homeostasis of gut microbiota is of great importance for liver health.
At present, the human liver is under great metabolic pressure from environmental pollutants. CCl4, a potent industrial environmental pollutant used in detergents, is metabolized by the cytochrome P450 into trichloromethyl radical and reactive oxygen species (ROS) in the hepatocytes, which causes liver injury [11]. In recent years, CCl4 has been widely used in the establishment of animal liver injury models for its clear toxicity mechanism. In this study, CCl4 was chosen to induce the mice liver injury model, so as to exclude unclear mechanism interference and better demonstrate the combined bioactivity of natural plant ingredients and probiotics. Our study hypothesized that EGCG from tea and the probiotic Lactiplantibacillus plantarum P101 (LP.P101, showed an excellent antioxidant performance, and was screened from Chinese family homemade sauerkraut) would have the best effect in alleviating liver damage caused by CCl4 in mice. Moreover, we explored the effect of the altered gut microbiota through the combination of EGCG and LP.P101 in liver damage reduction. In short, there is still no great progress in improving the utilization rate and biological activity of natural plant products. The study will be beneficial in understanding the promoting effect of probiotics on natural plant products and providing a potential strategy for enhancing the utilization and biological activity of natural products. Meanwhile, the alleviating effects and mechanisms of the combination of foodborne natural plant products and probiotics on mild liver oxidative and inflammatory damage are also worthy of further study.
2. Materials and Methods
2.1. Characterization of Catechins
Green tea catechins sample was obtained from Wuyuan Hongda Tea Co., Ltd. (Jiangxi, China). The specific characterization and analysis methods were described in the previous reference [12]. In brief, catechins was analyzed by ultra-high performance liquid chromatography (Agilent 1290, Agilent Technologies, Inc., Santa Clara, CA, USA), and equipped with a capillary column of Xtimate C18 (5 μm, 250 mm × 4.6 mm) at a column temperature of 35 °C. The injection volume was 10 μL and flow rate was 1 mL/min. Mobile phase A was 9% acetonitrile, 2% acetic acid, 0.2% EDTA-2Na and 88.8% water; mobile phase B was 80% acetonitrile, 2% acetic acid, 0.2% EDTA-2Na and 17.8% water. The elution gradient was 100% mobile phase A for 0–10 min, 0–68% mobile phase A for 10–25 min; 68–100% mobile phase A for 25–27 min. The ultraviolet detection wavelength was 278 nm. The composition of catechins sample was determined by comparing the retention time of standard catechins (Figure S1 and Tables S1 and S2). Since EGCG was the main component of the sample, EGCG was referred to catechins.
2.2. Preparation of LP.P101 and EGCG
LP.P101 was isolated from Chinese family homemade sauerkraut (Ji’an Jiangxi, China) and stored at China Center for Type Culture Collection (CCTCC, Wuhan, Hubei, China), numbered as CCTCC M 2021108. LP.P101 has a great antioxidant property. Activated LP.P101 was anaerobically cultured in Man Rogosa Sharpe broth (MRS) (Solarbio Science and Technology Co., Ltd., Beijing, China) liquid medium, at 37 °C for 16 h to obtain the concentration of 108 CFU/mL. The bacterium suspension was centrifuged at 9600× g for 5 min (a AS24-2 rotor, Centrifuge Scilogex CF1524R, SCILOGEX Co., Ltd., Rocky Hill, CT, USA), the supernatant was discarded, and the pelletized LP.P101 was resuspended with the same volume of PBS (phosphate buffer solution, Solarbio Science & Technology Co., Ltd.) or EGCG solution at selected dose.
To explore the effect of EGCG on the growth of LP.P101, activated LP.P101 was inoculated into MRS liquid medium or EGCG-MRS liquid medium (EGCG powder was added into MRS liquid medium at a concentration of 7 mg/mL). The inoculum was anaerobic incubation at 37 °C, and sampled 500 μL in a centrifuge tube every 3 h. The samples were centrifuged at 9600× g for 5 min, the supernatant was discarded and the pelletized LP.P101 was resuspended in 2 mL PBS. The absorbance of the bacterium suspension was measured at 600 nm (Varioskan LUX Multimode Microplate Reader, Thermo Fisher scientific Inc., Waltham, OH, USA).
2.3. Animal Experiment
Male Kunming mice (6-week-old, 35 ± 2 g) were purchased from an experimental animal center (Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi, China). The mice were raised in the standard environment with a 12 h light–12 h dark cycle, 25 °C and 55% relative humidity, free diet and water. Animal processes adhered to the institutional animal care committee guidelines, and the animal experiment was approved by the Animal Care Review Committee (approval number 0064257, Nanchang University, Nanchang, Jiangxi, China).
After acclimatization for a week, 40 mice were divided into 5 groups at random: control group (PBS), model group (PBS), P101 group (108 CFU/mL LP.P101 resuspended in PBS), EGCG group (EGCG powder was dissolved in PBS at a concentration of 7 mg/mL) and P + E group (108 CFU/mL LP.P101 resuspended in 7 mg/mL EGCG). Each mouse was given 200 μL of the corresponding substance intragastrically for 21 days. The body weight of mice was recorded every two days. CCl4 and olive oil were purchased from Xilong Scientific Co., Ltd. (Guangzhou, Guangdong, China) and Solarbio, respectively. The mice in control group were intraperitoneally injected with 3 mL/kg body weight olive oil on the last day. The mice in other groups were treated with 3 mL/kg body weight CCl4/olive oil (20% v/v) [13] 2 h after the last intragastric administration. After fasting for 16 h, the feces of mice were picked and stored at −80 °C for microbial diversity analysis. Mice were sacrificed after anesthesia with isoflurane, and blood and liver were collected and stored at −80 °C.
2.4. Histopathological Observation of Liver
The small portion of liver tissue was wrapped in paraffin and sliced into a thickness of 5 μm, stained with hematoxylin and eosin (H&E), and was observed and photographed with a Nikon Ti optical microscope (Nikon Ltd., Tokyo, Japan).
2.5. Biochemical Analysis of Serum and Liver
Serum was collected by centrifugation at 3000× g for 15 min under 4 °C. Assay kits of alkaline phosphatase (ALP), glutamic oxaloacetic transaminase (AST) and glutamic-pyruvic transaminase (ALT) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China), and such indicators were determined according to the instruction of assay kits.
A small portion of liver tissue was mixed with 0.9% normal saline to homogenize into 0.1 mg/mL, and supernatant was collected by centrifugation at 3000× g for 10 min under 4 °C. Analysis of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10) and LPS (lipopolysaccharide) followed the instruction of enzyme-linked immunosorbent assay kits (Shanghai YSRIBIO Industrial Co., Ltd., Shanghai, China). The determination of malonaldehyde (MDA) followed the instruction of assay kits (Nanjing Jiancheng Bioengineering Institute).
2.6. Quantitative Real Time Polymerase Chain Reaction (qPCR) Analysis of Liver
Total RNA of liver was extracted according to the AxyPrep Multisource Total RNA Miniprep Kit (Axygen Scientific Inc., San Francisco, CA, USA), and the extracted RNA was reverse transcribed by Takara PrimeScriptTM RT reagent kit (Takara Bio Inc., KUSATSU, Shiga, Japan) into cDNA. qPCR was performed with TB Green™ Premix Ex Taq™ II (Takara Bio Inc.) on the AriaMx RT-PCR System (Agilent Technologies, Inc.). The transcription level was calculated by the 2−∆∆Ct method. The sequence of primers was listed in Table S3.
2.7. High-Throughput Sequencing of Feces
The sequencing terminals were added to the primer ends for the 16S rRNA hypervariable region (V3–V4) PCR amplification. The total DNA of feces was extracted and amplificated. Then, the amplificated products were purified, quantified and homogenized to create the sequencing library. The gut microbiota analysis was performed on the platform BMKCloud (Beijing, China).
2.8. Statistical Analysis
Data was represented as mean ± SD; a one-way analysis of variance and multiple-comparison test was performed using SPSS 25.0 (IBM Co., Chicago, IL, USA). Significant difference existed when p < 0.05 and was noted as different letters.
3. Results
3.1. Characterization of Catechins and Effect Evaluation of EGCG on LP.P101
By comparing the chromatograms of sample catechins and standard catechins (Figure 1A and Figure S1), the highest peaks appeared at around 15.8 min and 17.2 min, indicating that the sample catechins had similar monomer composition to standard catechins, mainly EGCG. Then, to test the effect of EGCG on the growth of LP.P101, LP.P101 were co-incubated with EGCG. The result (Figure 1B) showed that the growth of LP.P101 in the presence of EGCG had no significant difference from that in the single MRS.
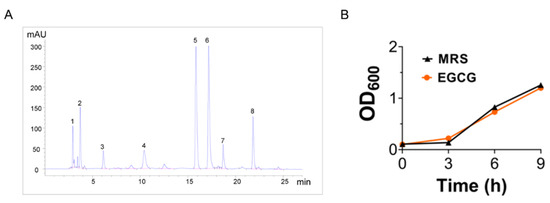
Figure 1.
Epigallocatechin gallate (EGCG) did not affect the growth of Lactiplantibacillus plantarum 101 (LP.P101). (A) Ultra-high performance liquid chromatography of catechins. (B) Comparison of growth curves of LP.P101 in Man Rogosa Sharpe broth (MRS) and EGCG-MRS (n = 3). Data was expressed as mean ± SD.
3.2. The Combination of EGCG and LP.P101 Alleviated CCl4-Induced Pathological Alterations
The body weight of the mice in EGCG and combination group remained constant (35 ± 2 g) (Figure 2A). The liver organ coefficient increased significantly (Figure 2B) in the model group, which indicated that CCl4 may cause edema or congestion in the liver. The combination of EGCG and LP.P101 showed the best effect on reducing the liver organ coefficient (Figure 2B). Accordingly, the mice liver in control group had a clear structure and intact cells (Figure 2C). Histopathological analysis showed congestion, hepatocyte ballooning degeneration and inflammatory cell infiltration in the model group. After 21 days of gavage with EGCG and LP.P101, the damage caused by CCl4 was alleviated with a few hepatocytes ballooning degeneration (Figure 2C). Although the LP.P101 or EGCG individual gavage showed some alleviation, the results were not as remarkable as that in combination group.
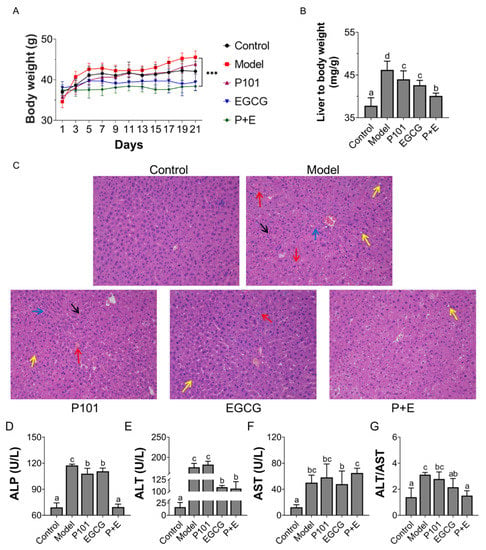
Figure 2.
The Combination of Epigallocatechin Gallate (EGCG) and Lactiplantibacillus plantarum P101 (LP.P101) prevented liver injury caused by carbon tetrachloride (CCl4). (A) Body weight (g) of mice over 21 days. (B) Liver weight to body weight (mg/g) of mice. (C) Histology of liver tissue (200×) in each group after hematoxylin and eosin staining. Red arrow: congestion; Black arrow: inflammatory cell infiltration; Yellow arrow: ballooning degeneration; Blue arrow: loose tissue structure. (D–F) The activities of alkaline phosphatase (ALP, U/L), glutamic oxaloacetic transaminase (AST, U/L) and glutamic-pyruvic transaminase (ALT, U/L) in serum. (G) The ratio of ALT/AST. Data was expressed as mean ± SD. The significant differences (p < 0.05) among groups showed as different letters.
In addition, compared with control group, the activities of ALP, AST and ALT in serum were significantly increased in model group (Figure 2D–G), which indicated that the liver was impaired. The activity of ALT in the combination group was similar to that of EGCG group. Additionally, there was no significant difference in enzyme activity level of AST between the model group and combination group. Interestingly, the ratio of ALT to AST in the combination group was more similar to that of the control group (Figure 2G).
3.3. The Combination of EGCG and LP.P101 Inhibited CCl4-Induced Liver Oxidative Injury and Inflammation
The Toll-like receptor-4/nuclear factor-kappa B (TLR4/NF-κB) signaling pathway was activated, and up-regulating the mRNA expression of pro-inflammatory factor TNF-α in the mice liver of the model group (Figure 3G). The level of inflammatory cytokines in liver tissue of the model group also showed a similar trend: the contents of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 were significantly increased, and the content of anti-inflammatory cytokine IL-10 was significantly decreased (Figure 3A–D). Compared with the model group, the combination group reversed the mRNA expression of inflammatory indicators, as well as lowering the content of pro-inflammatory cytokines and elevating the content of IL-10 in the liver. The content of LPS in liver was significantly increased in model group (Figure 3E), indicating the disruption of the intestinal barrier. However, the content of LPS was decreased in the presence of LP.P101 or EGCG, especially in the combination group (Figure 3E).
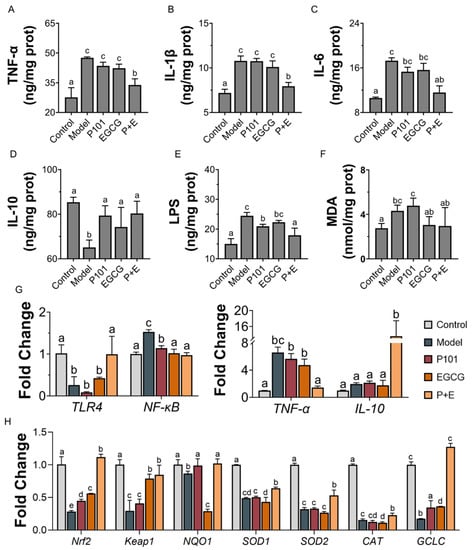
Figure 3.
The combination of EGCG and LP.P101 reduced liver inflammation and oxidative damage caused by CCl4. (A–F) The content of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), inter-leukin-10 (IL-10), lipopolysaccharide (LPS) and malonaldehyde (MDA) in liver tissue. (G) The mRNA expression level of inflammatory genes. (H) The mRNA expression level of antioxidative genes. Data was expressed as mean ± SD. The significant differences (p < 0.05) among groups shown as different letters.
CCl4 induced liver oxidative damage as expected; the content of MDA (Figure 3F) and the activities of SOD and CAT (Table S4) in liver were increased in the model group. CCl4 inhibited the liver oxidative stress pathway, decreased the expression level of nuclear factor E2-related factor 2 (Nrf2) and kelch-like ech-associated protein 1 (Keap1), and down-regulated mRNA expression level of the antioxidase system NAD(P)H: quinone oxidoreductase 1 (NQO1), SOD1, SOD2, CAT and glutamate-cysteine ligase catalytic (GCLC) (Figure 3H). The combination of EGCG and LP.P101 showed a more obvious effect than EGCG or LP.P101 alone in the recovery of gene expression level.
3.4. The Combination of EGCG and LP.P101 Improved the Gut Microbiota
Based on the results shown above, the control group, the model group and the combination group were selected to investigate the effect of intestinal microbial diversity and composition on liver damage reduction. Although there was no significant difference in α diversity among the three groups (Table S5), β-diversity analysis showed that the composition of the control group was similar to that of the model group, while the P + E group deviated from them (Figure 4A–B). At the phylum level, the relative abundance of Bacteroidetes was decreased, while Firmicutes and Proteobacteria increased in the combination group. (Figure 4C). Specifically, the relative abundance of Desulfovibrionaceae in the model group, and Alloprevotella in the combination group, was distinctly reduced at the genus level (Figure 4D).
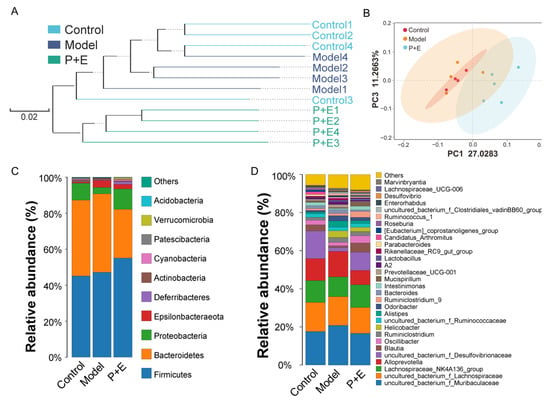
Figure 4.
The combination of EGCG and LP.P101 altered the diversity of gut microbiota. (A) The clustering tree using the unweighted pair-group method with arithmetic means at the operational taxonomic unit level. (B) Principal coordinates analysis of the gut microbiota at the operational taxonomic unit level. (C,D) Species distribution of bacterial communities at the phylum level and the genus level.
According to the results of the line discriminant analysis effect size (LEfSe) cladogram, there were only 11 significant different taxa between the control group and the model group, and less than 21 significant different taxa between the control group and the combination group (Figure 5(Aa,Bb)). In addition, the difference between the model group and the combination group was very significant, as evidenced by the 52 taxa of different bacteria in the LEfSe cladogram (Figure 5(Cc)). The different bacteria between the model group and the combination group are mainly Bacteroidates, Erysipelotrichaceae, Desulfovibrionales, Alteromonadales, Vibrionales and Verrucomicrobioles, at the order level (Figure 5(Cc)).
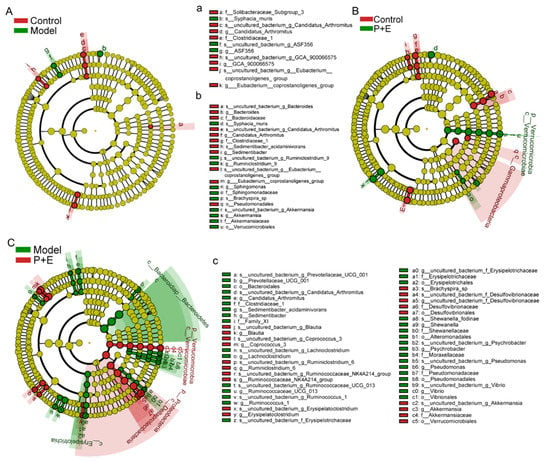
Figure 5.
The combination of EGCG and LP.P101 regulated the composition of gut microbiota. (A–C) The cladogram using line discriminant analysis effect size analysis of gut microbiota between different groups (cut-off line discriminant analysis score ≥ 3.0).
More specifically, the relative abundance of Alloprevotella and Alistipes was significantly increased, and Roseburia was significantly decreased in the model group compared with the control group (Figure 6(Aa,e,j)). However, there was a significant alteration after combined treatment of EGCG and LP.P101. The relative abundance of Blautia, Ruminiclostridium 9, Lactobacillus, Akkermansia and Roseburia was significantly increased, and Alloprevotella, Muribaculaceae, Bacteroides, Alistipes and Prevotellaceae UCG-001 was significantly decreased in the combination group compared to the control or model group (Figure 6(Aa–j)).
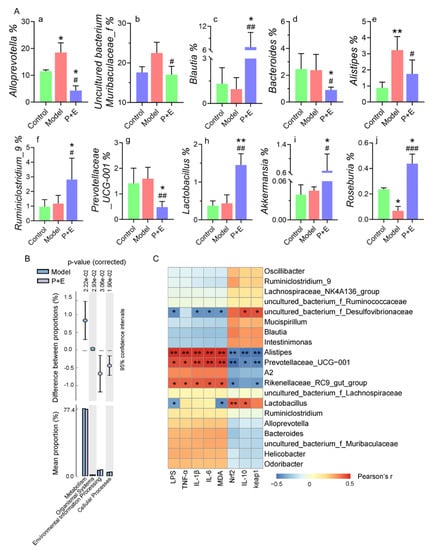
Figure 6.
The combination of EGCG and LP.P101 modulated the relative abundance of bacteria and affected the signal expression. (A) Relative abundance of different species. a-j: relative abundance of Alloprevotella, uncultured bacterium f Muribaculaceae, Blautia, Bacteroides, Alistipes, Ruminiclostridium 9, Prevotellaceae UCG-001, Lactobacillus, Akkermansia and Roseburia. Data were expressed as mean ± SD. *p < 0.05, **p < 0.01 vs. control group; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. model group. (B) Differential analysis of Kyoto encyclopedia of genes and genomes between the model and P + E group. (C) Pearson’s correlation analysis between biochemical parameters and the relative abundance of gut microbiota species. The color ranged from blue to red and indicated the change in correlation from negative to positive. Data were expressed as mean ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001.
Furthermore, through the prediction of functional genes, there were four different genes in the total six functional genes between the model group and the combination group (Figure 6B), while no difference was found between the other groups. The alteration in gut microbiota led to the change in different functional genes in relation to metabolism, organismal systems, environmental information processing and cellular processes. The mitigated effect of altered gut microbiota on liver damage was analyzed according to Pearson’s correlation (Figure 6C). The contents of LPS, TNF-α, IL-1β, IL-6 and MDA in the liver were positively related to the relative abundance of Alistipes, Prevotellaceae UCG-001 and Rikenellaceae RC9 gut group that decreased in the combination group, but were negatively related to the relative abundance of uncultured bacterium f Desulfovibrionaceae. In addition, the mRNA expression level of Nrf2 and keap1, and the content of IL-10 were positively correlated with the above flora (Figure 6C).
4. Discussion
Due to low lipid solubility and poor stability, the utilization rate of EGCG is negligible [14]. In recent years, a new method regarding the co-encapsulation of nutrients and probiotics, which may improve their bioavailability and function, has attracted the attention of researchers [15]. The probiotics contribute to the release of smaller molecules from the bioactive compounds to allow for a maximum utilization. For example, Lactobacillus rhamnosus GG converted cranberry proanthocyanins and produced additional active substances that enhanced their anticancer activity [16]. Therefore, we were inspired to combine the catechins and probiotics, two inexpensive and readily available materials, to explore their enhanced properties in liver damage reduction.
We screened a strain of Lactiplantibacillus plantarum from Chinese homemade sauerkraut, named LP.P101 that has excellent antioxidant property, high safety and could survive in simulated gastric and intestinal fluid. When LP.P101 was cultured in EGCG-MRS liquid medium, the growth of LP.P101 were not affected (Figure 1B), which demonstrated that LP.P101 could coexist with EGCG.
Interestingly, a better weight maintenance was evidenced by the consistent body weight in the combination group (Figure 2A). Furthermore, the increscent liver coefficient as well as inflammatory infiltration and cell degeneration caused by CCl4 have been reported [13,17], and our results are consistent with them (Figure 2B–C). As expected, CCl4 caused a significant increase of ALP (Figure 2D). Noteworthily, ALT exists in hepatocyte cytoplasm and AST is present in the mitochondria of hepatocyte. According to the report [18], mild hepatitis caused by CCl4 was manifested as hepatocyte damage and relatively complete mitochondria structure led to an increase of ALT/AST ratio, which was similar to our result (Figure 2G). Interestingly, the ratio of ALT/AST was decreased significantly in the combination group, and implied the alleviation effect of EGCG with the synergism of LP.P101 (Figure 2G).
Peroxidation products MDA (Figure 3F), produced after the treatment with CCl4, caused the release of antioxidant enzymes to maintain the balance of oxidative stress (Table S4). However, at the transcriptional level, the classical antioxidative signaling pathway Nrf2/Keap1, inhibited in model group (Figure 3H), suggested CCl4 caused oxidative damage in the liver. The combination of EGCG and LP.P101 was shown to be more effective than LP.P101 or EGCG alone in the recovery of antioxidative gene expression level. Furthermore, oxidative damage is always accompanied by inflammation [19]. Results showed that NF-κB was activated by CCl4, and the content of TNF-α, IL-1β and IL-6 was increased in the liver. It could be reversed by the combination of EGCG and LP.P101s (Figure 3). In summary, the combination of EGCG and LP.P101 were more effective in alleviating liver injury caused by CCl4.
Based on the above results, we proved that the combination group had a better prevention effect on liver injury. Next, control group, model group and combination group were selected to investigate the effect of intestinal microbial diversity and composition on liver damage reduction. Liver health was closely associated with the homeostasis of gut microbiota [20]. We speculated that 21 days of continuous oral administration of EGCG and LP.P101 could regulate gut microbiota beforehand and allow the mice to cope with liver damage caused by CCl4 better. As a result, the unweighted pair–group method with arithmetic means (UPGMA) and principal coordinates analysis (PCoA) showed that the microbial composition was altered in the combination group and deviated from the control group and model group (Figure 4A–B). A previous study indicated that CCl4 (twice a week for six weeks) induced liver fibrosis, as well as disruption of the gut microbiota in mice [21]. In this study, an acute injection of CCl4 did not induce strong gut microbiological changes (Figure 4, Figure 5 and Figure 6), possibly due to the short exposure time. Although there were differences in gut microbiota between the control and model group or between the control and combination group, the differences were far less than that between the model and combination group (Figure 5), indicating the effect of EGCG and LP.P101 in regulating gut microbiota. Similarly, tea and probiotics play a role in regulating gut microbiota in a previous study [22,23].
Specifically, at the phylum level, the relative abundance of Bacteroidetes was decreased, while firmicutes and proteobacteria increased in the combination group (Figure 4C–D). Similar to our results, in Tian et al.’s study [24], Lactiplantibacillus plantarum TW1-1 increased the abundance of firmicutes and alleviated testicular damage of mice. LEfSe (Figure 5) was used to show the specific bacteria with statistical differences between groups. The combination of EGCG and LP.P101 were given in advance, which mainly increased the abundance of Akkermansia at the phylum of Verrucomicrobia, and Desulfovibrionaceae at the phylum of Proteobacteria. Akkermansia is a typical probiotic, and its reduction is positively correlated with intestinal inflammation and liver disease [25]. Studies have found that Desulfovibrionaceae produces acetic acid and regulates metabolic diseases [26]. Furthermore, we listed some species that changed significantly, associated with the pathogenesis (Figure 6A). Alloprevotella, Prevotellaceae UCG-001 and Bacteroides was associated with irritable bowel syndrome and promote inflammation [27,28,29]. Alistipes is an opportunistic pathogen, and uncultured bacterium Muribaculaceae was associated with liver failure [30,31,32]. EGCG combined with LP.P101 reduced the abundance of these bacteria as shown in Figure 5A. Blautia, Ruminiclostridium 9 and Roseburia, which produce butyrate and benefit human health [33,34], were decreased in the model group, while their relative abundance in the combination group was even higher than that in the control group (Figure 6A). Pant et al. found that butyrate reduced ROS-mediated hepatocyte apoptosis [35]. There was no significant difference in the relative abundance of Lactobacillus and Akkermansia between the control group and the model group, but the combination of EGCG and LP.P101 significantly increased the abundance of these two probiotics (Figure 6A). Correspondingly, Pearson’s correlation analysis also showed that the liver health was associated with gut microbiota (Figure 6C). Gonzalez-Sarrias et al. [36] demonstrated that non-extractable and indigestible dietary polyphenol were fermented into small molecules by colon microbiota. These small molecules of nutrients could persist in circulation, and had effects of antioxidant and anti-inflammatory. Similarly, Ruminococcus and Faecalibacterium fermented non-digestible carbohydrates to produce short-chain fatty acids, and then inhibited the activation of NF-κb and reduced inflammatory damage [37], which was similar to our study. In fact, not only the combination of EGCG and LP.P101 improved the composition of gut microbiota, but also gut microbiota promoted the digestion and absorption of EGCG, which was a cycle conducive to body health. In addition, the alteration of gut microbiota led to the change of different functional genes about metabolism, organismal systems, environmental information processing and cellular processes (Figure 6B).
In summary, the combination of EGCG and LP.P101 responded to liver damage by promoting a beneficial shift of the microbiome in advance. However, the transformation of catechins in the presence of probiotics requires further exploration to explain their combination effect. The mechanism of gut microbiota alteration, caused by EGCG and LP.P101, in alleviating liver injury is also of great importance to future research.
5. Conclusions
The combination of EGCG and LP.P101 relieved CCl4-induced liver inflammation and oxidative damage better than EGCG or LP.P101 alone. The mechanism of damage mitigation may be due to the improved gut microbiota composition. The combination of EGCG and LP.P101 increased the relative abundance of beneficial bacteria (Lactobacillus, Akkermansia and Roseburia) and decreased the relative abundance of opportunistic bacteria (Alloprevotella, Prevotellaceae UCG-001 and Bacteroides). The improved gut microbiota by the combination of EGCG and LP.P101 was associated with the activation of antioxidation and the inhibition of inflammation. Generally, LP.P101 contributed to enhance the bioactivity of EGCG, and the combination of EGCG and LP.P101 alleviated CCl4-induced liver injury by improving the gut microbiota.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app122211636/s1, Figure S1: The chromatogram of catechins standard. Table S1: The retention time and peak area of catechins standard. Table S2: The retention time and peak area of catechins sample. Table S3: Primers used in this study. Table S4: The activities of liver antioxidant enzymes. Table S5: The α diversity index of gut microbiota.
Author Contributions
M.W.: investigation, conceptualization, data curation, methodology, visualization, writing—original draft, writing—review and editing. K.W.: conceptualization, methodology. Y.Z.: conceptualization, methodology. Z.P.A.: Writing—Review and editing. Y.W.: resources, supervision. H.X.; funding acquisition, resources, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 82060606.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care Review Committee (approval number 0064257), Nanchang University, Jiangxi, China.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of 16s rRNA high-throughput sequence that support the findings of this study is openly available in NCBI, sequence read archive number: PRJNA833265.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, F.; Gao, C.; Yan, P.; Zhang, M.; Wang, Y.; Hu, Y.; Wu, X.; Wang, X.; Sheng, J. EGCG Reduces Obesity and White Adipose Tissue Gain Partly Through AMPK Activation in Mice. Front. Pharmacol. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sang, S. Biotransformation of tea polyphenols by gut microbiota. J. Funct. Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Goldin, B.R. Health benefits of probiotics. Br. J. Nutr. 1998, 80, S203–S207. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Hesketh, J.; Huang, D.; Gan, F.; Hao, S.; Tang, S.; Guo, Y.; Huang, K. Protective effects of selenium-glutathione-enriched probiotics on CCl4-induced liver fibrosis. J. Nutr. Biochem. 2018, 58, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Song, J.; Li, J.; Wang, H.; Zhang, Y.; Suo, H. A synbiotic consisting of Lactobacillus plantarum S58 and hull-less barley beta-glucan ameliorates lipid accumulation in mice fed with a high-fat diet by activating AMPK signaling and modulating the gut microbiota. Carbohydr. Polym. 2020, 243, 116398. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43, S54–S62. [Google Scholar] [CrossRef] [PubMed]
- Delalande, J.-M.; Milla, P.J.; Burns, A.J. Hepatic nervous system development. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2004, 280, 848–853. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, W.-D.; Wang, Y.-D. Gut microbiota: An integral moderator in health and disease. Front. Microbiol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Biedermann, L.; Rogler, G. The intestinal microbiota: Its role in health and disease. Eur. J. Pediatr. 2015, 174, 151–167. [Google Scholar] [CrossRef]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut microbiome and liver diseases. Gut 2016, 65, 2035–2044. [Google Scholar] [CrossRef]
- Sohn, D.H.; Yun, Y.-P.; Park, K.S.; Veech, R.L.; Song, B.J. Post-translational reduction of cytochrome P450IIE by CCl4, its substrate. Biochem. Biophys. Res. Commun. 1991, 179, 449–454. [Google Scholar] [CrossRef]
- Xu, C.; Liang, L.; Li, Y.; Yang, T.; Fan, Y.; Mao, X.; Wang, Y. Studies of quality development and major chemical composition of green tea processed from tea with different shoot maturity. LWT 2021, 142, 111055. [Google Scholar] [CrossRef]
- Wu, T.; Shen, M.; Guo, X.; Huang, L.; Yang, J.; Yu, Q.; Chen, Y.; Xie, J. Cyclocarya paliurus polysaccharide alleviates liver inflammation in mice via beneficial regulation of gut microbiota and TLR4/MAPK signaling pathways. Int. J. Biol. Macromol. 2020, 160, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Parmar, I.; Neir, S.V. Biotransformation of cranberry proanthocyanidins to probiotic metabolites by Lactobacillus rhamnosus enhances their anticancer activity in HepG2 cells in vitro. Oxidative Med. Cell. Longev. 2019, 2019, 4750795. [Google Scholar] [CrossRef]
- Karakus, E.; Karadeniz, A.; Simsek, N.; Can, I.; Kara, A.; Yildirim, S.; Kalkan, Y.; Kisa, F. Protective effect of Panax ginseng against serum biochemical changes and apoptosis in liver of rats treated with carbon tetrachloride (CCl4). J. Hazard. Mater. 2011, 195, 208–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Li, H.; Zhao, H.; Wang, F.; He, Q.; Zhang, T.; Wang, S. Serum metabonomics study of the hepatoprotective effect of amarogentin on CCl4-induced liver fibrosis in mice by GC-TOF-MS analysis. J. Pharm. Biomed. Anal. 2018, 149, 120–127. [Google Scholar] [CrossRef]
- Galli, F.; Piroddi, M.; Annetti, C.; Aisa, C.; Floridi, E.; Floridi, A. Oxidative stress and reactive oxygen species. Contrib. Nephrol. 2005, 149, 240–260. [Google Scholar] [CrossRef]
- Luo, J.; Lin, X.; Bordiga, M.; Brennan, C.; Xu, B. Manipulating effects of fruits and vegetables on gut microbiota—A critical review. Int. J. Food Sci. Technol. 2021, 56, 2055–2067. [Google Scholar] [CrossRef]
- Li, Y.; Hu, H.; Yang, H.; Lin, A.; Xia, H.; Cheng, X.; Kong, M.; Liu, H. Vine Tea (Ampelopsis grossedentata) Extract attenuates CCl4-induced liver injury by restoring gut microbiota dysbiosis in mice. Mol. Nutr. Food Res. 2022, 66, 2100892. [Google Scholar] [CrossRef]
- Xue, L.; He, J.; Gao, N.; Lu, X.; Li, M.; Wu, X.; Liu, Z.; Jin, Y.; Liu, J.; Xu, J.; et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci. Rep. 2017, 7, 45176. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cheng, M.; Zhang, X.; Cao, J.; Wu, Z.; Weng, P. Green tea polyphenols reduce obesity in high-fat diet-induced mice by modulating intestinal microbiota composition. Int. J. Food Sci. Technol. 2017, 52, 1723–1730. [Google Scholar] [CrossRef]
- Tian, X.; Yu, Z.; Feng, P.; Ye, Z.; Li, R.; Liu, J.; Hu, J.; Kakade, A.; Liu, P.; Li, X. Lactobacillus plantarum TW1-1 alleviates diethylhexylphthalate-induced testicular damage in mice by modulating gut microbiota and decreasing inflammation. Front. Cell. Infect. Microbiol. 2019, 9, 221. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G.; et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes 2021, 13, 1930874. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zhou, J.; Su, Y.; Mao, K.; Wu, J.; Zhu, C.; He, L.; Cui, Y. Gut microbiota composition and functional prediction in diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2021, 21, 105. [Google Scholar] [CrossRef]
- Chi, X.; Liu, Z.; Wang, H.; Wang, Y.; Xu, B.; Wei, W. Regulation of a new type of selenium-rich royal jelly on gut microbiota profile in mice. Biol. Trace Elem. Res. 2022, 200, 1763–1775. [Google Scholar] [CrossRef]
- Tvede, M.; Bondesen, S.; Haagen, N.O.; Nørby, R.S. Serum Antibodies to bacteroides species in chronic inflammatory bowel disease. Scand. J. Gastroenterol. 1983, 18, 783–789. [Google Scholar] [CrossRef]
- Wei, X.; Tao, J.; Xiao, S.; Jiang, S.; Shang, E.; Zhu, Z.; Qian, D.; Duan, J. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci. Rep. 2018, 8, 3685. [Google Scholar] [CrossRef]
- Jing, Y.; Li, A.; Liu, Z.; Yang, P.; Wei, J.; Chen, X.; Zhao, T.; Bai, Y.; Zha, L.; Zhang, C. Absorption of Codonopsis pilosula saponins by coexisting polysaccharides alleviates gut microbial dysbiosis with dextran sulfate sodium-induced colitis in model mice. BioMed Res. Int. 2018, 2018, 1781036. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Han, Y.; Gu, M.; Du, H.; Song, M.; Zhu, X.; Ma, G.; Pan, C.; Wang, W.; Zhao, E.; et al. Foodborne titanium dioxide nanoparticles induce stronger adverse effects in obese mice than non-obese mice: Gut microbiota dysbiosis, colonic inflammation, and proteome alterations. Small 2020, 16, e2001858. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Shen, Y.; Chen, M.; Zhang, Z.; Xiao, S.; Liu, C.; Wan, Y.; Yang, L.; Jiang, S.; Shang, E.; et al. Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 5999–6012. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Liu, S.; Zhang, B.; Hu, Y.; Ma, H.; Wang, S. Green tea leaf powder prevents dyslipidemia in high-fat diet-fed mice by modulating gut microbiota. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Yadav, A.K.; Gupta, P.; Islam, R.; Saraya, A.; Venugopal, S.K. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 2017, 12, 340–349. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).