Formation and Characterization of Fucus virsoides J. Agardh Pigment–Polyethylene Glycol Microparticles Produced Using PGSS Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
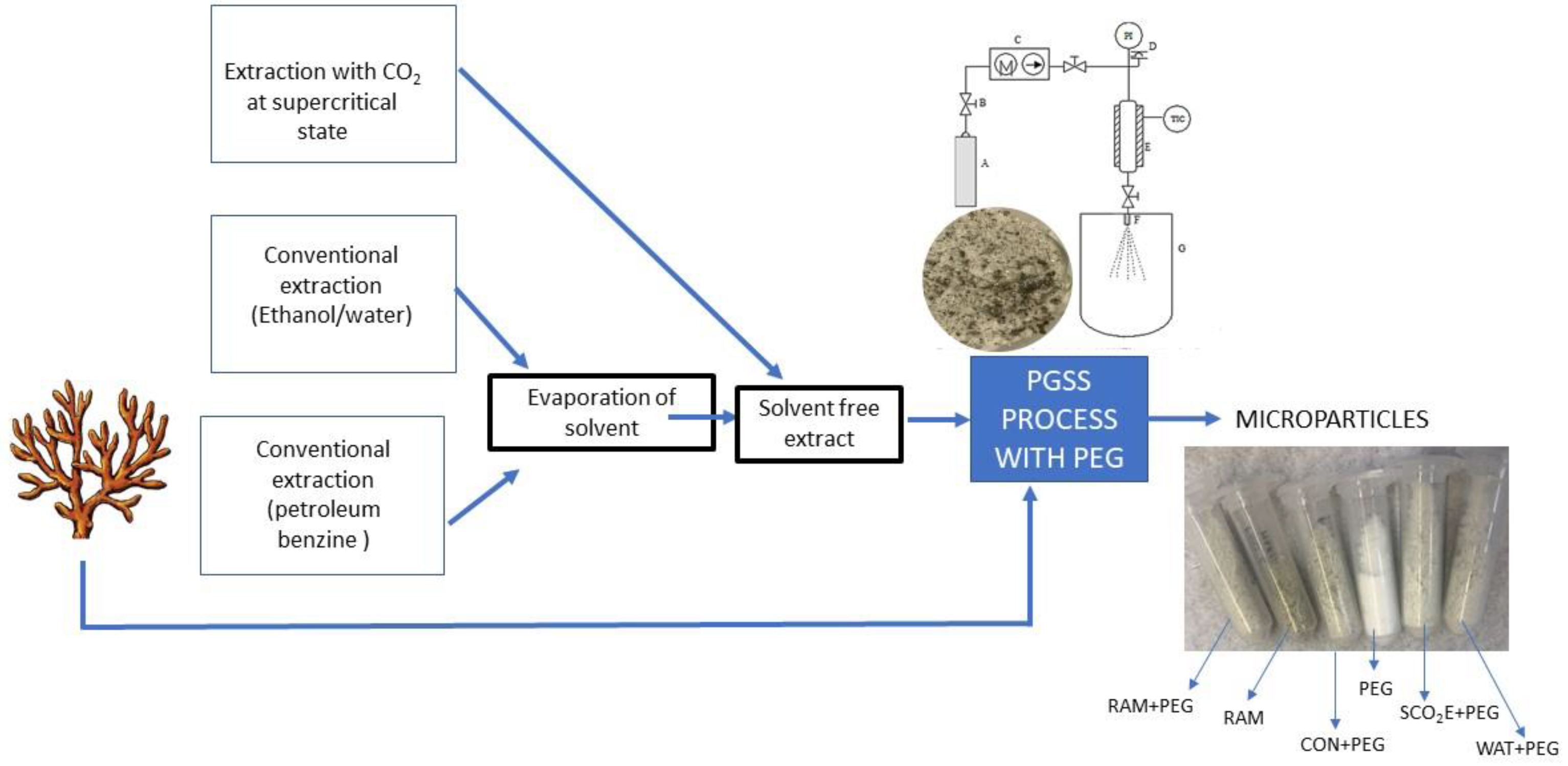
2.2. Extraction Procedure
2.3. Particles from Gas-Saturated Solution (PGSS) Process
2.4. Chemical Characterization of Microparticles
2.5. Determination of Physical Properties of Microparticles
2.5.1. Solubility Properties
2.5.2. Color Properties
2.5.3. Bulk Density
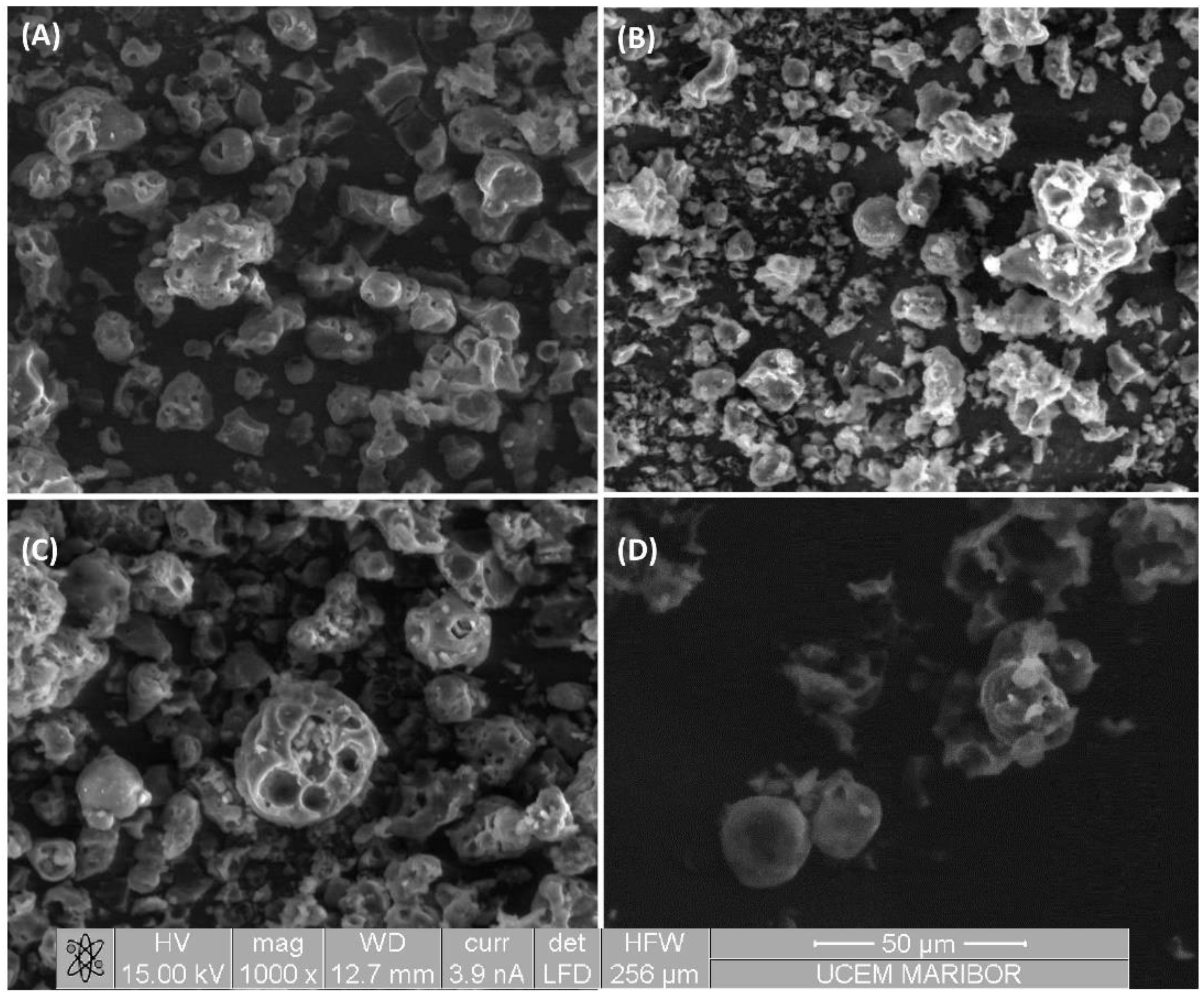
2.6. Environmental Scanning Electron Microscopy (ESEM)
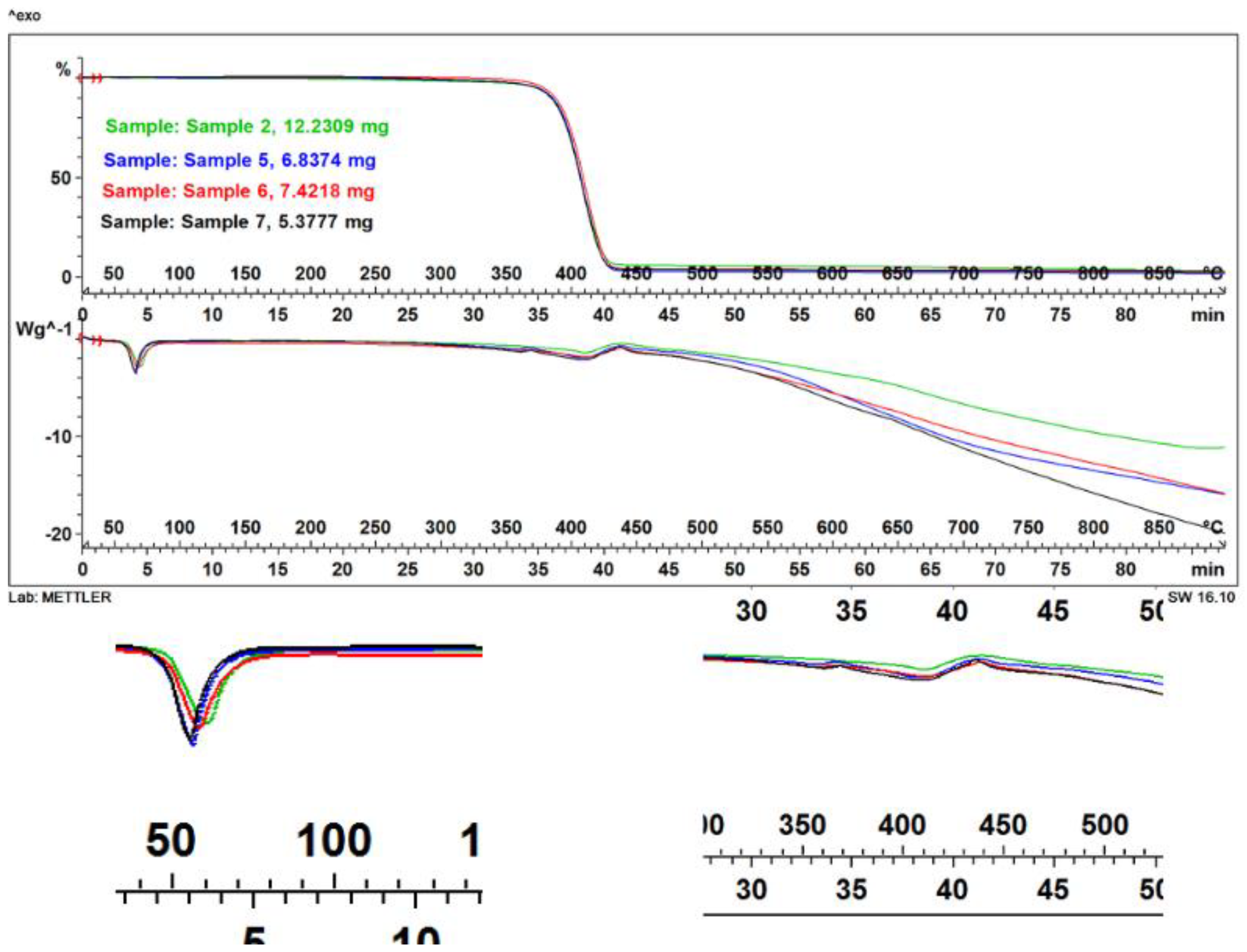
2.7. Thermogravimetric Analysis of Microparticles
2.8. Fourier-Transform Infrared Spectroscopy
3. Results and Discussion
3.1. Encapsulation Yield and Encapsulation Efficiency of Algae Pigment Microparticles
3.2. Fourier-Transform Infrared Spectroscopy (FTIR) of Algal Pigment Microparticles
3.3. Morphology of Algal Pigment Microparticles
3.4. Thermal Behavior of Algal Pigment Microparticles
3.5. Physical Properties of Algal Pigment Microparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of different commercial dried algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, metabolite of fucoxanthin, improves obesity-induced inflammation in adipocyte cells. Mar. Drugs 2015, 13, 4799–4813. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bai, Y.; Xu, Z.; Shi, Y.; Sun, Y.; Janaswamy, S.; Yu, C.; Qi, H. Phlorotannins from Undaria pinnatifida Sporophyll: Extraction, Antioxidant, and Anti-Inflammatory Activities. Mar. Drugs 2019, 17, 434. [Google Scholar] [CrossRef]
- Azeem, M.; Iqbal, N.; Mir, R.A.; Adeel, S.; Batool, F.; Khan, A.A.; Gul, S. Harnessing natural colorants from algal species for fabric dyeing: A sustainable eco-friendly approach for textile processing. J. Appl. Phycol. 2019, 31, 3941–3948. [Google Scholar] [CrossRef]
- El-Khatib, E.; Ali, N.F.; El-Mohamedy, R. Enhancing dyeing of wool fibers with colorant pigment extracted from green algae. J. Chem. Pharm. Res. 2016, 8, 614–619. [Google Scholar]
- Cikoš, A.M.; Šubarić, D.; Roje, M.; Babić, J.; Jerković, I.; Jokić, S. Recent advances on macroalgal pigments and their biological activities (2016–2021). Algal Res. 2022, 65, 102748. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E. Carotenoids microencapsulation by spray drying method and supercritical micronization. Int. Food Res. J. 2017, 99, 891–901. [Google Scholar] [CrossRef]
- Akolade, J.O.; Balogun, M.; Swanepoel, A.; Ibrahim, R.B.; Yusuf, A.A.; Labuschagne, P. Microencapsulation of eucalyptol in polyethylene glycol and polycaprolactone using particles from gas-saturated solutions. RSC Adv. 2019, 23, 34039–34049. [Google Scholar] [CrossRef]
- Priamo, W.L.; Dalmolin, I.; Boschetto, D.L.; Mezzomo, N.; Ferreira, S.R.S.; de Oliveira, J.V. Micronization processes by supercritical fluid technologies: A short review on process design (2008–2012). Acta Sci. Technol. 2013, 35, 695–709. [Google Scholar]
- Islam, T.; Al Ragib, A.; Ferdosh, S.; Uddin, A.H.; Haque Akanda, M.J.; Mia, M.A.R.; Islam Sarker, M.Z. Development of nanoparticles for pharmaceutical preparations using supercritical techniques. Chem. Eng. Com. 2022, 209, 1642–1663. [Google Scholar] [CrossRef]
- Kwon, K.T.; Uddin, M.S.; Jung, G.W.; Chun, B.S. Preparation of micro particles of functional pigments by gas-saturated solution process using supercritical carbon dioxide and polyethylene glycol. Korean J. Chem. Eng. 2011, 28, 2044. [Google Scholar] [CrossRef]
- Knez, Ž.; Škerget, M.; Hrnčič, M.K.; Čuček, D. Particle formation using sub-and supercritical fluids. In Supercritical Fluid Technology for Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 31–67. [Google Scholar]
- Knez, Ž.; Knez Hrnčič, M.; Škerget, M. Particle formation and product formulation using supercritical fluids. Annu. Rev. Chem. Biomol. Eng. 2015, 6, 379–407. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Perrut, M. Particle design using supercritical fluids: Literature and patent survey. J. Supercrit. Fluids 2011, 20, 179–219. [Google Scholar] [CrossRef]
- Perko, T.; Ravber, M.; Knez, Ž.; Škerget, M. Isolation, characterization and formulation of curcuminoids and in vitro release study of the encapsulated particles. J. Supercrit. Fluids 2015, 103, 48–54. [Google Scholar] [CrossRef]
- Weidner, E.; Wiesmet, V.; Knez, Ž.; Skerget, M. Phase equilibrium (solid-liquidgas)in polyethyleneglycol-carbon dioxide systems. J. Supercrit. Fluids 1997, 10, 139–147. [Google Scholar] [CrossRef]
- Vo, D.T.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Fucoxanthin-rich oil encapsulation using biodegradable polyethylene glycol and particles from gas-saturated solutions technique. J. CO2 Util. 2018, 26, 359–369. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. chlorophylls and carotenoids: Measurement and characterization by uv-vis spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- García-González, C.A.; Argemí, A.; Sousa, A.R.S.; de Duarte, C.M.M.; Saurina, J.; Domingo, C. Encapsulation efficiency of solid lipid hybrid particles prepared using the PGSS® technique and loaded with different polarity active agents. J. Supercrit. Fluids 2010, 54, 342–347. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Quílez, C.; Barros, J.; Velasco, D.; Alvarez-Lorenzo, C.; Jorcano, J.L.; Monteiro, F.J.; García-González, C.A. Lidocaine-loaded solid lipid microparticles (SLMPs) produced from gas-saturated solutions for wound applications. Pharmaceutics 2020, 12, 870. [Google Scholar] [CrossRef]
- Lee, S.C.; Surendhiran, D.; Chun, B.S. Extraction and encapsulation of squalene-rich cod liver oil using supercritical CO2 process for enhanced oxidative stability. J. CO2 Util. 2022, 62, 102104. [Google Scholar] [CrossRef]
- Kartikaningsih, H.; Mufti, E.D.; Nurhanief, A.E. Fucoxanthin from brown seaweed Sargassum cristaefolium tea in acid pH. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; Volume 1844, p. 030009. [Google Scholar]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M.; Panciani, P.P.; Ugazio, E.; Sapino, S.; Peira, E.; Chirio, D. Techniques for the Preparation of Solid Lipid Nano and Microparticles. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; IntechOpen: London, UK, 2014; ISBN 978-953-51-1628-8. [Google Scholar]
- Ghosh, M.K.; Wahed, M.I.I.; Ali, M.A.; Barman, R.K. Formulation and characterization of fenofibrate loaded solid dispersion with enhanced dissolution profile. J. Pharm. Pharmacol. 2019, 10, 343–355. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2018, 128, 259–274. [Google Scholar] [CrossRef]
- Karaman, S.; Karaipekli, A.; Sarı, A.; Biçer, A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1647–1653. [Google Scholar] [CrossRef]
- Matishov, G.G.; Makarov, M.V. Changes in the pigment composition of Fucus Vesiculosus l. and Fucus Serratus l. from the barents sea during prolonged exposure to the dark. Dokl. Biol. Sci. 2004, 397, 338–339. [Google Scholar] [CrossRef]
- Shaygannia, S.; Eshaghi, M.R.; Fazel, M.; Hashemiravan, M. The Effect of Microencapsulation of Phenolic Compounds from Lemon Waste by Persian and Basil Seed Gums on the Chemical and Microbiological Properties of Mayonnaise. Prev. Nutr. Food Sci. 2021, 31, 82–91. [Google Scholar] [CrossRef]
- Ahmed, M.; Akter, M.S.; Lee, J.C.; Eun, J.B. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT Food Sci. Technol. 2010, 43, 1307–1312. [Google Scholar] [CrossRef]
- Haq, M.; Chun, B.S. Microencapsulation of omega-3 polyunsaturated fatty acids and astaxanthin-rich salmon oil using particles from gas saturated solutions (PGSS) process. LWT 2018, 92, 523–530. [Google Scholar] [CrossRef]
- Abdullah, E.C.; Geldart, D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999, 102, 151–165. [Google Scholar] [CrossRef]
- Gupta, A.K.; Seth, K.; Maheshwari, K.; Baroliya, P.K.; Meena, M.; Kumar, A.; Vinayak, V. Biosynthesis and extraction of high-value carotenoid from algae. Front. Biosci. Landmark 2021, 26, 171–190. [Google Scholar]
- Esquivel-Hernández, D.A.; Ibarra-Garza, I.P.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.d.; Alemán-Nava, G.S.; García-Pérez, J.S.; Parra-Saldívar, R. Green extraction technologies for high-value metabolites from algae: A review. Biofuels Bioprod. Biorefining 2017, 11, 215–231. [Google Scholar] [CrossRef]



| Microparticles | Encapsulation Yield (%) | EE Chlorophyll a (%) | EE Chlorophyll b (%) | EE Carotenoids (%) |
|---|---|---|---|---|
| RAM+PEG | 61.60 | 56.98 | 65.48 | 43.02 |
| SCO2E+PEG | 66.03 | 95.00 | 75.91 | 62.31 |
| CON+PEG | 64.24 | 70.73 | 83.40 | 57.33 |
| WAT+PEG | 73.73 | 57.01 | 70.95 | 55.37 |
| TOm (°C) | Tm (°C) | ΔHm (J/g) | TOmax (°C) | Tmax (°C) | ΔHmax (J/g) | |
|---|---|---|---|---|---|---|
| RAM+PEG | 45.47 | 63.59 | 181 | 382.53 | 411.64 | 101.17 |
| SCO2E+PEG | 37.47 | 60.44 | 191 | 372.77 | 408.90 | 222.81 |
| CON+PEG | 46.36 | 62.01 | 173 | 370.49 | 413.86 | 188.07 |
| WAT+PEG | 48.65 | 60.26 | 171 | 372.12 | 410.87 | 250.85 |
| For PEG Tm (°C)= 61.3, ΔHm=170 | ||||||
| Micro | Dry Matter (%) | Mean Diameter (μm) | COLOR | ΔE | WI | WAI (g/g) | WSI (%) |
|---|---|---|---|---|---|---|---|
| PEG | 98.67 | - |  | - | - | 0.073 | 99.71 |
| RAM+PEG | 98.66 | 24.09 |  | 13.34 | 80.59 | 0.490 | 93.32 |
| SCO2E+PEG | 99.47 | 16.49 |  | 7.97 | 85.84 | 0.097 | 98.71 |
| CON+PEG | 98.92 | 17.68 |  | 9.41 | 84.42 | 0.190 | 97.86 |
| WAT+PEG | 98.99 | 17.98 |  | 15.92 | 77.96 | 0.195 | 97.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banožić, M.; Čolnik, M.; Škerget, M.; Cikoš, A.-M.; Aladić, K.; Jokić, S. Formation and Characterization of Fucus virsoides J. Agardh Pigment–Polyethylene Glycol Microparticles Produced Using PGSS Process. Appl. Sci. 2022, 12, 11496. https://doi.org/10.3390/app122211496
Banožić M, Čolnik M, Škerget M, Cikoš A-M, Aladić K, Jokić S. Formation and Characterization of Fucus virsoides J. Agardh Pigment–Polyethylene Glycol Microparticles Produced Using PGSS Process. Applied Sciences. 2022; 12(22):11496. https://doi.org/10.3390/app122211496
Chicago/Turabian StyleBanožić, Marija, Maja Čolnik, Mojca Škerget, Ana-Marija Cikoš, Krunoslav Aladić, and Stela Jokić. 2022. "Formation and Characterization of Fucus virsoides J. Agardh Pigment–Polyethylene Glycol Microparticles Produced Using PGSS Process" Applied Sciences 12, no. 22: 11496. https://doi.org/10.3390/app122211496
APA StyleBanožić, M., Čolnik, M., Škerget, M., Cikoš, A.-M., Aladić, K., & Jokić, S. (2022). Formation and Characterization of Fucus virsoides J. Agardh Pigment–Polyethylene Glycol Microparticles Produced Using PGSS Process. Applied Sciences, 12(22), 11496. https://doi.org/10.3390/app122211496








